Ophiocephalus striatus Extract Supplementation Decreases Serum IL-6 Levels in Older People with Sarcopenia—A Single-Center Experience
Abstract
1. Introduction
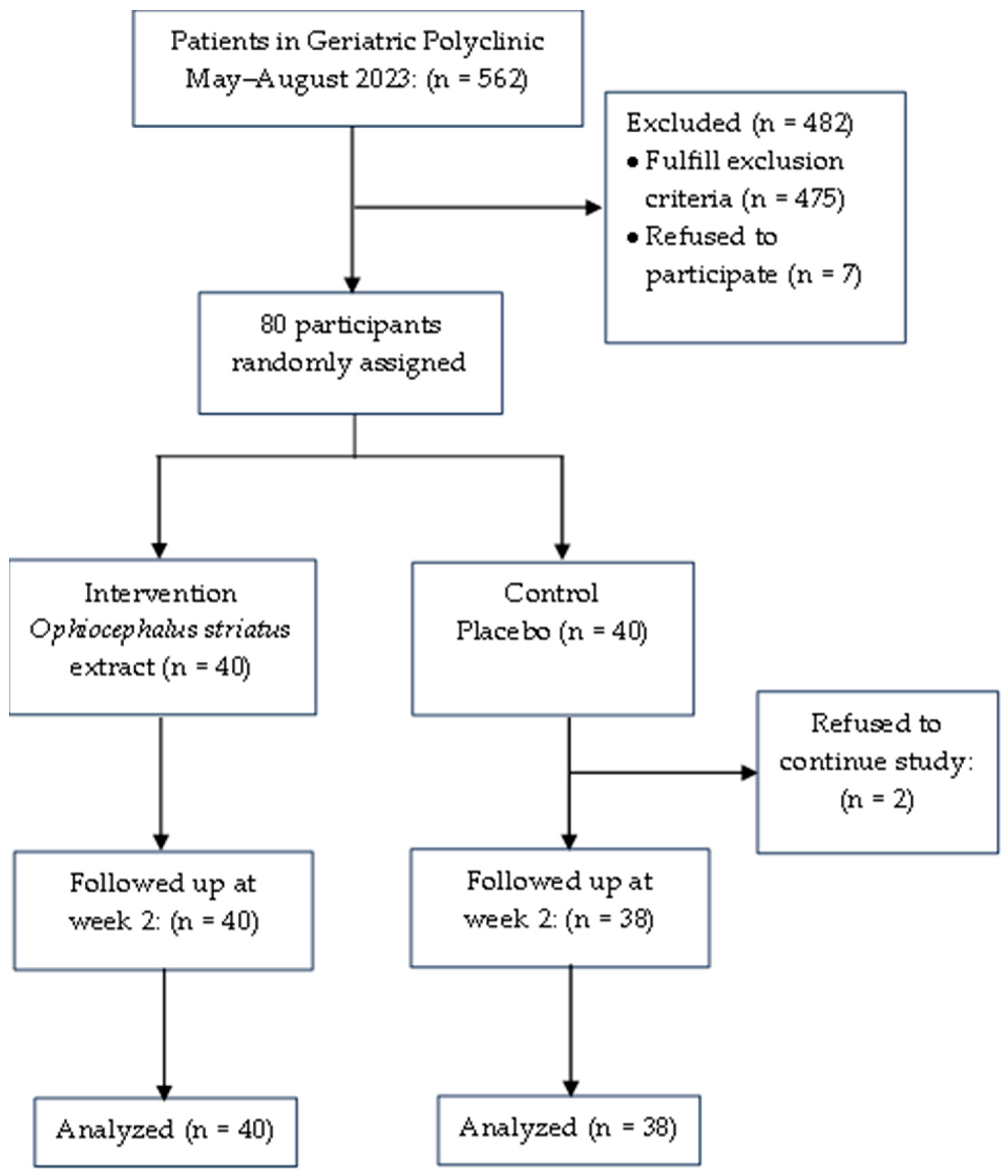
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Drop-Out Criteria
2.4. Data Collection
2.5. Intervention
2.6. Outcome Measures
2.7. Data Processing and Analysis
3. Results
4. Discussion
4.1. Prevalence of Sarcopenia by Age and Sex
4.2. Effect of Ophiocephalus Striatus Extract on Calf Circumference
4.3. Effect of Ophiocephalus Striatus Extract on Serum IGF-1
4.4. Effect of Ophiocephalus Striatus Extract on Serum IL-6
4.5. Effect of Ophiocephalus Striatus Extract on ASMI
4.6. Effect of Ophiocephalus Striatus Extract on Muscle Strength
4.7. Effect of Ophiocephalus Striatus Extract on Gait Speed
4.8. Effect of Sarcopenia Severity on Intervention Response
4.9. Influence of Nutritional Intake
4.10. Influence of Physical Activity Level on Intervention Response
4.11. Tolerability and Safety
4.12. Clinical Relevance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Nelke, C.; Dziewas, R.; Minnerup, J.; Meuth, S.G.; Ruck, T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine 2019, 49, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Uemura, K.; Doi, T.; Lee, S.; Shimada, H. Sarcopenia and Low Serum Albumin Level Synergistically Increase the Risk of Incident Disability in Older Adults. J. Am. Med. Dir. Assoc. 2019, 20, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Petermann-Rocha, F.; Chen, M.; Gray, S.R.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. New versus old guidelines for sarcopenia classification: What is the impact on prevalence and health outcomes? Age Ageing 2020, 49, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Bonato, M.; Turrini, F.; Galli, L.; Banfi, G.; Cinque, P. The role of physical activity for the management of sarcopenia in people living with HIV. Int. J. Environ. Res. Public Health 2020, 17, 1283. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Zaaria, M.; Pasleau, F.; Reginster, J.Y.; Bruyère, O. Health outcomes of sarcopenia: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0169548. [Google Scholar] [CrossRef]
- Coin, A.; Sergi, G.; Inelmen, E.M.; Enzi, G. Pathophysiology of Body Composition Changes in Elderly People. In Cachexia and Wasting: A Modern Approach; Springer: Berlin/Heidelberg, Germany, 2006; pp. 369–375. [Google Scholar]
- Rachim, R.; Sudarso, A.; Pricillia Makagiansar, S.; Bakri, S.; Rasyid, H.; Aman, A.; Kasim, H.; Seweng, A. Expression of Interleukin-6 Levels in Elderly Sarcopenia. Eur. J. Mol. Clin. Med. 2020, 7, 2837–2844. [Google Scholar]
