Assessment and Management of Atrial Fibrillation in Older Adults with Frailty
Abstract
1. Introduction
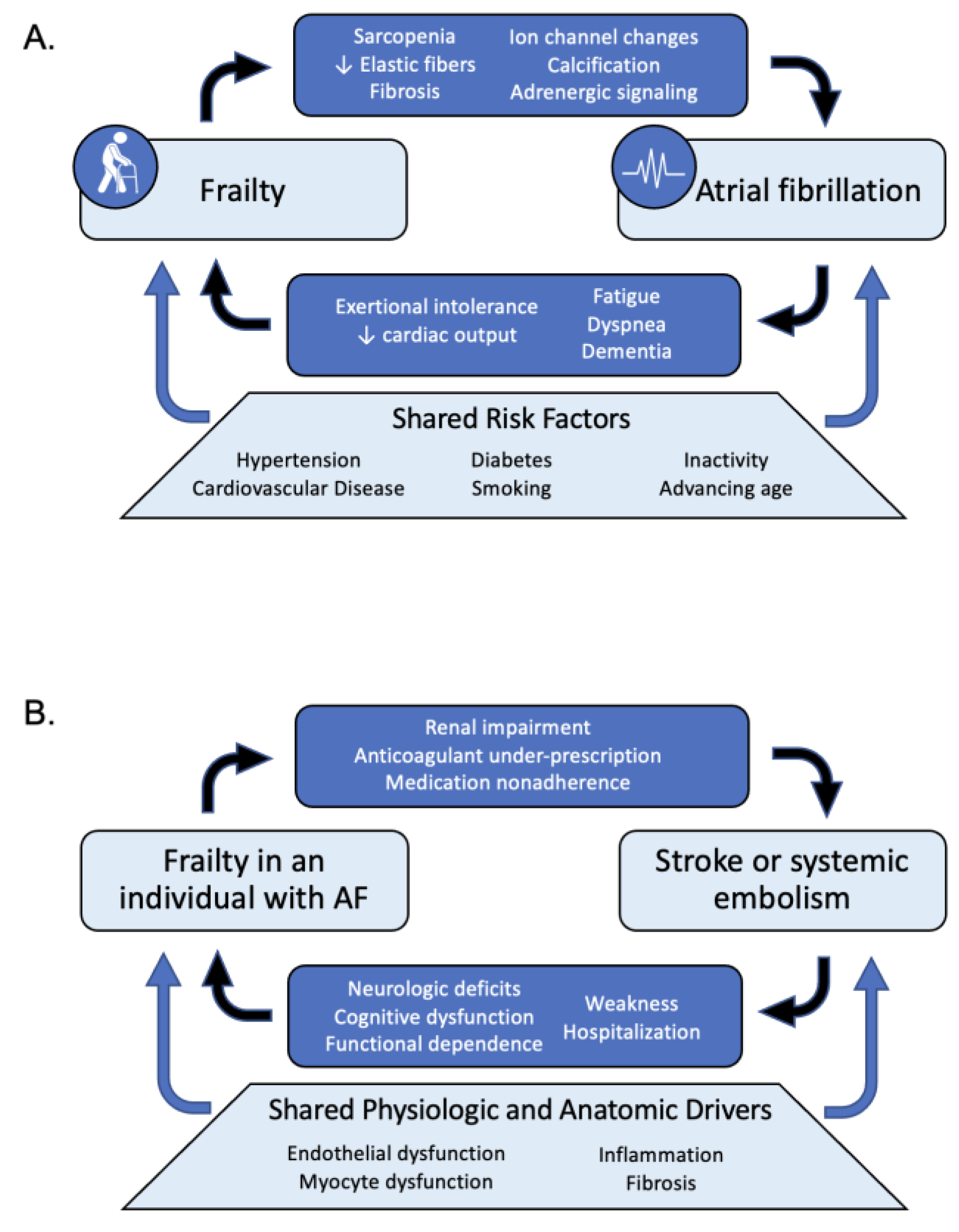
2. Frailty and Atrial Fibrillation Risk
3. Frailty Assessment for Older Adults with Atrial Fibrillation
4. Atrial Fibrillation Assessment for Older Adults with Frailty
5. Rate Control and Frailty
6. Rhythm Control and Frailty
7. Stroke Prevention and Frailty
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, J.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Ko, D.; Bostrom, J.A.; Qazi, S.; Kramer, D.B.; Kim, D.H.; Orkaby, A.R. Frailty and Cardiovascular Mortality: A Narrative Review. Curr. Cardiol. Rep. 2023, 25, 249–259. [Google Scholar] [CrossRef]
- Guo, Q.; Du, X.; Ma, C.S. Atrial fibrillation and frailty. J. Geriatr. Cardiol. 2020, 17, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Krijthe, B.P.; Kunst, A.; Benjamin, E.J.; Lip, G.Y.; Franco, O.H.; Hofman, A.; Witteman, J.C.; Stricker, B.H.; Heeringa, J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur. Heart J. 2013, 34, 2746–2751. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, S.; Ashburner, J.M.; Ellinor, P.T.; McManus, D.D.; Atlas, S.J.; Singer, D.E.; Lubitz, S.A. Prevalence and Incidence of Atrial Fibrillation Among Older Primary Care Patients. JAMA Netw. Open. 2023, 6, e2255838. [Google Scholar] [CrossRef]
- Madhavan, M.; Holmes, D.N.; Piccini, J.P.; Ansell, J.E.; Fonarow, G.C.; Hylek, E.M.; Kowey, P.R.; Mahaffey, K.W.; Thomas, L.; Peterson, E.D.; et al. Association of frailty and cognitive impairment with benefits of oral anticoagulation in patients with atrial fibrillation. Am. Heart J. 2019, 211, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Gugganig, R.; Aeschbacher, S.; Leong, D.P.; Meyre, P.; Blum, S.; Coslovsky, M.; Beer, J.H.; Moschovitis, G.; Müller, D.; Anker, D.; et al. Frailty to predict unplanned hospitalization, stroke, bleeding, and death in atrial fibrillation. Eur. Heart J. Qual. Care Clin. Outcomes 2021, 7, 42–51. [Google Scholar] [CrossRef]
- Flint, K. Which Came First, the Frailty or the Heart Disease? J. Am. Coll. Cardiol. 2015, 65, 984–986. [Google Scholar] [CrossRef][Green Version]
- Bouillon, K.; Kivimaki, M.; Hamer, M.; Sabia, S.; I Fransson, E.; Singh-Manoux, A.; Gale, C.R.; Batty, G.D. Measures of frailty in population-based studies: An overview. BMC Geriatr. 2013, 13, 64. [Google Scholar] [CrossRef]
- Stewart, R. Cardiovascular Disease and Frailty: What Are the Mechanistic Links? Clin. Chem. 2019, 65, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Gale, C.R.; Cooper, C.; Sayer, A.A. Framingham cardiovascular disease risk scores and incident frailty: The English longitudinal study of ageing. Age 2014, 36, 9692. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Refaat, M.; Shen, W.-K.; Kutyifa, V.; Cha, Y.-M.; Di Biase, L.; Baranchuk, A.; Lampert, R.; Natale, A.; Fisher, J.; et al. Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 75, 1689–1713. [Google Scholar] [CrossRef] [PubMed]
- Brundel, B.J.J.M.; Ai, X.; Hills, M.T.; Kuipers, M.F.; Lip, G.Y.H.; de Groot, N.M.S. Atrial fibrillation. Nat. Rev. Dis. Primers 2022, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Wasmer, K.; Eckardt, L.; Breithardt, G. Predisposing factors for atrial fibrillation in the elderly. J. Geriatr. Cardiol. 2017, 14, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Jansen, H.J.; Moghtadaei, M.; Rafferty, S.A.; Rose, R.A. Atrial Fibrillation in Aging and Frail Mice. Circ. Arrhythm. Electrophysiol. 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Fleg, J.L.; Strait, J. Age-associated changes in cardiovascular structure and function: A fertile milieu for future disease. Heart Fail. Rev. 2012, 17, 545–554. [Google Scholar] [CrossRef]
