Useful Electrocardiographic Signs to Support the Prediction of Favorable Response to Cardiac Resynchronization Therapy
Abstract
:1. Introduction
2. The problem of Defining the “True” Left Bundle Branch Block
3. ECG Signs Predicting CRT Response beyond the Classical LBBB Markers
3.1. QRS Fractionation
3.2. Signs of Residual Left-Bundle Branch Conduction
3.3. S-Waves
3.4. Axis Deviation
3.5. Universal QRS Signs of Delayed Left Ventricular Activation
3.6. The Prolongation of the PR Interval
4. Limitations
5. Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leclercq, C.; Cazeau, S.; Le Breton, H.; Ritter, P.; Mabo, P.; Gras, D.; Pavin, D.; Lazarus, A.; Daubert, J.-C. Acute hemodynamic effects of biventricular DDD pacing in patients with end-stage heart failure. J. Am. Coll. Cardiol. 1998, 32, 1825–1831. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.T.; Fisher, W.G.; Smith, A.L.; Delurgio, D.B.; Leon, A.R.; Loh, E.; Kocovic, D.Z.; Packer, M.; Clavell, A.L.; Hayes, D.L.; et al. Cardiac Resynchronization in Chronic Heart Failure. N. Engl. J. Med. 2002, 346, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Bristow, M.R.; Saxon, L.A.; Boehmer, J.; Krueger, S.; Kass, D.A.; De Marco, T.; Carson, P.; DiCarlo, L.; DeMets, D.; White, B.G.; et al. Cardiac-Resynchronization Therapy with or without an Implantable Defibrillator in Advanced Chronic Heart Failure. N. Engl. J. Med. 2004, 350, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.J.; Hall, W.J.; Cannom, D.S.; Klein, H.; Brown, M.W.; Daubert, J.P.; Estes, N.A.M.; Foster, E.; Greenberg, H.; Higgins, S.L.; et al. Cardiac-Resynchronization Therapy for the Prevention of Heart-Failure Events. N. Engl. J. Med. 2009, 361, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.F.; Daubert, J.-C.; Erdmann, E.; Freemantle, N.; Gras, D.; Kappenberger, L.; Tavazzi, L. The Effect of Cardiac Resynchronization on Morbidity and Mortality in Heart Failure. N. Engl. J. Med. 2005, 352, 1539–1549. [Google Scholar] [CrossRef]
- Tang, A.S.L.; Wells, G.A.; Talajic, M.; Arnold, M.O.; Sheldon, R.; Connolly, S.; Hohnloser, S.H.; Nichol, G.; Birnie, D.H.; Sapp, J.L.; et al. Cardiac-Resynchronization Therapy for Mild-to-Moderate Heart Failure. N. Engl. J. Med. 2010, 363, 2385–2395. [Google Scholar] [CrossRef] [PubMed]
- Linde, C.; Abraham, W.T.; Gold, M.R.; St. John Sutton, M.; Ghio, S.; Daubert, C. Randomized Trial of Cardiac Resynchronization in Mildly Symptomatic Heart Failure Patients and in Asymptomatic Patients with Left Ventricular Dysfunction and Previous Heart Failure Symptoms. J. Am. Coll. Cardiol. 2008, 52, 1834–1843. [Google Scholar] [CrossRef]
- Epstein, A.E.; DiMarco, J.P.; Ellenbogen, K.A.; Estes, N.A.M.; Freedman, R.A.; Gettes, L.S.; Gillinov, A.M.; Gregoratos, G.; Hammill, S.C.; Hayes, D.L.; et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities. Circulation 2008, 117, e350–e408. [Google Scholar] [CrossRef]
