A Comparative Assessment of Myocardial Work Performance during Spontaneous Rhythm, His Bundle Pacing, and Left Bundle Branch Area Pacing: Insights from the EMPATHY Study
Abstract
:1. Clinical Perspective
2. Background
3. Methods
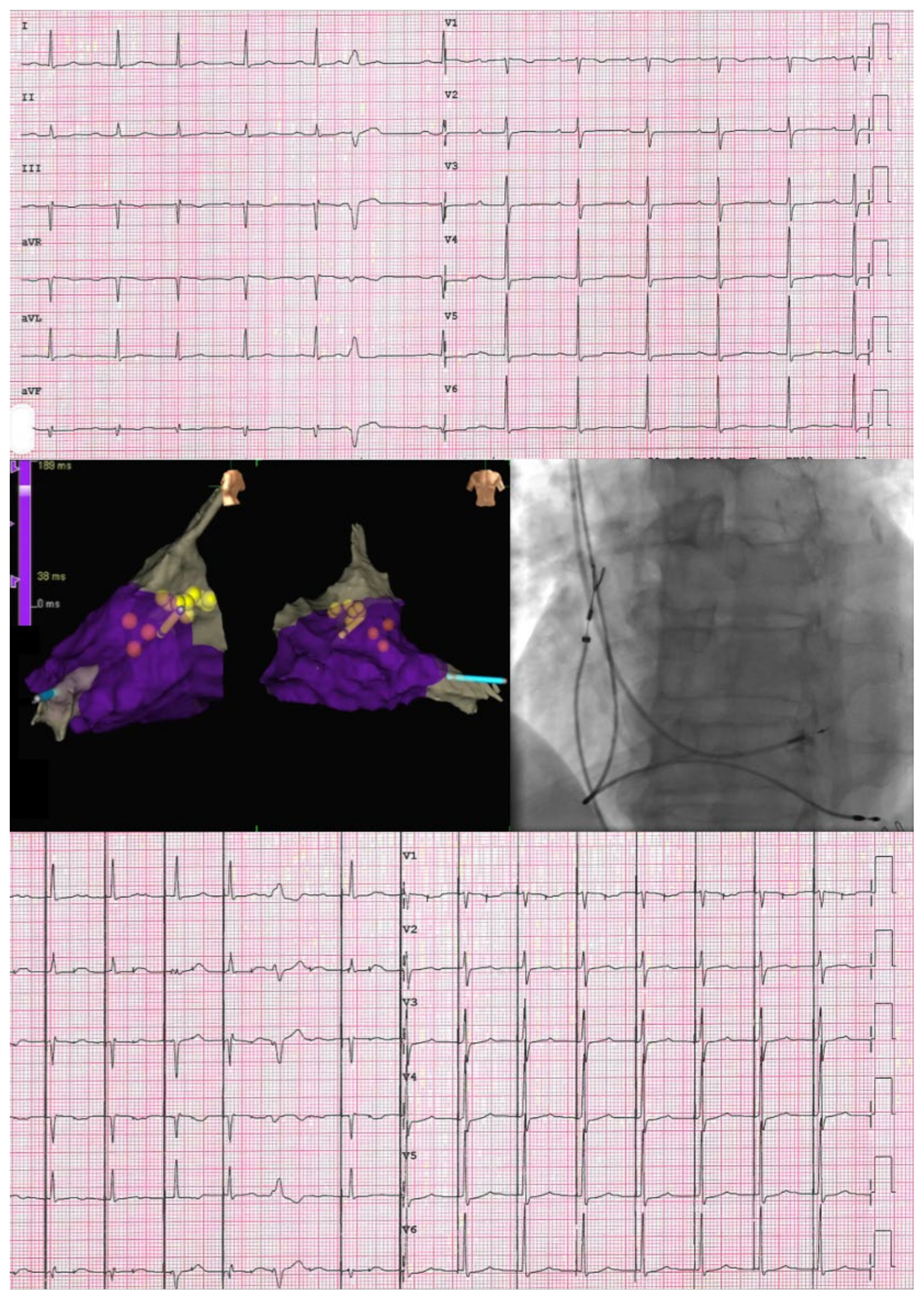
3.1. Implantation
3.1.1. His Bundle Pacing
3.1.2. Left Bundle Branch Area Pacing
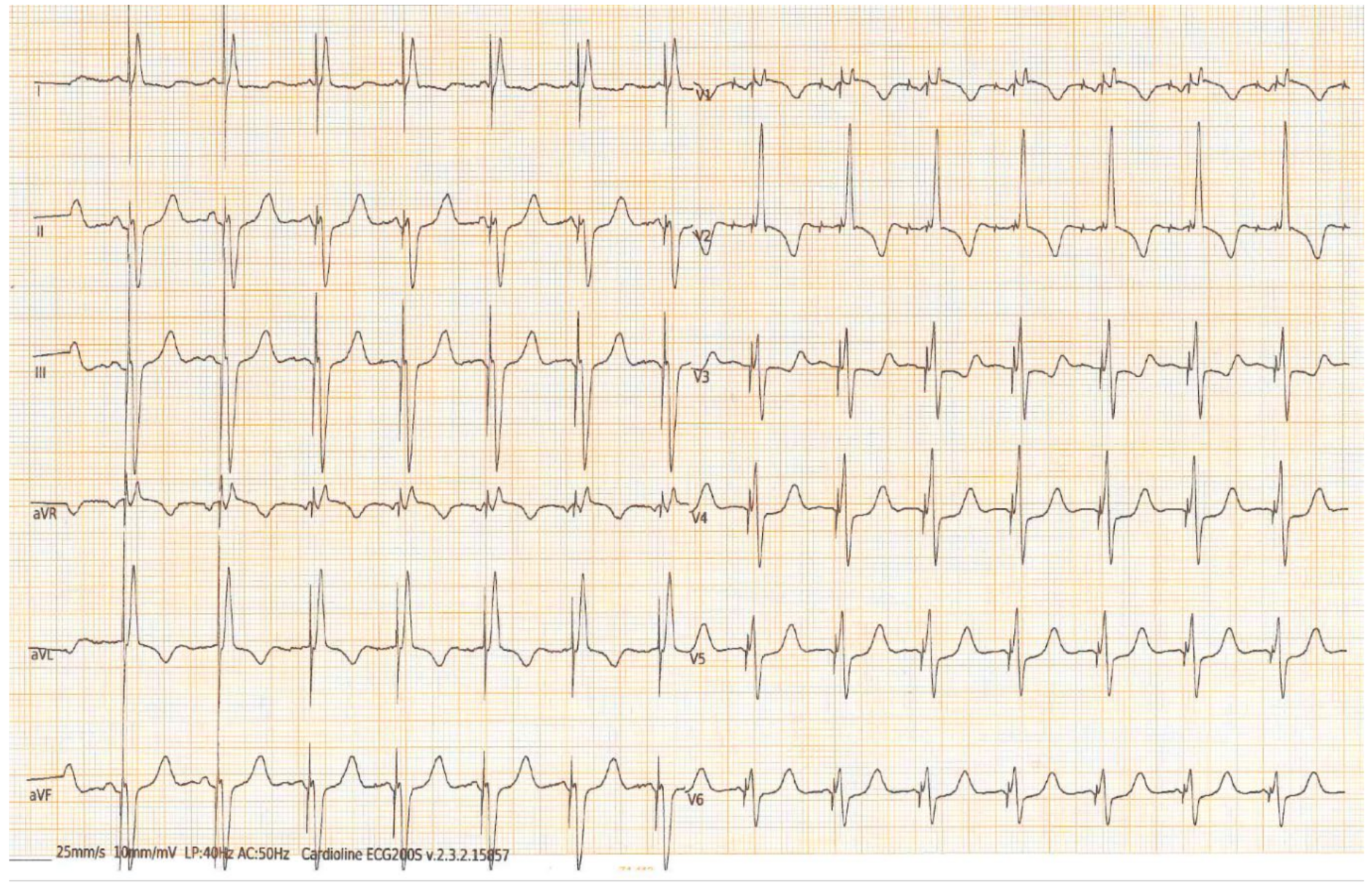
3.2. Imaging and Electrocardiogram
- Global constructive work (GCW): the sum of work performed during myocardial shortening in systole and myocardial lengthening during isovolumetric diastole;
- Global wasted work (GWW): the sum of work performed by myocardial lengthening in systole and myocardial shortening during isovolumetric diastole;
- Global work index (GWI): the work performed throughout systole, specifically between mitral valve closure and opening;
- Global work efficiency (GWE): expressed as the percentage ratio of GCW to the sum of GCW and GWW.