- Polidoro, A.; Stefanelli, F.; Ciacciarelli, M.; Pacelli, A.; Di Sanzo, D.; Alessandri, C. Frailty in patients affected by atrial fibrillation. Arch. Gerontol. Geriatr. 2013, 57, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.C.; Schiller, N.B.; Leung, D.; Ross, D.L.; Thomas, L. Atrial Dilation and Altered Function Are Mediated by Age and Diastolic Function but Not before the Eighth Decade. JACC Cardiovasc. Imaging 2011, 4, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Yang, P.-S.; Yu, H.T.; Kim, T.-H.; Jang, E.; Sung, J.-H.; Pak, H.-N.; Lee, M.-Y.; Lee, M.-H.; Lip, G.Y.H.; et al. Risk of dementia in stroke-free patients diagnosed with atrial fibrillation: Data from a population-based cohort. Eur. Heart J. 2019, 40, 2313–2323. [Google Scholar] [CrossRef]
- Healey, J.S.; Connolly, S.J.; Gold, M.R.; Israel, C.W.; Van Gelder, I.C.; Capucci, A.; Lau, C.; Fain, E.; Yang, S.; Bailleul, C.; et al. Subclinical Atrial Fibrillation and the Risk of Stroke. N. Engl. J. Med. 2012, 366, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Kamel, H.; Okin, P.M.; Elkind, M.S.V.; Iadecola, C. Atrial Fibrillation and Mechanisms of Stroke. Stroke 2016, 47, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Perera, V.; Bajorek, B.V.; Matthews, S.; Hilmer, S.N. The impact of frailty on the utilisation of antithrombotic therapy in older patients with atrial fibrillation. Age Ageing 2008, 38, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Winovich, D.T.; Longstreth, W.T.; Arnold, A.M.; Varadhan, R.; Al Hazzouri, A.Z.; Cushman, M.; Newman, A.B.; Odden, M.C. Factors Associated with Ischemic Stroke Survival and Recovery in Older Adults. Stroke 2017, 48, 1818–1826. [Google Scholar] [CrossRef]
- Kim, D.; Yang, P.-S.; Sung, J.-H.; Jang, E.; Yu, H.T.; Kim, T.-H.; Uhm, J.-S.; Kim, J.-Y.; Pak, H.-N.; Lee, M.-H.; et al. Effectiveness and Safety of Anticoagulation Therapy in Frail Patients with Atrial Fibrillation. Stroke 2022, 53, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.R.; Todd, O.M.; Minhas, J.S.; Fearon, P.; Harston, G.W.; Mant, J.; Mead, G.; Hewitt, J.; Quinn, T.J.; A Warburton, E. Frailty and cerebrovascular disease: Concepts and clinical implications for stroke medicine. Int. J. Stroke 2022, 17, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, P.; Burton, J.K.; Quinn, T.J.; Mair, F.S.; McAllister, D.; Lewsey, J.; I Gallacher, K. Prevalence, measurement, and implications of frailty in stroke survivors: An analysis of three global aging cohorts. Int. J. Stroke 2023, 18, 720–727. [Google Scholar] [CrossRef]
- Appelros, P.; Nydevik, I.; Viitanen, M. Poor Outcome after First-Ever Stroke. Stroke 2003, 34, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Rockwood, K. A global clinical measure of fitness and frailty in elderly people. Can. Med. Assoc. J. 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Ijaz, N.; Buta, B.; Xue, Q.-L.; Mohess, D.T.; Bushan, A.; Tran, H.; Batchelor, W.; Defilippi, C.R.; Walston, J.D.; Bandeen-Roche, K.; et al. Interventions for Frailty among Older Adults with Cardiovascular Disease. J. Am. Coll. Cardiol. 2022, 79, 482–503. [Google Scholar] [CrossRef]
- Orkaby, A.R.; James, K.; Leuchtenburg, J.; Solooki, E.; Gaziano, J.M.; Driver, J.A. Taking prevention to the next step: Implementation of a brief, sustainable frailty assessment in a cardiology clinic. BMJ Open Qual. 2021, 10, e001140. [Google Scholar] [CrossRef] [PubMed]
- Orkaby, A.R.; Kornej, J.; Lubitz, S.A.; McManus, D.D.; Travison, T.G.; Sherer, J.A.; Trinquart, L.; Murabito, J.M.; Benjamin, E.J.; Preis, S.R. Association between Frailty and Atrial Fibrillation in Older Adults: The Framingham Heart Study Offspring Cohort. J. Am. Heart Assoc. 2021, 10, e018557. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chai, K.; Zhu, W.; DU, M.; Meng, C.; Yang, L.; Cui, L.; Guo, D.; Sun, N.; Wang, H.; et al. Implication of different frailty criteria in older people with atrial fibrillation: A prospective cohort study. BMC Geriatr. 2023, 23, 604. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, C.; Wu, J.; Searle, S.D.; Todd, O.; Hall, M.; Kunadian, V.; Clegg, A.; Rockwood, K.; Gale, C.P. Clinical outcomes in patients with atrial fibrillation and frailty: Insights from the ENGAGE AF-TIMI 48 trial. BMC Med. 2020, 18, 401. [Google Scholar] [CrossRef] [PubMed]