- Dickstein, K.; Members, A.T.F.; Cohen-Solal, A.; Filippatos, G.; McMurray, J.J.V.; Ponikowski, P.; Poole-Wilson, P.A.; Strömberg, A.; van Veldhuisen, D.J.; Atar, D.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. Eur. J. Heart Fail. 2008, 10, 933–989. [Google Scholar] [CrossRef]
- Ruschitzka, F.; Abraham, W.T.; Singh, J.P.; Bax, J.J.; Borer, J.S.; Brugada, J.; Dickstein, K.; Ford, I.; Gorcsan, J.; Gras, D.; et al. Cardiac-Resynchronization Therapy in Heart Failure with a Narrow QRS Complex. N. Engl. J. Med. 2013, 369, 1395–1405. [Google Scholar] [CrossRef]
- Beshai, J.F.; Grimm, R.A.; Nagueh, S.F.; Baker, J.H.; Beau, S.L.; Greenberg, S.M.; Pires, L.A.; Tchou, P.J. Cardiac-Resynchronization Therapy in Heart Failure with Narrow QRS Complexes. N. Engl. J. Med. 2007, 357, 2461–2471. [Google Scholar] [CrossRef]
- Thibault, B.; Harel, F.; Ducharme, A.; White, M.; Ellenbogen, K.A.; Frasure-Smith, N.; Roy, D.; Philippon, F.; Dorian, P.; Talajic, M.; et al. Cardiac Resynchronization Therapy in Patients with Heart Failure and a QRS Complex <120 Milliseconds. Circulation 2013, 127, 873–881. [Google Scholar] [CrossRef]
- Auricchio, A.; Prinzen, F.W. Non-Responders to Cardiac Resynchronization Therapy: The Magnitude of the Problem and the Issues. Circ. J. 2011, 75, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Corbisiero, R.; Buck, D.C.; Muller, D.; Bharmi, R.; Dalal, N.; Kazemian, P. What is the cost of non-response to cardiac resynchronization therapy? Hospitalizations and healthcare utilization in the CRT-D population. J. Interv. Card. Electrophysiol. 2016, 47, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Sipahi, I.; Carrigan, T.P.; Rowland, D.Y.; Stambler, B.S.; Fang, J.C. Impact of QRS Duration on Clinical Event Reduction with Cardiac Resynchronization Therapy: Meta-analysis of Randomized Controlled Trials. Arch. Intern. Med. 2011, 171, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Adelstein, E.C.; Saba, S. Usefulness of baseline electrocardiographic QRS complex pattern to predict response to cardiac resynchronization. Am. J. Cardiol. 2009, 103, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Wokhlu, A.; Rea, R.F.; Asirvatham, S.J.; Webster, T.; Brooke, K.; Hodge, D.O.; Wiste, H.J.; Dong, Y.; Hayes, D.L.; Cha, Y.-M. Upgrade and de novo cardiac resynchronization therapy: Impact of paced or intrinsic QRS morphology on outcomes and survival. Heart Rhythm 2009, 6, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Zareba, W.; Klein, H.; Cygankiewicz, I.; Hall, W.J.; McNitt, S.; Brown, M.; Cannom, D.; Daubert, J.P.; Eldar, M.; Gold, M.R.; et al. Effectiveness of Cardiac Resynchronization Therapy by QRS Morphology in the Multicenter Automatic Defibrillator Implantation Trial- Cardiac Resynchronization Therapy (MADIT-CRT). Circulation 2011, 123, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Kutyifa, V.; Stockburger, M.; Daubert, J.P.; Holmqvist, F.; Olshansky, B.; Schuger, C.; Klein, H.; Goldenberg, I.; Brenyo, A.; McNitt, S.; et al. PR interval identifies clinical response in patients with non-left bundle branch block: A Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy substudy. Circ. Arrhythmia Electrophysiol. 