3.3. Statistical Analysis
4. Results
ECG and SVA Myocardial Work
5. Discussion
- (1)
- There were no statistically significant differences in terms of myocardial work index and global constructive work between spontaneous and paced activation in both LBBAP and HBP groups;
- (2)
- The relative difference in all myocardial work parameters between sinus and paced activation did not show statistically significant differences between the two groups.
- (3)
- The relative difference between spontaneous and paced QRS durations did not exhibit statistically significant differences between the HBP and LBBAP groups.
Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, A.D.; Rizo-Patron, C.; Hallstrom, A.P.; O’Neill, G.P.; Rothbart, S.; Martins, J.B.; Roelke, M.; Steinberg, J.S.; Greene, H.L.; DAVID Investigators. Percent right ventricular pacing predicts outcomes in the DAVID trial. Heart Rhythm 2005, 2, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.O.; Hellkamp, A.S.; Ellenbogen, K.A.; Greenspon, A.J.; Freedman, R.A.; Lee, K.L.; Lamas, G.A. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2003, 107, 2932–2937. [Google Scholar] [CrossRef] [PubMed]
- Wilkoff, B.L.; Cook, J.R.; Epstein, A.E.; Greene, H.L.; Hallstrom, A.P.; Hsia, H.; Kutalek, S.P.; Sharma, A.; Chamber, D.; VVI Implantable Defibrillator Trial Investigators. Dual chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: The dual chamber and VVI implantable defibrillator (DAVID) trial. JAMA 2002, 288, 3115–3123. [Google Scholar] [PubMed]
- Nahlawi, M.; Waligora, M.; Spies, S.M.; Bonow, R.O.; Kadish, A.H.; Goldberger, J.J. Left ventricular function during and after right ventricular pacing. J. Am. Coll. Cardiol. 2004, 44, 1883–1888. [Google Scholar] [CrossRef] [PubMed]
- Herweg, B.; Welter-Frost, A.; Vijayaraman, P. The evolution of cardiac resynchronization therapy and an introduction to conduction system pacing: A conceptual review. Europace 2021, 23, 496–510. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, P.; Bordachar, P.; Ellenbogen, K.A. The Continued Search for Physiological Pacing: Where Are We Now? J. Am. Coll. Cardiol. 2017, 69, 3099–3114. [Google Scholar] [CrossRef] [PubMed]
- Togashi, I.; Sato, T. Conduction system pacing: Current status and prospects. J. Cardiol. 2023, 81, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, P.; Chung, M.K.; Dandamudi, G.; Upadhyay, G.A.; Krishnan, K.; Crossley, G.; Campbell, K.B.; Lee, B.K.; Refaat, M.M.; Saksena, S.; et al. His Bundle Pacing. J. Am. Coll. Cardiol. 2018, 72, 927–947. [Google Scholar] [CrossRef]
- Catanzariti, D.; Maines, M.; Manica, A.; Angheben, C.; Varbaro, A.; Vergara, G. Permanent His-bundle pacing maintains long-term ventricular synchrony and left ventricular performance, unlike conventional right ventricular apical pacing. Europace 2012, 15, 546–553. [Google Scholar] [CrossRef]
- Li, Y.; Chen, K.; Dai, Y.; Li, C.; Sun, Q.; Chen, R.; Gold, M.R.; Zhang, S. Left bundle branch pacing for symptomatic bradycardia: Implant success rate, safety, and pacing characteristics. Heart Rhythm 2019, 16, 1758–1765. [Google Scholar] [CrossRef]