- Okumura, K.; Akao, M.; Yoshida, T.; Kawata, M.; Okazaki, O.; Akashi, S.; Eshima, K.; Tanizawa, K.; Fukuzawa, M.; Hayashi, T.; et al. Low-Dose Edoxaban in Very Elderly Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1735–1745. [Google Scholar] [CrossRef] [PubMed]
- Proietti, M.; Romiti, G.F.; Raparelli, V.; Diemberger, I.; Boriani, G.; Vecchia, L.A.D.; Bellelli, G.; Marzetti, E.; Lip, G.Y.; Cesari, M. Frailty prevalence and impact on outcomes in patients with atrial fibrillation: A systematic review and meta-analysis of 1,187,000 patients. Ageing Res. Rev. 2022, 79, 101652. [Google Scholar] [CrossRef] [PubMed]
- Presta, R.; Brunetti, E.; Polidori, M.C.; Bo, M. Impact of frailty models on the prescription of oral anticoagulants and on the incidence of stroke, bleeding, and mortality in older patients with atrial fibrillation: A systematic review. Ageing Res. Rev. 2022, 82, 101761. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Cumming, R.G.; Hilmer, S.N. Atrial fibrillation in older inpatients: Are there any differences in clinical characteristics and pharmacological treatment between the frail and the non-frail? Intern. Med. J. 2016, 46, 86–95. [Google Scholar] [CrossRef]
- Joosten, L.P.; van Doorn, S.; van de Ven, P.M.; Köhlen, B.T.; Nierman, M.C.; Koek, H.L.; Hemels, M.E.; Huisman, M.V.; Kruip, M.; Faber, L.M.; et al. Safety of Switching from a Vitamin K Antagonist to a Non-Vitamin K Antagonist Oral Anticoagulant in Frail Older Patients with Atrial Fibrillation: Results of the FRAIL-AF Randomized Controlled Trial. Circulation 2024, 149, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Troy, A.L.; Herzig, S.J.; Trivedi, S.; Anderson, T.S. Initiation of oral anticoagulation in US older adults newly diagnosed with atrial fibrillation during hospitalization. J. Am. Geriatr. Soc. 2023, 71, 2748–2758. [Google Scholar] [CrossRef] [PubMed]
- Force, U.P.S.T.; Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Epling, J.W.; et al. Screening for Atrial Fibrillation. JAMA 2022, 327, 360. [Google Scholar] [CrossRef]
- Hobbs, F.; Fitzmaurice, D.; Mant, J.; Murray, E.; Jowett, S.; Bryan, S.; Raftery, J.; Davies, M.; Lip, G. A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study. Health Technol. Assess. 2005, 9, iii–iv, ix–x, 1–74. [Google Scholar] [CrossRef] [PubMed]
- Gladstone, D.J.; Wachter, R.; Schmalstieg-Bahr, K.; Quinn, F.R.; Hummers, E.; Ivers, N.; Marsden, T.; Thornton, A.; Djuric, A.; Suerbaum, J.; et al. Screening for Atrial Fibrillation in the Older Population. JAMA Cardiol. 2021, 6, 558. [Google Scholar] [CrossRef] [PubMed]
- Steinhubl, S.R.; Waalen, J.; Edwards, A.M.; Ariniello, L.M.; Mehta, R.R.; Ebner, G.S.; Carter, C.; Baca-Motes, K.; Felicione, E.; Sarich, T.; et al. Effect of a Home-Based Wearable Continuous ECG Monitoring Patch on Detection of Undiagnosed Atrial Fibrillation. JAMA 2018, 320, 146. [Google Scholar] [CrossRef]
- Uittenbogaart, S.B.; Gurp, N.V.-V.; Lucassen, W.A.M.; Winkens, B.; Nielen, M.; Erkens, P.M.G.; Knottnerus, J.A.; van Weert, H.C.P.M.; Stoffers, H.E.J.H. Opportunistic screening versus usual care for detection of atrial fibrillation in primary care: Cluster randomised controlled trial. BMJ 2020, 370, m3208. [Google Scholar] [CrossRef]
- Perez, M.V.; Mahaffey, K.W.; Hedlin, H.; Rumsfeld, J.S.; Garcia, A.; Ferris, T.; Balasubramanian, V.; Russo, A.M.; Rajmane, A.; Cheung, L.; et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N. Engl. J. Med. 2019, 381, 1909–1917. [Google Scholar] [CrossRef]
- Svendsen, J.H.; Diederichsen, S.Z.; Højberg, S.; Krieger, D.W.; Graff, C.; Kronborg, C.; Olesen, M.S.; Nielsen, J.B.; Holst, A.G.; Brandes, A.; et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): A randomised controlled trial. Lancet 2021, 398, 1507–1516. [Google Scholar] [CrossRef]