2014, 7, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Tracy, C.M.; Epstein, A.E.; Darbar, D.; DiMarco, J.P.; Dunbar, S.B.; Estes, N.A.M.; Ferguson, T.B.; Hammill, S.C.; Karasik, P.E.; Link, M.S.; et al. 2012 ACCF/AHA/HRS Focused Update of the 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2012, 60, 1297–1313. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: Developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) With the special contribution of the European Heart Rhythm Association (EHRA). Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.R.; Thébault, C.; Linde, C.; Abraham, W.T.; Gerritse, B.; Ghio, S.; St John Sutton, M.; Daubert, J.C. Effect of QRS duration and morphology on cardiac resynchronization therapy outcomes in mild heart failure: Results from the Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE) study. Circulation 2012, 126, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Strauss, D.G.; Selvester, R.H.; Wagner, G.S. Defining Left Bundle Branch Block in the Era of Cardiac Resynchronization Therapy. Am. J. Cardiol. 2011, 107, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Willems, J.L.; Robles de Medina, E.O.; Bernard, R.; Coumel, P.; Fisch, C.; Krikler, D.; Mazur, N.A.; Meijler, F.L.; Mogensen, L.; Moret, P.; et al. Criteria for intraventricular conduction disturbances and pre-excitation. J. Am. Coll. Cardiol. 1985, 5, 1261–1275. [Google Scholar] [CrossRef] [PubMed]
- Padanilam, B.J.; Morris, K.E.; Olson, J.A.; Rippy, J.S.; Walsh, M.N.; Subramanian, N.; Vidal, A.; Prystowsky, E.N.; Steinberg, L.A. The Surface Electrocardiogram Predicts Risk of Heart Block During Right Heart Catheterization in Patients with Preexisting Left Bundle Branch Block: Implications for the Definition of Complete Left Bundle Branch Block. J. Cardiovasc. Electrophysiol. 2010, 21, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.; Freemantle, N.; Ghio, S.; Fruhwald, F.; Shankar, A.; Marijanowski, M.; Verboven, Y.; Tavazzi, L. Predicting the Long-Term Effects of Cardiac Resynchronization Therapy on Mortality from Baseline Variables and the Early Response: A Report From the CARE-HF (Cardiac Resynchronization in Heart Failure) Trial. J. Am. Coll. Cardiol. 2008, 52, 438–445. [Google Scholar] [CrossRef]
- Goldenberg, I.; Moss, A.J.; Hall, W.J.; Foster, E.; Goldberger, J.J.; Santucci, P.; Shinn, T.; Solomon, S.; Steinberg, J.S.; Wilber, D.; et al. Predictors of Response to Cardiac Resynchronization Therapy in the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT). Circulation 2011, 124, 1527–1536. [Google Scholar] [CrossRef]
- van Stipdonk, A.M.W.; Hoogland, R.; ter Horst, I.; Kloosterman, M.; Vanbelle, S.; Crijns, H.J.G.M.; Prinzen, F.W.; Meine, M.; Maass, A.H.; Vernooy, K. Evaluating Electrocardiography-Based Identification of Cardiac Resynchronization Therapy Responders Beyond Current Left Bundle Branch Block Definitions. JACC Clin. Electrophysiol. 2020, 6, 193–203. [Google Scholar] [CrossRef]