- Nishikawa, H.; Fukunishi, S.; Asai, A.; Yokohama, K.; Nishiguchi, S.; Higuchi, K. Pathophysiology and mechanisms of primary sarcopenia (Review). Int. J. Mol. Med. 2021, 48, 156. [Google Scholar] [CrossRef]
- Wiedmer, P.; Jung, T.; Castro, J.P.; Pomatto, L.C.D.; Sun, P.Y.; Davies, K.J.A.; Grune, T. Sarcopenia–Molecular mechanisms and open questions. Ageing Res. Rev. 2020, 65, 101200. [Google Scholar] [CrossRef]
- Mulyana, R.; Setiati, S.; Martini, R.D.; Harimurti, K.; Dwimartutie, N. The Effect of Ophiocephalus Striatus Extract on the Levels of IGF-1 and Albumin in Elderly Patients with Hypoalbuminemia. Acta Med. Indones-Indones J. Intern. Med. 2017, 49, 324–329. [Google Scholar]
- Musarò, A.; Scicchitano, B.M. Counteracting sarcopenia: The role of IGF-1 isoforms. Aging 2019, 11, 3410–3411. [Google Scholar] [CrossRef]
- Coelho-Junior, H.J.; Calvani, R.; Azzolino, D.; Picca, A.; Tosato, M.; Landi, F.; Cesari, M.; Marzetti, E. Protein Intake and Sarcopenia in Older Adults: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 8718. [Google Scholar] [CrossRef]
- Stangl, M.K.; Böcker, W.; Chubanov, V.; Ferrari, U.; Fischereder, M.; Gudermann, T.; Hesse, E.; Meinke, P.; Reincke, M.; Reisch, N.; et al. Sarcopenia-Endocrinological and Neurological Aspects. Exp. Clin. Endocrinol. Diabetes 2018, 6, 8–22. [Google Scholar] [CrossRef]
- Hardee, J.P.; Lynch, G.S. Current pharmacotherapies for sarcopenia. In Expert Opinion on Pharmacotherapy; Taylor and Francis Ltd.: Abingdon, UK, 2019; pp. 1645–1657. [Google Scholar] [CrossRef]
- Jamshidi, S.; Mohsenpour, M.A.; Masoumi, S.J.; Fatahi, S.; Nasimi, N.; Zahabi, E.S.; Pourrajab, B.; Shidfar, F. Effect of whey protein consumption on IL-6 and TNF-α: A systematic review and meta-analysis of randomized controlled trials. In Diabetes and Metabolic Syndrome: Clinical Research and Reviews; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Murdhani, H.; Gatot, D.; Syahrini, H. International journal of research science & management the effect of ophiocephalus striatus extract on IL-6 serum levels in patients with cancer cachexia. Int. J. Res. Sci. Manag. 2020, 7, 117–122. [Google Scholar] [CrossRef]
- Tungadi, R. Potensi Ikan Gabus (Ophiocephalus Striatus) Dalam Mempercepat Penyembuhan Luka. Jambura Fish Process. J. 2020, 1, 46–55. [Google Scholar] [CrossRef]
- Kementerian Kelautan dan Perikanan. Produksi Perikanan. Available online: https://statistik.kkp.go.id/home.php?m=total&i=2#panel-footer (accessed on 18 February 2023).