- Kim, D.H.; Schneeweiss, S.; Glynn, R.J.; Lipsitz, L.A.; Rockwood, K.; Avorn, J. Measuring Frailty in Medicare Data: Development and Validation of a Claims-Based Frailty Index. J. Gerontol. Ser. A 2018, 73, 980–987. [Google Scholar] [CrossRef]
- Lip, G.Y.H. The ABC pathway: An integrated approach to improve AF management. Nat. Rev. Cardiol. 2017, 14, 627–628. [Google Scholar] [CrossRef] [PubMed]
- Savelieva, I.; Fumagalli, S.; Kenny, R.A.; Anker, S.; Benetos, A.; Boriani, G.; Bunch, J.; Dagres, N.; Dubner, S.; Fauchier, L.; et al. EHRA expert consensus document on the management of arrhythmias in frailty syndrome, endorsed by the Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA). EP Eur. 2023, 25, 1249–1276. [Google Scholar] [CrossRef]
- Romiti, G.F.; Proietti, M.; Vitolo, M.; Bonini, N.; Fawzy, A.M.; Ding, W.Y.; Fauchier, L.; Marin, F.; Nabauer, M.; Dan, G.A.; et al. Clinical complexity and impact of the ABC (Atrial fibrillation Better Care) pathway in patients with atrial fibrillation: A report from the ESC-EHRA EURObservational Research Programme in AF General Long-Term Registry. BMC Med. 2022, 20, 326. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, S.P.; Proietti, M.; Maggioni, A.P.; Lip, G.Y.H. A multinational European network to implement integrated care in elderly multimorbid atrial fibrillation patients: The AFFIRMO Consortium. Eur. Heart J. 2022, 43, 2916–2918. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Baker, E.; Collins, R.; Merino, J.L.; Desteghe, L.; Heidbuchel, H. The challenge of managing multimorbid atrial fibrillation: A pan-European European Heart Rhythm Association (EHRA) member survey of current management practices and clinical priorities. EP Eur. 2022, 24, 2004–2014. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.M.; Steinhubl, S.; Lakkireddy, D.; Turakhia, M.P.; Passman, R.; Jones, W.S.; Bunch, T.J.; Curtis, A.B.; Peterson, E.D.; Ruskin, J.; et al. Does early detection of atrial fibrillation reduce the risk of thromboembolic events? Rationale and design of the Heartline study. Am. Heart J. 2023, 259, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Opolski, G.; Torbicki, A.; Kosior, D.A.; Szulc, M.; Wozakowska-Kapłon, B.; Kołodziej, P.; Achremczyk, P. Rate Control vs Rhythm Control in Patients with Nonvalvular Persistent Atrial Fibrillation. Chest 2004, 126, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Wyse, D.G.; Waldo, A.L.; DiMarco, J.P.; Domanski, M.J.; Rosenberg, Y.; Schron, E.B.; Kellen, J.C.; Greene, H.L.; Mickel, M.C.; E Dalquist, J.; et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N. Engl. J. Med. 2002, 347, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Shariff, N.; Desai, R.V.; Patel, K.; Ahmed, M.I.; Fonarow, G.C.; Rich, M.W.; Aban, I.B.; Banach, M.; Love, T.E.; White, M.; et al. Rate-control versus Rhythm-control Strategies and Outcomes in Septuagenarians with Atrial Fibrillation. Am. J. Med. 2013, 126, 887–893. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Groenveld, H.F.; Crijns, H.J.; Tuininga, Y.S.; Tijssen, J.G.; Alings, A.M.; Hillege, H.L.; Bergsma-Kadijk, J.A.; Cornel, J.H.; Kamp, O.; et al. Lenient versus Strict Rate Control in Patients with Atrial Fibrillation. N. Engl. J. Med. 2010, 362, 1363–1373. [Google Scholar] [CrossRef]
- Steinberg, B.A.; Kim, S.; Thomas, L.; Fonarow, G.C.; Gersh, B.J.; Holmqvist, F.; Hylek, E.; Kowey, P.R.; Mahaffey, K.W.; Naccarelli, G.; et al. Increased Heart Rate Is Associated with Higher Mortality in Patients with Atrial Fibrillation (AF): Results From the Outcomes Registry for Better Informed Treatment of AF (ORBIT-AF). J. Am. Heart Assoc. 2015, 4, e002031. [Google Scholar] [CrossRef] [PubMed]