- Mascioli, G.; Padeletti, L.; Sassone, B.; Zecchin, M.; Lucca, E.; Sacchi, S.; Boggian, G.; Tondo, A.L.; Belvito, C.; Bakhtadze, N.; et al. Electrocardiographic Criteria of True Left Bundle Branch Block: A Simple Sign to Predict a Better Clinical and Instrumental Response to CRT. Pacing Clin. Electrophysiol. 2012, 35, 927–934. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, P.; Li, X.; Gao, Y.; Zhu, T.; Wang, L.; Li, D.; Wang, J.; Yuan, C.; Guo, J. True complete left bundle branch block morphology strongly predicts good response to cardiac resynchronization therapy. EP Europace 2013, 15, 1499–1506. [Google Scholar] [CrossRef]
- García-Seara, J.; Iglesias Alvarez, D.; Alvarez Alvarez, B.; Gude Sampedro, F.; Martínez Sande, J.L.; Rodríguez-Mañero, M.; Kreidieh, B.; Fernández-López, X.A.; González Melchor, L.; González Juanatey, J.R. Cardiac resynchronization therapy response in heart failure patients with different subtypes of true left bundle branch block. J. Interv. Card. Electrophysiol. 2018, 52, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Perrin, M.J.; Green, M.S.; Redpath, C.J.; Nery, P.B.; Keren, A.; Beanlands, R.S.; Birnie, D.H. Greater response to cardiac resynchronization therapy in patients with true complete left bundle branch block: A PREDICT substudy. EP Europace 2011, 14, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Celikyurt, U.; Agacdiken, A.; Sahin, T.; Al, N.; Kozdag, G.; Vural, A.; Ural, D. Number of Leads with Fragmented QRS Predicts Response to Cardiac Resynchronization Therapy. Clin. Cardiol. 2013, 36, 36–39. [Google Scholar] [CrossRef]
- Celikyurt, U.; Açar, B.; Karauzum, I.; Karauzum, K.; Ural, D.; Agir, A.A.; Vural, A. El inicio rápido de la fragmentación del QRS predice la no respuesta a la terapia de resincronización cardíaca en pacientes con insuficiencia cardíaca no isquémica. Rev. Clínica Española 2019, 219, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Rickard, J.; Zardkoohi, O.; Popovic, Z.; Verhaert, D.; Sraow, D.; Baranowski, B.; Martin, D.O.; Grimm, R.A.; Chung, M.K.; Tchou, P.; et al. QRS fragmentation is not associated with poor response to cardiac resynchronization therapy. Ann. Noninvasive Electrocardiol. Off. J. Int. Soc. Holter Noninvasive Electrocardiol. Inc 2011, 16, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Mollo, R.; Cosenza, A.; Coviello, I.; Stazi, A.; Russo, G.; Villano, A.; Sestito, A.; Bencardino, G.; Lanza, G.A.; Crea, F. A novel electrocardiographic predictor of clinical response to cardiac resynchronization therapy. EP Europace 2013, 15, 1615–1621. [Google Scholar] [CrossRef]
- Strauss, D.G.; Selvester, R.H.; Lima, J.A.C.; Arheden, H.; Miller, J.M.; Gerstenblith, G.; Marbán, E.; Weiss, R.G.; Tomaselli, G.F.; Wagner, G.S.; et al. ECG Quantification of Myocardial Scar in Cardiomyopathy Patients with or Without Conduction Defects. Circ. Arrhythmia Electrophysiol. 2008, 1, 327–336. [Google Scholar] [CrossRef]
- Jiang, Z.; Qiu, Y.; Qian, Z.; Wang, Y.; Zhao, Y.; Hou, X.; Liang, Y.; Zheng, L.; Xu, G.; Su, Y.; et al. An S wave in ECG lead V6 predicts poor response to cardiac resynchronization therapy and long-term outcome. Heart Rhythm 2020, 17, 265–272. [Google Scholar] [CrossRef]