- BPS Prov Sumatera Selatan. Total Produksi Perikanan Budidaya 2017–2019. Available online: https://sumsel.bps.go.id/indicator/56/685/1/total-produksi-perikanan-budidaya-.html (accessed on 18 February 2023).
- Dwijayanti, D.R.; Djati, M.S.; Rifai, M. Decreasing the expression level of macrophage cell, pro-inflammatory cytokines and NF-κb by using Vipalbumin® in vitro. Asian J. Cell Biol. 2015, 10, 43–56. [Google Scholar] [CrossRef][Green Version]
- Rong, Y.D.; Bian, A.L.; Hu, H.Y.; Ma, Y.; Zhou, X.Z. Study on relationship between elderly sarcopenia and inflammatory cytokine IL-6, anti-inflammatory cytokine IL-10. BMC Geriatr. 2018, 18, 308. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef]
- Gao, Q.; Hu, K.; Yan, C.; Zhao, B.; Mei, F.; Chen, F.; Zhao, L.; Shang, Y.; Ma, Y.; Ma, B. Associated factors of sarcopenia in community-dwelling older adults: A systematic review and meta-analysis. Nutrients 2021, 13, 4291. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Zhao, L.; Shang, Y.; Ma, Y.; Ma, B.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Hiraoka, A.; Nagamatsu, K.; Izumoto, H.; Yoshino, T.; Adachi, T.; Tsuruta, M.; Aibiki, T.; Okudaira, T.; Yamago, H.; Suga, Y.; et al. SARC-F combined with a simple tool for assessment of muscle abnormalities in outpatients with chronic liver disease. Hepatol. Res. 2020, 50, 502–511. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Ahmad, K.; Lee, E.J.; Lee, Y.; Choi, I. Implications of Insulin-Like Growth Factor-1 in Skeletal Muscle and Various Diseases. Cells 2020, 9, 1773. [Google Scholar] [CrossRef]
- Song, Y.; Song, J.; Delafontaine, P.; Godard, M.P. The Therapeutic Potential of IGF-I in Skeletal Muscle Repair. Trends Endocrinol. Metab. 2013, 24, 310–319. [Google Scholar] [CrossRef]
- Muldoon, M.F.; Laderian, B.; Kuan, D.C.H.; Sereika, S.M.; Marsland, A.L.; Manuck, S.B. Fish oil supplementation does not lower C-reactive protein or interleukin-6 levels in healthy adults. J. Intern. Med. 2016, 279, 98–109. [Google Scholar] [CrossRef]
- Ramirez-Ramirez, V.; Macias-Islas, M.A.; Ortiz, G.G.; Pacheco-Moises, F.; Torres-Sanchez, E.D.; Sorto-Gomez, T.E.; Cruz-Ramos, J.A.; Orozco-Aviña, G.; de la Rosa, A.J.C. Efficacy of fish oil on serum of TNF α, IL-1 β, and IL-6 oxidative stress markers in multiple sclerosis treated with interferon beta-1b. Oxidative Med. Cell. Longev. 2013, 2013, 709493. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Belury, M.A.; Andridge, R.; Malarkey, W.B.; Hwang, B.S.; Glaser, R. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: A randomized controlled trial. Brain Behav. Immun. 2012, 26, 988–995. [Google Scholar] [CrossRef]