- Ulimoen, S.R.; Enger, S.; Carlson, J.; Platonov, P.G.; Pripp, A.H.; Abdelnoor, M.; Arnesen, H.; Gjesdal, K.; Tveit, A. Comparison of Four Single-Drug Regimens on Ventricular Rate and Arrhythmia-Related Symptoms in Patients with Permanent Atrial Fibrillation. Am. J. Cardiol. 2013, 111, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Grossman, E.; Beloosesky, Y.; Grinblat, J. Orthostatic Hypotension in Acute Geriatric Ward. Arch. Intern. Med. 2002, 162, 2369. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Adachi, T.; Kobayashi, K.; Okumura, T.; Izawa, H.; Murohara, T.; McMurray, J.J.V.; Yamada, S. Physical Frailty and Use of Guideline-Recommended Drugs in Patients with Heart Failure and Reduced Ejection Fraction. J. Am. Heart Assoc. 2023, 12, e026844. [Google Scholar] [CrossRef] [PubMed]
- Hikoso, S.; Kida, H.; Sunaga, A.; Nakatani, D.; Okada, K.; Dohi, T.; Sotomi, Y.; Oeun, B.; Sato, T.; Matsuoka, Y.; et al. β-blockers may be detrimental in frail patients with heart failure with preserved ejection fraction. Clin. Res. Cardiol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Ziff, O.J.; A Lane, D.; Samra, M.; Griffith, M.; Kirchhof, P.; Lip, G.Y.H.; Steeds, R.P.; Townend, J.; Kotecha, D. Safety and efficacy of digoxin: Systematic review and meta-analysis of observational and controlled trial data. BMJ 2015, 351, h4451. [Google Scholar] [CrossRef]
- Hashim, T.; Elbaz, S.; Patel, K.; Morgan, C.J.; Fonarow, G.C.; Fleg, J.L.; McGwin, G.; Cutter, G.R.; Allman, R.M.; Prabhu, S.D.; et al. Digoxin and 30-day All-cause Hospital Admission in Older Patients with Chronic Diastolic Heart Failure. Am. J. Med. 2014, 127, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Bourge, R.C.; Fleg, J.L.; Fonarow, G.C.; Cleland, J.G.; McMurray, J.J.; van Veldhuisen, D.J.; Gheorghiade, M.; Patel, K.; Aban, I.B.; Allman, R.M.; et al. Digoxin Reduces 30-day All-cause Hospital Admission in Older Patients with Chronic Systolic Heart Failure. Am. J. Med. 2013, 126, 701–708. [Google Scholar] [CrossRef]
- American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 2023, 71, 2052–2081. [CrossRef]
- Freeman, J.V.; Reynolds, K.; Fang, M.; Udaltsova, N.; Steimle, A.; Pomernacki, N.K.; Borowsky, L.H.; Harrison, T.N.; Singer, D.E.; Go, A.S.; et al. Digoxin and Risk of Death in Adults with Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2015, 8, 49–58. [Google Scholar] [CrossRef]
- Brignole, M.; Menozzi, C.; Gianfranchi, L.; Musso, G.; Mureddu, R.; Bottoni, N.; Lolli, G. Assessment of Atrioventricular Junction Ablation and VVIR Pacemaker Versus Pharmacological Treatment in Patients with Heart Failure and Chronic Atrial Fibrillation. Circulation 1998, 98, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Bradley, D.J.; Shen, W.K. Atrioventricular junction ablation combined with either right ventricular pacing or cardiac resynchronization therapy for atrial fibrillation: The need for large-scale randomized trials. Heart Rhythm. 2007, 4, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Armaganijan, L.V.; Toff, W.D.; Nielsen, J.C.; Andersen, H.R.; Connolly, S.J.; Ellenbogen, K.A.; Healey, J.S. Are Elderly Patients at Increased Risk of Complications Following Pacemaker Implantation? A Meta-Analysis of Randomized Trials. Pacing Clin. Electrophysiol. 2012, 35, 131–134. [Google Scholar] [CrossRef]
- Depoorter, L.; Sels, L.; Deschodt, M.; Van Grootven, B.; Van der Linden, L.; Tournoy, J. Clinical Outcomes of Rate vs Rhythm Control for Atrial Fibrillation in Older People: A Systematic Review and Meta-Analysis. Drugs Aging 2020, 37, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, R.; Al Khadra, Y.; Al-Tamimi, R.; Albast, N.; Labedi, M. Atrial fibrillation: Rate control or rhythm control? Clevel. Clin. J. Med. 2022, 89, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Santangeli, P.; DI Biase, L.; Mohanty, P.; Burkhardt, J.D.; Horton, R.; Bai, R.; Mohanty, S.; Pump, A.; Gibson, D.; Couts, L.; et al. Catheter Ablation of Atrial Fibrillation in Octogenarians: Safety and Outcomes. J. Cardiovasc. Electrophysiol. 2012, 23, 687–693. [Google Scholar] [CrossRef]
- Packer, D.L.; Mark, D.B.; Robb, R.A.; Monahan, K.H.; Bahnson, T.D.; Poole, J.E.; Noseworthy, P.A.; Rosenberg, Y.D.; Jeffries, N.; Mitchell, L.B.; et al. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest among Patients with Atrial Fibrillation. JAMA 2019, 321, 1261. [Google Scholar] [CrossRef] [PubMed]