- Leonelli, F.M.; Bagliani, G.; De Ponti, R.; Padeletti, L. Intraventricular Delay and Blocks. Card. Electrophysiol. Clin. 2018, 10, 211–231. [Google Scholar] [CrossRef]
- Cardone-Noott, L.; Bueno-Orovio, A.; Mincholé, A.; Zemzemi, N.; Rodriguez, B. Human ventricular activation sequence and the simulation of the electrocardiographic QRS complex and its variability in healthy and intraventricular block conditions. EP Europace 2016, 18, iv4–iv15. [Google Scholar] [CrossRef]
- Poposka, L.; Boskov, V.; Risteski, D.; Taleski, J.; Janusevski, F.; Srbinovska, E.; Georgievska-Ismail, L. Electrocardiographic Parameters as Predictors of Response to Cardiac Resynchronization Therapy. Open Access Maced. J. Med. Sci. 2018, 6, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Loring, Z.; Atwater, B.D.; Xia, X.; Axelsson, J.; Klem, I.; Nijveldt, R.; Schelbert, E.B.; Couderc, J.-P.; Strauss, D.G.; Ugander, M.; et al. Low lead one ratio predicts clinical outcomes in left bundle branch block. J. Cardiovasc. Electrophysiol. 2019, 30, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Brenyo, A.; Rao, M.; Barsheshet, A.; Cannom, D.; Quesada, A.; McNITT, S.; Huang, D.T.; Moss, A.J.; Zareba, W. QRS Axis and the Benefit of Cardiac Resynchronization Therapy in Patients with Mildly Symptomatic Heart Failure Enrolled in MADIT-CRT. J. Cardiovasc. Electrophysiol. 2013, 24, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Storkås, H.S.; Hansen, T.F.; Tahri, J.B.; Lauridsen, T.K.; Olsen, F.J.; Borgquist, R.; Vinther, M.; Lindhardt, T.B.; Bruun, N.E.; Søgaard, P.; et al. Left axis deviation in patients with left bundle branch block is a marker of myocardial disease associated with poor response to cardiac resynchronization therapy. J. Electrocardiol. 2020, 63, 147–152. [Google Scholar] [CrossRef]
- Perrotta, L.; Kandala, J.; Di Biase, L.; Valleggi, A.; Michelotti, F.; Pieragnoli, P.; Ricciardi, G.; Mascioli, G.; Lakkireddy, D.; Pillarisetti, J.; et al. Prognostic Impact of QRS Axis Deviation in Patients Treated with Cardiac Resynchronization Therapy. J. Cardiovasc. Electrophysiol. 2016, 27, 315–320. [Google Scholar] [CrossRef]
- Del-Carpio Munoz, F.; Powell, B.D.; Cha, Y.M.; Wiste, H.J.; Redfield, M.M.; Friedman, P.A.; Asirvatham, S.J. Delayed intrinsicoid deflection onset in surface ECG lateral leads predicts left ventricular reverse remodeling after cardiac resynchronization therapy. Heart Rhythm 2013, 10, 979–987. [Google Scholar] [CrossRef]
- Vereckei, A.; Szelényi, Z.; Kutyifa, V.; Zima, E.; Szénási, G.; Kiss, M.; Katona, G.; Karádi, I.; Merkely, B. Novel electrocardiographic dyssynchrony criteria improve patient selection for cardiac resynchronization therapy. EP Europace 2016, 20, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.P.; Fan, D.; Heist, E.K.; Alabiad, C.R.; Taub, C.; Reddy, V.; Mansour, M.; Picard, M.H.; Ruskin, J.N.; Mela, T. Left ventricular lead electrical delay predicts response to cardiac resynchronization therapy. Heart Rhythm 2006, 3, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.R.; Birgersdotter-Green, U.; Singh, J.P.; Ellenbogen, K.A.; Yu, Y.; Meyer, T.E.; Seth, M.; Tchou, P.J. The relationship between ventricular electrical delay and left ventricular remodelling with cardiac resynchronization therapy. Eur. Heart J. 2011, 32, 2516–2524. [Google Scholar] [CrossRef] [PubMed]