- Su, L.; Wang, S.; Wu, S.; Xu, L.; Huang, Z.; Chen, X.; Zheng, R.; Jiang, L.; Ellenbogen, K.A.; Whinnett, Z.I.; et al. Long-Term Safety and Feasibility of Left Bundle Branch Pacing in a Large Single-Center Study. Circ. Arrhythmia Electrophysiol. 2021, 14, e009261. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, S.S.; Arora, V.; Namboodiri, N.; Kumar, V.; Kapoor, A.; Vijayaraman, P. Left bundle branch pacing: A comprehensive review. J Cardiovasc. Electrophysiol. 2020, 31, 2462–2473. [Google Scholar] [CrossRef] [PubMed]
- Malagù, M.; Vitali, F.; Massafra, R.F.; Cardelli, L.S.; Pavasini, R.; Guardigli, G.; Rapezzi, C.; Bertini, M. Three-Dimensional Electroanatomical Mapping and Myocardial Work Performance during Spontaneous Rhythm, His Bundle Pacing and Right Ventricular Pacing: The EMPATHY Study. J. Cardiovasc. Dev. Dis. 2022, 9, 377. [Google Scholar] [PubMed]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, P.; Dandamudi, G.; Zanon, F.; Sharma, P.S.; Tung, R.; Huang, W.; Koneru, J.; Tada, H.; Ellenbogen, K.A.; Lustgarten, D.L. Permanent His bundle pacing: Recommendations from a Multicenter His Bundle Pacing Collaborative Working Group for standardization of definitions, implant measurements, and follow-up. Heart Rhythm. 2018, 15, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace 2021, 23, 1612–1676. [Google Scholar] [CrossRef] [PubMed]
- Malagù, M.; Vitali, F.; Brieda, A.; Cimaglia, P.; De Raffele, M.; Tazzari, E.; Musolino, C.; Balla, C.; Serenelli, M.; Cultrera, R.; et al. Antibiotic prophylaxis based on individual infective risk stratification in cardiac implantable electronic device: The PRACTICE study. Europace 2022, 24, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Burri, H.; Jastrzebski, M.; Cano, Ó.; Čurila, K.; de Pooter, J.; Huang, W.; Israel, C.; Joza, J.; Romero, J.; Vernooy, K.; et al. EHRA clinical consensus statement on conduction system pacing implantation: Endorsed by the Asia Pacific Heart Rhythm Society (APHRS), Canadian Heart Rhythm Society (CHRS), and Latin American Heart Rhythm Society (LAHRS). Europace 2023, 25, 1208–1236. [Google Scholar] [CrossRef]
- Huang, W.; Chen, X.; Su, L.; Wu, S.; Xia, X.; Vijayaraman, P. A beginner’s guide to permanent left bundle branch pacing. Heart Rhythm. 2019, 16, 1791–1796. [Google Scholar] [CrossRef]
- Papadopoulos, K.; Tok, Ö.Ö.; Mitrousi, K.; Ikonomidis, I. Myocardial Work: Methodology and Clinical Applications. Diagnostics 2021, 11, 573. [Google Scholar] [CrossRef]
- Boe, E.; Skulstad, H.; Smiseth, O.A. Myocardial work by echocardiography: A novel method ready for clinical testing. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Remme, E.W.; Haugaa, K.H.; Opdahl, A.; Fjeld, J.G.; Gjesdal, O.; et al. A novel clinical method for quantification of regional left ventricular pressure–strain loop area: A non-invasive index of myocardial work. Eur. Heart J. 2012, 33, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Ghio, S.; Constantin, C.; Klersy, C.; Serio, A.; Fontana, A.; Campana, C.; Tavazzi, L. Interventricular and intraventricular dys- synchrony are common in heart failure patients, regardless of QRS duration. Eur. Heart J. 2004, 25, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.; Subzposh, F.A.; Beer, D.; Durr, B.; Naperkowski, A.; Sun, H.; Oren, J.W.; Dandamudi, G.; Vijayaraman, P. Clinical Outcomes of His Bundle Pacing Compared to Right Ventricular Pacing. J. Am. Coll. Cardiol. 