- Fink, T.; Metzner, A.; Willems, S.; Eckardt, L.; Ince, H.; Brachmann, J.; Spitzer, S.G.; Deneke, T.; Schmitt, C.; Hochadel, M.; et al. Procedural success, safety and patients satisfaction after second ablation of atrial fibrillation in the elderly: Results from the German Ablation Registry. Clin. Res. Cardiol. 2019, 108, 1354–1363. [Google Scholar] [CrossRef]
- Yang, P.-S.; Sung, J.-H.; Kim, D.; Jang, E.; Yu, H.T.; Kim, T.-H.; Uhm, J.-S.; Kim, J.-Y.; Pak, H.-N.; Lee, M.-H.; et al. Frailty and the Effect of Catheter Ablation in the Elderly Population with Atrial Fibrillation—A Real-World Analysis. Circ. J. 2021, 85, CJ-20-1062. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Brachmann, J.; Andresen, D.; Siebels, J.; Boersma, L.; Jordaens, L.; Merkely, B.; Pokushalov, E.; Sanders, P.; Proff, J.; et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N. Engl. J. Med. 2018, 378, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.L.; Buckley, B.J.R.; Austin, P.; Lane, D.A.; Lip, G.Y.H. Catheter ablation and lower risk of incident dementia and mortality in older adults with atrial fibrillation. J. Am. Geriatr. Soc. 2023, 71, 3357–3366. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.H.; Clementy, N.; Pericart, L.; Banerjee, A.; Fauchier, L. Stroke and Major Bleeding Risk in Elderly Patients Aged ≥75 Years with Atrial Fibrillation. Stroke 2015, 46, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Pundi, K.; Perino, A.C.; Fan, J.; Din, N.; Szummer, K.; Heidenreich, P.; Turakhia, M.P. Association of CHA2DS2-VASc and HAS-BLED to frailty and frail outcomes: From the TREAT-AF study. Am. Heart J. 2023, 261, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Shrauner, W.; Lord, E.M.; Nguyen, X.-M.T.; Song, R.J.; Galloway, A.; Gagnon, D.R.; A Driver, J.; Gaziano, J.M.; Wilson, P.W.F.; Djousse, L.; et al. Frailty and cardiovascular mortality in more than 3 million US Veterans. Eur. Heart J. 2022, 43, 818–826. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, R.; Huang, J.; Zou, C.; Fan, Y. Impact of frailty on all-cause mortality and major bleeding in patients with atrial fibrillation: A meta-analysis. Ageing Res. Rev. 2022, 73, 101527. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.H.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J.G.M. Refining Clinical Risk Stratification for Predicting Stroke and Thromboembolism in Atrial Fibrillation Using a Novel Risk Factor-Based Approach. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Oqab, Z. What is the Impact of Frailty on Prescription of Anticoagulation in Elderly Patients with Atrial Fibrillation? A Systematic Review and Meta-Analysis. J. Atr. Fibrillation 2018, 10, 1870. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.; Lin, K.J.; Bessette, L.G.; Lee, S.B.; Walkey, A.J.; Cheng, S.; Kim, E.; Glynn, R.J.; Kim, D.H. Trends in Use of Oral Anticoagulants in Older Adults with Newly Diagnosed Atrial Fibrillation, 2010–2020. JAMA Netw. Open 2022, 5, e2242964. [Google Scholar] [CrossRef]
- Sen, S.; Dahlberg, K.W. Physician’s Fear of Anticoagulant Therapy in Nonvalvular Atrial Fibrillation. Am. J. Med. Sci. 2014, 348, 513–521. [Google Scholar] [CrossRef]
- Ekerstad, N.; Karlsson, T.; Söderqvist, S.; Karlson, B.W. Hospitalized frail elderly patients—Atrial fibrillation, anticoagulation and 12 months’ outcomes. Clin. Interv. Aging 2018, 13, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Pawar, A.; Gagne, J.J.; Bessette, L.G.; Lee, H.; Glynn, R.J.; Schneeweiss, S. Frailty and Clinical Outcomes of Direct Oral Anticoagulants Versus Warfarin in Older Adults with Atrial Fibrillation. Ann. Intern. Med. 2021, 174, 1214–1223. [Google Scholar] [CrossRef]
- Lin, K.J.; Singer, D.E.; Ko, D.; Glynn, R.; Najafzadeh, M.; Lee, S.B.; Bessette, L.G.; Cervone, A.; DiCesare, E.; Kim, D.H. Frailty, Home Time, and Health Care Costs in Older Adults with Atrial Fibrillation Receiving Oral Anticoagulants. JAMA Netw. Open 2023, 6, e2342264. [Google Scholar] [CrossRef]