- Pastore, G.; Maines, M.; Marcantoni, L.; Zanon, F.; Noventa, F.; Corbucci, G.; Baracca, E.; Aggio, S.; Picariello, C.; Lanza, D.; et al. ECG parameters predict left ventricular conduction delay in patients with left ventricular dysfunction. Heart Rhythm 2016, 13, 2289–2296. [Google Scholar] [CrossRef] [PubMed]
- Unger, P.N.; Lesser, M.E.; Kugel, V.H.; Lev, M. The Concept of “Masquerading” Bundle-Branch Block. Circulation 1958, 17, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.L.; Wolff, L. Left bundle branch block masquerading as right bundle branch block. Am. Heart J. 1954, 47, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Elizari, M.V.; Baranchuk, A.; Chiale, P.A. Masquerading bundle branch block: A variety of right bundle branch block with left anterior fascicular block. Expert Rev. Cardiovasc. Ther. 2013, 11, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Tzogias, L.; Steinberg, L.A.; Williams, A.J.; Morris, K.E.; Mahlow, W.J.; Fogel, R.I.; Olson, J.A.; Prystowsky, E.N.; Padanilam, B.J. Electrocardiographic Features and Prevalence of Bilateral Bundle-Branch Delay. Circ. Arrhythmia Electrophysiol. 2014, 7, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Pastore, G.; Morani, G.; Maines, M.; Marcantoni, L.; Bolzan, B.; Zanon, F.; Noventa, F.; Corbucci, G.; Baracca, E.; Picariello, C.; et al. Patients with right bundle branch block and concomitant delayed left ventricular activation respond to cardiac resynchronization therapy. EP Europace 2017, 20, e171–e178. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Niwano, S.; Ito, H.; Karakawa, M.; Ako, J. Evaluation of R-wave offset in the left chest leads for estimating the left ventricular activation delay: An evaluation based on coronary sinus electrograms and the 12-lead electrocardiogram. J. Electrocardiol. 2016, 49, 148–153. [Google Scholar] [CrossRef]
- Pastore, G.; Maines, M.; Marcantoni, L.; Lanza, D.; Zanon, F.; Noventa, F.; Corbucci, G.; Rigatelli, G.; Baracca, E.; Zuin, M.; et al. The QR-max index, a novel electrocardiographic index for the determination of left ventricular conduction delay and selection of cardiac resynchronization in patients with non-left bundle branch block. J. Interv. Card. Electrophysiol. 2020, 58, 147–156. [Google Scholar] [CrossRef]
- Cheng, S.; Keyes, M.J.; Larson, M.G.; McCabe, E.L.; Newton-Cheh, C.; Levy, D.; Benjamin, E.J.; Vasan, R.S.; Wang, T.J. Long-term outcomes in individuals with prolonged PR interval or first-degree atrioventricular block. JAMA 2009, 301, 2571–2577. [Google Scholar] [CrossRef]