2018, 71, 2319–2330. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yang, L.; Zheng, S.; Mai, J.; Wei, Y.; Liu, Y.; Deng, B.; Lv, H.; Chen, Y.; Qiu, Q. Left bundle branch pacing on mechanical synchrony and myocardial work in bradycardia patients. Int. J. Cardiovasc. Imaging 2023, 39, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, Y.; Dai, Y.; Sun, Q.; Luo, B.; Li, C.; Zhang, S. Comparison of electrocardiogram characteristics and pacing parameters between left bundle branch pacing and right ventricular pacing in patients receiving pacemaker therapy. Europace 2019, 21, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.S.; Dandamudi, G.; Naperkowski, A.; Oren, J.W.; Storm, R.H.; Ellenbogen, K.A.; Vijayaraman, P. Permanent His-bundle pacing is feasible, safe, and superior to right ventricular pacing in routine clinical practice. Heart Rhythm. 2015, 12, 305–312. [Google Scholar] [CrossRef]
- Keene, D.; Arnold, A.D.; Jastrzębski, M.; Burri, H.; Zweibel, S.; Crespo, E.; Chandrasekaran, B.; Bassi, S.; Joghetaei, N.; Swift, M.; et al. His bundle pacing, learning curve, procedure characteristics, safety, and feasibility: Insights from a large international observational study. J. Cardiovasc. Electrophysiol. 2019, 30, 1984–1993. [Google Scholar] [CrossRef]
- Bertini, M.; Ng, A.C.; Antoni, M.L.; Nucifora, G.; Ewe, S.H.; Auger, D.; Marsan, N.A.; Schalij, M.J.; Bax, J.J.; Delgado, V. Global longitudinal strain predicts long-term survival in patients with chronic ischemic cardiomyopathy. Circ. Cardiovasc. Imaging 2012, 5, 383–391. [Google Scholar] [CrossRef]
| HBP (12 Patients) | LBBAP (12 Patients) | p-Value | |
|---|---|---|---|
| Baseline characteristics | |||
| Age (years) | 79 (73–85) | 81 (73–85) | 0.88 |
| Female sex | 2 (17) | 2 (17) | 1.00 |
| BMI (kg/m2) | 25.1 (23.4–28.3) | 24.7 (22.3–29.1) | 0.69 |
| CAD | 4 (33) | 2 (17) | 0.64 |
| Atrial fibrillation | 7 (58) | 5 (42) | 0.68 |
| Diabetes mellitus | 0 (0) | 4 (33) | 0.093 |
| Hypertension | 10 (83) | 9 (75) | 1.00 |
| Dyslipidemia | 6 (50) | 8 (67) | 0.68 |
| Procedural data | |||
| Procedure duration (min) | 125 (120–140) | 81 (70–120) | 0.004 |
| Fluoroscopy time (min) | 13 (9–21) | 6 (5–11) | 0.010 |
| Selective HPB capture | 6 (50) | - | - |
| Electrical parameters | |||
| Lead impedance (ohm) | 509 (447–568) | 718 (609–795) | 0.002 |
| Lead sensing (mV) | 2.4 (1.8–4.8) | 9.4 (8–13.5) | <0.001 |
| Lead threshold (V@0.4 ms) | 1.0 (0.5–2.5) | 0.6 (0.4–0.8) | 0.045 |
| EF (%) | 51 (42–63) | 55 (51–56) | 0.750 |
| GLS (%) | −14 (−19–−11) | −15 (−18–−10) | 0.410 |
| Left atrial volume (mL/m2) | 41 ± 10 | 39 ± 13 | 0.721 |
| HBP (12 Patients) | LBBAP (12 Patients) | p-Value | |