- Xu, Y.; Chang, A.R.; Inker, L.A.; McAdams-DeMarco, M.; Grams, M.E.; Shin, J.I. Associations of Apixaban Dose with Safety and Effectiveness Outcomes in Patients with Atrial Fibrillation and Severe Chronic Kidney Disease. Circulation 2023, 148, 1445–1454. [Google Scholar] [CrossRef]
- Akashi, S.; Oguri, M.; Ikeno, E.; Manita, M.; Taura, J.; Watanabe, S.; Hayashi, T.; Akao, M.; Okumura, K.; Akishita, M.; et al. Outcomes and Safety of Very-Low-Dose Edoxaban in Frail Patients with Atrial Fibrillation in the ELDERCARE-AF Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2228500. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Doshi, S.K.; Kar, S.; Gibson, D.N.; Price, M.J.; Huber, K.; Horton, R.P.; Buchbinder, M.; Neuzil, P.; Gordon, N.T.; et al. 5-Year Outcomes after Left Atrial Appendage Closure. J. Am. Coll. Cardiol. 2017, 70, 2964–2975. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Ferro, E.G.; Song, Y.; Xu, J.; Sun, T.; Yeh, R.W.; Strom, J.B.; Kramer, D.B. Frailty in patients undergoing percutaneous left atrial appendage closure. Heart Rhythm. 2022, 19, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Darden, D.; Munir, M.B.; Zimmerman, S.; Eskander, M.; Pothineni, N.V.K.; Gopinathannair, R.; Kabra, R.; Lakkireddy, D.; Duong, T.; Han, F.T.; et al. Frailty and associated outcomes in patients undergoing percutaneous left atrial appendage occlusion: Findings from the NCDR LAAO registry. J. Interv. Card. Electrophysiol. 2024, 67, 625–635. [Google Scholar] [CrossRef]
- Agarwal, S.; Munir, M.B.; Bansal, A.; DeSimone, C.V.; Baber, U.; Deshmukh, A.; Asad, Z.U.A. Impact of Frailty on In-Hospital Outcomes in Patients Who Underwent Percutaneous Left Atrial Appendage Occlusion. Am. J. Cardiol. 2023, 196, 19–21. [Google Scholar] [CrossRef]
- Sulaiman, S.; Roy, K.; Wang, H.; de Backer, O.; Alloco, D.; Reddy, V.Y.; Holmes, D.R.; Alkhouli, M. Left Atrial Appendage Occlusion in the Elderly. JACC Clin. Electrophysiol. 2023, 9, 669–676. [Google Scholar] [CrossRef]
- Brouwer, T.F.; Whang, W.; Kuroki, K.; Halperin, J.L.; Reddy, V.Y. Net Clinical Benefit of Left Atrial Appendage Closure versus Warfarin in Patients with Atrial Fibrillation: A Pooled Analysis of the Randomized PROTECT-AF and PREVAIL Studies. J. Am. Heart Assoc. 2019, 8, e013525. [Google Scholar] [CrossRef] [PubMed]
- Harrington, J.; Piccini, J.P.; Alexander, J.H.; Granger, C.B.; Patel, M.R. Clinical Evaluation of Factor XIa Inhibitor Drugs. J. Am. Coll. Cardiol. 2023, 81, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Tucker, E.I.; Marzec, U.M.; White, T.C.; Hurst, S.; Rugonyi, S.; McCarty, O.J.T.; Gailani, D.; Gruber, A.; Hanson, S.R. Prevention of vascular graft occlusion and thrombus-associated thrombin generation by inhibition of factor XI. Blood 2009, 113, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Crosby, J.R.; Marzec, U.M.; Revenko, A.S.; Zhao, C.; Gao, D.; Matafonov, A.; Gailani, D.; MacLeod, A.R.; Tucker, E.I.; Gruber, A.; et al. Antithrombotic Effect of Antisense Factor XI Oligonucleotide Treatment in Primates. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1670–1678. [Google Scholar] [CrossRef]
- Piccini, J.P.; Caso, V.; Connolly, S.J.; A A Fox, K.; Oldgren, J.; Jones, W.S.; A Gorog, D.; Viethen, T.; Neumann, C.; Mundl, H.; et al. Safety of the oral factor XIa inhibitor asundexian compared with apixaban in patients with atrial fibrillation (PACIFIC-AF): A multicentre, randomised, double-blind, double-dummy, dose-finding phase 2 study. Lancet 2022, 399, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Dharam, J.K. A Multicenter, RandomiZed, Active-ControLled Study to Evaluate the Safety and Tolerability of Two Blinded Doses of Abelacimab Compared with Open-Label Rivaroxaban in Patients with Atrial Fibrillation—AZALEA-TIMI 71. Available online: https://www.acc.org/Latest-in-Cardiology/Clinical-Trials/2023/11/10/22/46/azalea-timi-71#references-for-article (accessed on 1 December 2023).