- Lin, J.; Buhr, K.A.; Kipp, R. Effect of PR Interval on Outcomes Following Cardiac Resynchronization Therapy: A Secondary Analysis of the COMPANION Trial. J. Cardiovasc. Electrophysiol. 2017, 28, 185–191. [Google Scholar] [CrossRef]
- Atwater, B.D.; Emerek, K.; Sørensen, P.L.; Hansen, S.M.; Loring, Z.; Graff, C.; Polcwiartek, C.; Kisslo, J.; Søgaard, P.; Friedman, D.J. PR Prolongation predicts inadequate resynchronization with biventricular pacing in left bundle branch block. Pacing Clin. Electrophysiol. 2019, 42, 1477–1485. [Google Scholar] [CrossRef]
- De Pooter, J.; El Haddad, M.; Timmers, L.; Van Heuverswyn, F.; Jordaens, L.; Duytschaever, M.; Stroobandt, R. Different Methods to Measure QRS Duration in CRT Patients: Impact on the Predictive Value of QRS Duration Parameters. Ann. Noninvasive Electrocardiol. 2016, 21, 305–315. [Google Scholar] [CrossRef] [PubMed]
- van Stipdonk, A.M.W.; Vanbelle, S.; ter Horst, I.A.H.; Luermans, J.G.; Meine, M.; Maass, A.H.; Auricchio, A.; Prinzen, F.W.; Vernooy, K. Large variability in clinical judgement and definitions of left bundle branch block to identify candidates for cardiac resynchronisation therapy. Int. J. Cardiol. 2019, 286, 61–65. [Google Scholar] [CrossRef] [PubMed]
- van Deursen, C.J.M.; Vernooy, K.; Dudink, E.; Bergfeldt, L.; Crijns, H.J.G.M.; Prinzen, F.W.; Wecke, L. Vectorcardiographic QRS area as a novel predictor of response to cardiac resynchronization therapy. J. Electrocardiol. 2015, 48, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Maass, A.H.; Vernooy, K.; Wijers, S.C.; van ’t Sant, J.; Cramer, M.J.; Meine, M.; Allaart, C.P.; De Lange, F.J.; Prinzen, F.W.; Gerritse, B.; et al. Refining success of cardiac resynchronization therapy using a simple score predicting the amount of reverse ventricular remodelling: Results from the Markers and Response to CRT (MARC) study. EP Europace 2017, 20, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Emerek, K.; Friedman, D.J.; Sørensen, P.L.; Hansen, S.M.; Larsen, J.M.; Risum, N.; Thøgersen, A.M.; Graff, C.; Kisslo, J.; Søgaard, P.; et al. Vectorcardiographic QRS area is associated with long-term outcome after cardiac resynchronization therapy. Heart Rhythm 2019, 16, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Nguyên, U.C.; Potse, M.; Regoli, F.; Caputo, M.L.; Conte, G.; Murzilli, R.; Muzzarelli, S.; Moccetti, T.; Caiani, E.G.; Prinzen, F.W.; et al. An in-silico analysis of the effect of heart position and orientation on the ECG morphology and vectorcardiogram parameters in patients with heart failure and intraventricular conduction defects. J. Electrocardiol. 2015, 48, 617–625. [Google Scholar] [CrossRef]
- Jurak, P.; Curila, K.; Leinveber, P.; Prinzen, F.W.; Viscor, I.; Plesinger, F.; Smisek, R.; Prochazkova, R.; Osmancik, P.; Halamek, J.; et al. Novel ultra-high-frequency electrocardiogram tool for the description of the ventricular depolarization pattern before and during cardiac resynchronization. J. Cardiovasc. Electrophysiol. 2020, 31, 300–307. [Google Scholar] [CrossRef]