|---|---|---|---|
| Spontaneous QRS duration (ms) | 106 (88–140) | 115 (90–132) | 0.76 |
| Paced QRS duration (ms) | 124 (98–140) | 128 (118–136) | 0.62 |
| Spontaneous GWI (mmHg%) | 1120 (902–1763) | 1548 (1072–2273) | 0.094 |
| Spontaneous GCW (mmHg%) | 1648 (1044–2152) | 2089 (1705–2594) | 0.052 |
| Spontaneous GWW (mmHg%) | 217 (125–249) | 137 (105–286) | 0.670 |
| Spontaneous GWE (mmHg%) | 87 (80–90) | 93 (83–95) | 0.065 |
| HBP (12 Patients) | |||
|---|---|---|---|
| Spontaneous | Paced | p-value | |
| QRS duration (ms) | 106 (88–140) | 124 (98–140) | 0.75 |
| GWI (mmHg%) | 1110 (902–1763) | 1020 (822–1969) | 0.534 |
| GCW (mmHg%) | 1648 (1044–2152) | 1505 (1151–2133) | 0.075 |
| GWW (mmHg%) | 217 (125–249) | 283 (205–354) | 0.016 |
| GWE (mmHg%) | 87 (80–90) | 82 (73–90) | 0.049 |
| LBBAP (12 patients) | |||
| Spontaneous | Paced | p-value | |
| QRS duration (ms) | 115 (90–132) | 128 (118–136) | 0.05 |
| GWI (mmHg%) | 1548 (1072–2273) | 1545 (1363–1961) | 0.374 |
| GCW (mmHg%) | 2089 (1705–2594) | 2320 (2073–2530) | 0.929 |
| GWW (mmHg%) | 137 (105–286) | 264 (195–341) | 0.091 |
| GWE (mmHg%) | 93 (83–95) | 87 (83–92) | 0.109 |
| HBP (12 Patients) | LBBAP (12 Patients) | p-Value | |
|---|---|---|---|
| dQRS (%) | 0 (−7.5–+42.8) | +16.4 (−4.9–+32.0) | 0.54 |
| dGWI (%) | +0.1 (−18.4–+36.4) | −6.3 (−22.6–+24.4) | 0.67 |
| dGCW (%) | 12.3 (−2.8–+16.2) | −0.9 (−10.2–+6.2) | 0.25 |
| dGWW (%) | +66.4 (+8.4–+91.7) | +113.3 (−5.3–+218.2) | 0.53 |
| dGWE (%) | −2.4 (−16.7–+1.1) | −5.3 (−9.7–+1.1) | 0.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azzolini, G.; Bianchi, N.; Vitali, F.; Malagù, M.; Balla, C.; De Raffele, M.; Bertini, M. A Comparative Assessment of Myocardial Work Performance during Spontaneous Rhythm, His Bundle Pacing, and Left Bundle Branch Area Pacing: Insights from the EMPATHY Study. J. Cardiovasc. Dev. Dis. 2023, 10, 444. https://doi.org/10.3390/jcdd10110444
Azzolini G, Bianchi N, Vitali F, Malagù M, Balla C, De Raffele M, Bertini M. A Comparative Assessment of Myocardial Work Performance during Spontaneous Rhythm, His Bundle Pacing, and Left Bundle Branch Area Pacing: Insights from the EMPATHY Study. Journal of Cardiovascular Development and Disease. 2023; 10(11):444. https://doi.org/10.3390/jcdd10110444
Chicago/Turabian StyleAzzolini, Giorgia, Nicola Bianchi, Francesco Vitali, Michele Malagù, Cristina Balla, Martina De Raffele, and Matteo Bertini. 2023. "A Comparative Assessment of Myocardial Work Performance during Spontaneous Rhythm, His Bundle Pacing, and Left Bundle Branch Area Pacing: Insights from the EMPATHY Study" Journal of Cardiovascular Development and Disease 10, no. 11: 444. https://doi.org/10.3390/jcdd10110444
APA StyleAzzolini, G., Bianchi, N., Vitali, F., Malagù, M., Balla, C., De Raffele, M., & Bertini, M. (2023). A Comparative Assessment of Myocardial Work Performance during Spontaneous Rhythm, His Bundle Pacing, and Left Bundle Branch Area Pacing: Insights from the EMPATHY Study. Journal of Cardiovascular Development and Disease, 10(11), 444. https://doi.org/10.3390/jcdd10110444