- Yu, G.-I.; Kim, D.; Sung, J.-H.; Jang, E.; Yu, H.T.; Kim, T.-H.; Pak, H.-N.; Lee, M.-H.; Lip, G.Y.H.; Yang, P.-S.; et al. Impact of frailty on early rhythm control outcomes in older adults with atrial fibrillation: A nationwide cohort study. Front. Cardiovasc. Med. 2023, 9, 1050744. [Google Scholar] [CrossRef]
- Troy, A.; Anderson, T.S. National Trends in Use of and Spending on Oral Anticoagulants Among US Medicare Beneficiaries From 2011 to 2019. JAMA Health Forum. 2021, 2, e211693. [Google Scholar] [CrossRef]

| AF Stage | Name | Definition |
|---|---|---|
| 1 | At risk for AF | Presence of AF risk factors |
| 2 | Pre-AF | Structural or electrical findings predisposing to AF |
| 3A | Paroxysmal AF | Intermittent AF, lasting up to 7 days |
| 3B | Persistent AF | Continuous and sustained AF for more than 7 days requiring intervention |
| 3C | Long-standing persistent AF | Continuous AF lasting > 12 months |
| 3D | Successful AF ablation | Free from AF after ablation or surgical intervention |
| 4 | Permanent AF | No further attempts at rhythm control |
| Medication Class | Important Adverse Effects | Considerations in Presence of Frailty |
|---|---|---|
| Beta Blockers |
|
|
| Calcium Channel Blockers |
|
|
| Digoxin |
|
|
| Antiarrhythmic | Class | Elimination | Adverse Effects | Frailty Considerations | ||||
|---|---|---|---|---|---|---|---|---|
| Use in Structural Heart Disease | Dosage Adjustments for Renal Function | Screening for Fall Risk | Screening for Drug Interactions | Drug Monitoring | ||||
| Amiodarone | III | Liver | AV block Bradycardia Prolonged QT interval Torsades de pointes Corneal deposits Hepatotoxicity Hyper/hypothyroidism Pulmonary toxicity Nausea/Vomiting Photosensitivity | ✔ | × | ✔ | ✔ | TSH LFTs EKG CXR and PFTs |
| Dofetilide | III | Kidney | Bradycardia Prolonged QT interval Torsades de pointes | ✔ | ✔ | ✔ | ✔ | EKG (and telemetry for 3 days during initiation) Electrolytes Creatinine |
| Flecainide | I | Liver (70%) Kidney (30%) | QT prolongation AV Block Atrial flutter Ventricular tachycardia HFrEF exacerbation Dizziness Nausea Visual disturbances | × | ✔ | ✔ | ✔ | EKG |
| Propafenone | I | Liver | Bradycardia AV Block Atrial flutter Ventricular tachycardia HRrEF exacerbation Dizziness Nausea and taste disturbances Visual disturbances | × | × | ✔ | ✔ | EKG |
| Sotalol | III | Kidney | Bradycardia AV Block Prolonged QT interval Torsades de pointes HFrEF exacerbation Bronchospasm GI upset | ✔ | ✔ | ✔ | ✔ | EKG Electrolytes Creatinine |
| Dronedarone | III | Liver | Bradycardia Prolonged QT interval Torsades de pointes GI upset Fatigue/weakness | × | × | ✔ | ✔ | EKG LFTs |
| Catheter Ablation | N/A | N/A | Bleeding complications Infection risk General anesthesia risks Thromboembolic event Cardiac perforation Post-ablation syndrome | ✔ | × | × | × | EKG |
| Study | Setting | Study Design | Intervention | Primary Outcome | Results |
|---|---|---|---|---|---|
| Hobbs et al. [44] | UK | Randomized controlled trial | AF screening (opportunistic and systematic) in adults aged ≥ 65 | Incidence of new cases of AF and incremental cost per case detected | AF screening increased new AF detection rates |
| Svendsen et al. [49] | Denmark | Randomized controlled trial | AF screening in adults aged 70–90 with at least one stroke risk factor | Time to first stroke or systemic arterial embolism | Loop recorder increased AF detection |
| Wyse et al. [57] | US and Canada | Randomized controlled trial | Rate control vs. rhythm control in adults aged ≥ 65 with AF | Overall mortality | No survival advantage between rhythm and control and rate control |
| Van Gelder et al. [59] | Netherlands | Randomized controlled non-inferiority trial | Lenient rate control vs. strict rate control in adults age ≤ 80 with permanent AF | Composite of death from cardiovascular causes, hospitalization for heart failure, stroke, systemic embolism, bleeding, and life-threatening arrhythmic events | Lenient rate control was non-inferior to the prevention of the primary outcome |
| Kirchhof et al. [75] | European countries | Randomized, open-label trial with blinded-outcome trial | Early rhythm control vs. usual care in asymptomatic and symptomatic adults with AF | Composite of death from cardiovascular causes, stroke, or hospitalization with worsening of heart failure or acute coronary syndrome | The rhythm-control strategy had a lower risk of the primary outcome |
| Packer et al. [79] | 10 Countries | Randomized controlled trial | Catheter ablation vs. drug therapy in adults with AF | Composite of death, disabling stroke, serious bleeding, or cardiac arrest | No difference in the primary outcome |
| Kim et al. [25] | Korea | Retrospective cohort study | N/A | First occurrence of ischemic stroke, major bleeding, or cardiovascular death | Oral anticoagulants in frail adults with AF decreased the risk of the primary outcome |
| Okumura et al. [37] | Japan | Randomized, double-blind, placebo-controlled trial | Low dose Edoxaban vs. placebo in adults age ≥ 80 with AF | Primary efficacy endpoint: composite of stroke or systemic embolism. Primary safety endpoint: major bleeding | Low-dose Edoxaban decreased the risk of stroke or systemic embolism with no increased risk of major bleeding |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosas Diaz, A.N.; Troy, A.L.; Kaplinskiy, V.; Pritchard, A.; Vani, R.; Ko, D.; Orkaby, A.R. Assessment and Management of Atrial Fibrillation in Older Adults with Frailty. Geriatrics 2024, 9, 50. https://doi.org/10.3390/geriatrics9020050
Rosas Diaz AN, Troy AL, Kaplinskiy V, Pritchard A, Vani R, Ko D, Orkaby AR. Assessment and Management of Atrial Fibrillation in Older Adults with Frailty. Geriatrics. 2024; 9(2):50. https://doi.org/10.3390/geriatrics9020050
Chicago/Turabian StyleRosas Diaz, Andrea Nathalie, Aaron L. Troy, Vladimir Kaplinskiy, Abiah Pritchard, Rati Vani, Darae Ko, and Ariela R. Orkaby. 2024. "Assessment and Management of Atrial Fibrillation in Older Adults with Frailty" Geriatrics 9, no. 2: 50. https://doi.org/10.3390/geriatrics9020050
APA StyleRosas Diaz, A. N., Troy, A. L., Kaplinskiy, V., Pritchard, A., Vani, R., Ko, D., & Orkaby, A. R. (2024). Assessment and Management of Atrial Fibrillation in Older Adults with Frailty. Geriatrics, 9(2), 50. https://doi.org/10.3390/geriatrics9020050