- Feeny, A.K.; Rickard, J.; Trulock, K.M.; Patel, D.; Toro, S.; Moennich, L.A.; Varma, N.; Niebauer, M.J.; Gorodeski, E.Z.; Grimm, R.A.; et al. Machine Learning of 12-Lead QRS Waveforms to Identify Cardiac Resynchronization Therapy Patients with Differential Outcomes. Circ. Arrhythm. Electrophysiol. 2020, 13, e008210. [Google Scholar] [CrossRef]
- Vamos, M.; Erath, J.W.; Benz, A.P.; Duray, G.Z. Editorial: Developments in cardiac implantable electronic device therapy: How can we improve clinical implementation? Front. Cardiovasc. Med. 2023, 10, 1177882. [Google Scholar] [CrossRef]
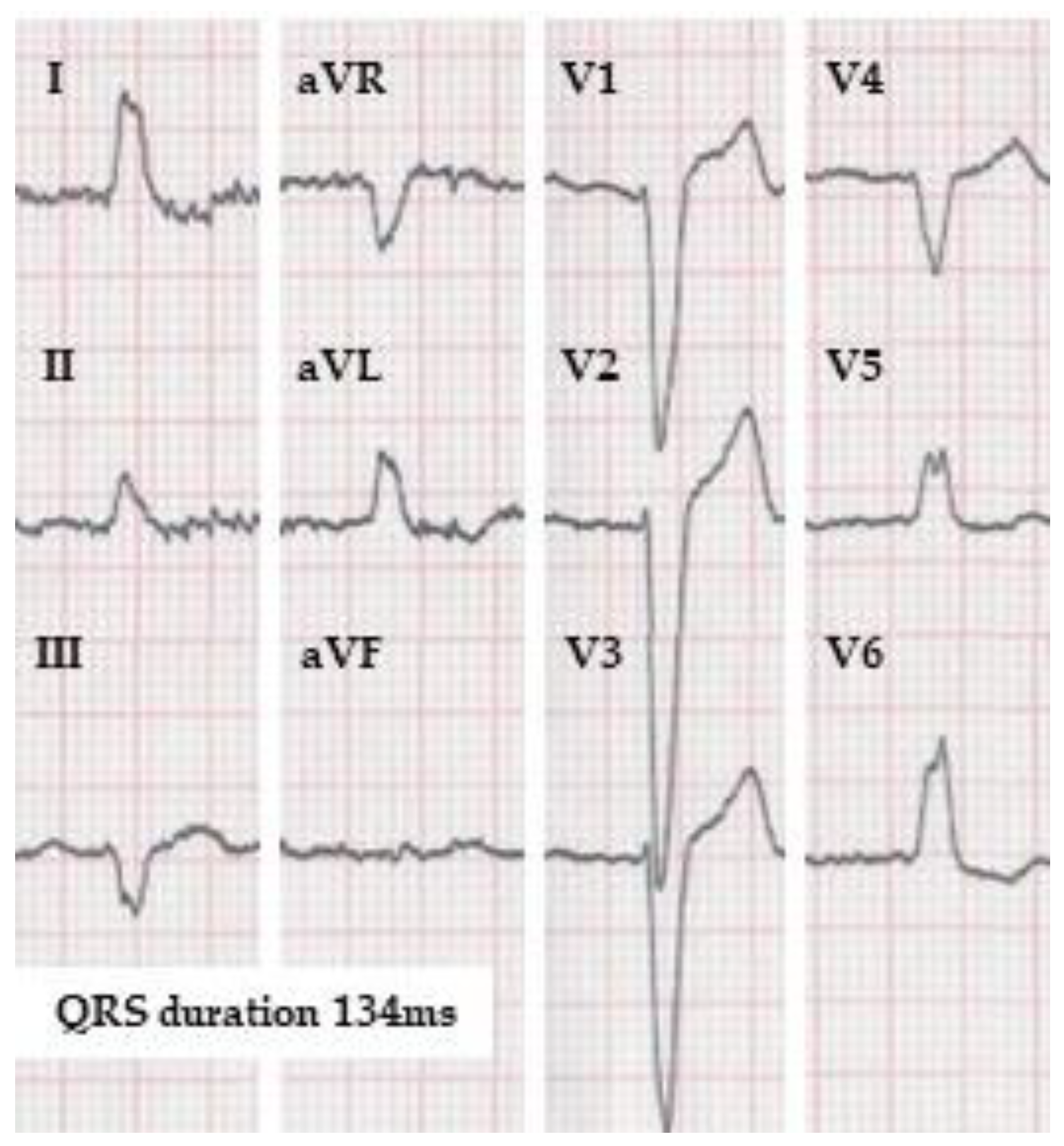
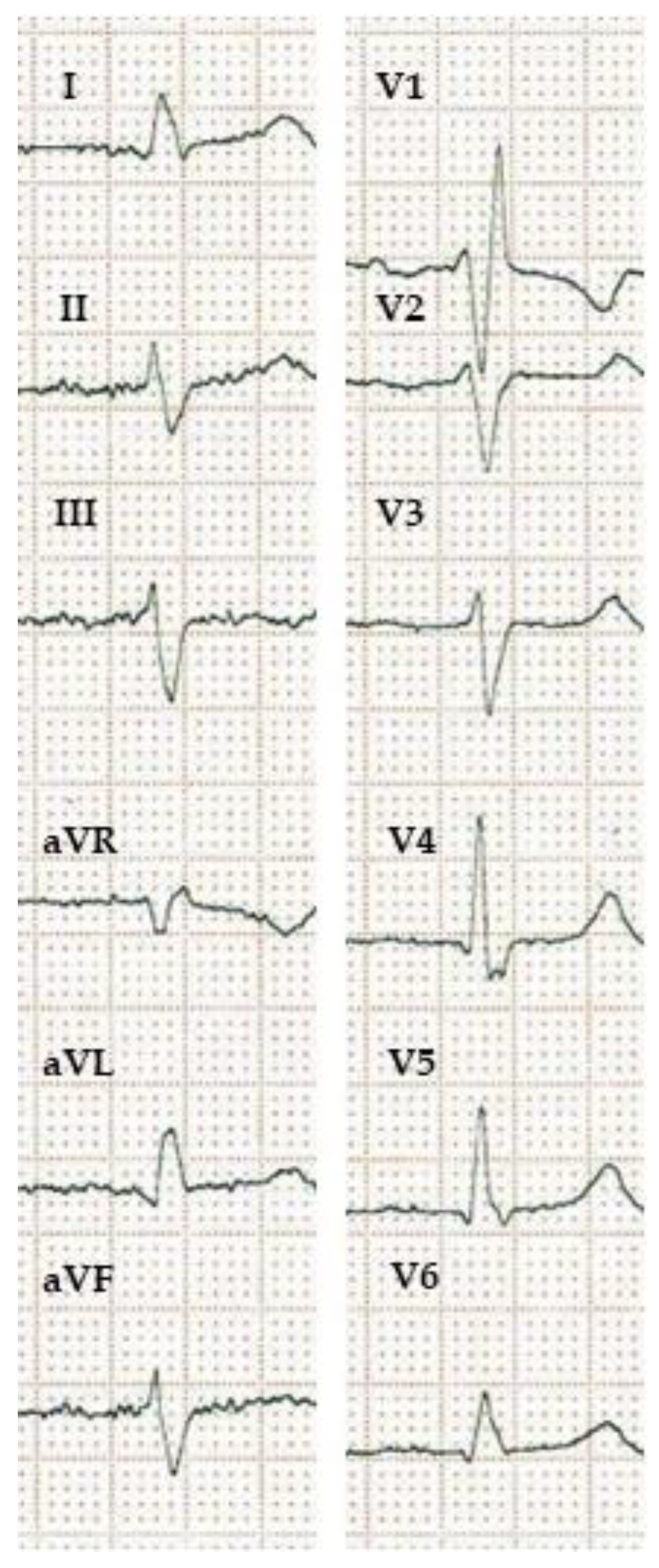
| WHO [24] | AHA/ACCF/HRS [20] | ESC [21] | MADIT-CRT [18] | REVERSE [22] | Strauss et al. [23] | |
|---|---|---|---|---|---|---|
| QRS duration (ms) | 120 | 120 | 120 | 130 | 120 | female: 130 male: 140 |
| rS or QS in V1 | + | + | + | + | + | + |
| Transition zone in the precordial leads is displaced to the left | + | - | - | - | - | - |
| Broad R in I, aVL, V5-6 | + | + | + | + | + | - |
| Notched/slurred R in I, aVL, V5-6 | + | + | + | + | - | + |
| Mid-QRS notching or slurring in at least 2 of leads V1, V2, V5, V6, I, and aVL | - | - | + | - | - | + |
| RS pattern allowed in V5-6 | + | + | - | + | + | + |
| No q allowed in V5-6 | + | + | + | + | + | - |
| No q allowed in aVL | - | - | + | - | - | - |
| No q allowed in I | + | + | + | - | - | - |
| R peak time greater than 60 ms in leads V5-V6 | + | + | + | - | - | - |
| Normal R peak time in leads V1-V2 | + | + | - | - | - | - |
| Discordant repolarization, but after a positive QRS the T wave can be positive | - | + | + | - | - | - |
| Slightly elevated ST and positive, asymmetrical T wave in V1 | - | - | + | - | - | - |
| QS in aVR with positive T wave | - | - | + | - | - | - |
| Predictors of CRT Response | Predictors of CRT Non-Response |
|---|---|
| True LBBB | |
| |
| Non-true LBBB and non-LBBB | |
|
|
| All QRS morphologies | |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simon, A.; Pilecky, D.; Kiss, L.Z.; Vamos, M. Useful Electrocardiographic Signs to Support the Prediction of Favorable Response to Cardiac Resynchronization Therapy. J. Cardiovasc. Dev. Dis. 2023, 10, 425. https://doi.org/10.3390/jcdd10100425
Simon A, Pilecky D, Kiss LZ, Vamos M. Useful Electrocardiographic Signs to Support the Prediction of Favorable Response to Cardiac Resynchronization Therapy. Journal of Cardiovascular Development and Disease. 2023; 10(10):425. https://doi.org/10.3390/jcdd10100425
Chicago/Turabian StyleSimon, Andras, David Pilecky, Loretta Zsuzsa Kiss, and Mate Vamos. 2023. "Useful Electrocardiographic Signs to Support the Prediction of Favorable Response to Cardiac Resynchronization Therapy" Journal of Cardiovascular Development and Disease 10, no. 10: 425. https://doi.org/10.3390/jcdd10100425
APA StyleSimon, A., Pilecky, D., Kiss, L. Z., & Vamos, M. (2023). Useful Electrocardiographic Signs to Support the Prediction of Favorable Response to Cardiac Resynchronization Therapy. Journal of Cardiovascular Development and Disease, 10(10), 425. https://doi.org/10.3390/jcdd10100425