- Dewita, D.; Sidauruk, S.W.; Desmelati, D. Amino Acid and Mineral Profiles of Fresh Snakehead (Channa Striata) Meat to Potential as an Immune System. Iop Conf. Ser. Earth Environ. Sci. 2022, 1118, 012034. [Google Scholar] [CrossRef]
- Alshahrani, A.; Bin Khunayfir, A.; Al Rayih, M.; Al Sayed, H.; Alsadoon, A.; Al Dubayee, M.; Zahra, M.; Alrumayyan, Y.; Al Zayer, M.; Nasr, A.; et al. Phenotypic Characterization of Human Monocytes Following Macronutrient Intake in Healthy Humans. Front. Immunol. 2017, 8, 1293. [Google Scholar] [CrossRef]
- Ria, N.; Siahaan, G.; Nasution, Z.; Saragih, H.S. Clinical Manifestation of Bmi, Tlc, Albumin and Cd4 After Provision of Snakehead Nugget and Colored Fruit Juice to People with Hiv. Media Gizi Indones. 2022, 17, 76–81. [Google Scholar] [CrossRef]
- Sa’ad, M.; Muhtadi, M. Protein Profiles of Snakehead (Channa striata), Catfish (Pangasius hypopthalmus), and Mackerel (Rastrelliger spp.) and Their Effectiveness as Antidiabetic Agents. In Atlantis Highlights in Chemistry and Pharmaceutical Sciences; Springer Nature: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Ebaid, H.; Salem, A.M.H.; Sayed, A.A.; Metwalli, A. Whey Protein Enhances Normal Inflammatory Responses During Cutaneous Wound Healing in Diabetic Rats. Lipids Health Dis. 2011, 10, 235. [Google Scholar] [CrossRef]
- Hanaoka, B.Y.; Zhao, J.; Heitman, K.N.; Khan, F.; Jarjour, W.; Volek, J.; Brock, G.; Gower, B.A. Interaction Effect of Systemic Inflammation and Modifiable Rheumatoid Cachexia Risk Factors on Resting Energy Expenditure in Patients with Rheumatoid Arthritis. JCSM Clin. Rep. 2022, 7, 12–23. [Google Scholar] [CrossRef]
- Zhu, K.; Kerr, D.A.; Meng, X.; Devine, A.; Solah, V.; Binns, C.W.; Prince, R.L. Two-year whey protein supplementation did not enhance muscle mass and physical function in well-nourished healthy older postmenopausal women. J. Nutr. 2015, 145, 2520–2526. [Google Scholar] [CrossRef]
- Coelho-Júnior, H.J.; Calvani, R.; Tosato, M.; Landi, F.; Picca, A.; Marzetti, E. Protein intake and physical function in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2022, 81, 101731. [Google Scholar] [CrossRef]
- Ellis, A.C.; Hunter, G.R.; Goss, A.M.; Gower, B.A. Oral Supplementation with Beta-Hydroxy-Beta-Methylbutyrate, Arginine, and Glutamine Improves Lean Body Mass in Healthy Older Adults. J. Diet. Suppl. 2019, 16, 281–293. [Google Scholar] [CrossRef]
- Caballero-García, A.; Pascual-Fernández, J.; Noriega-González, D.C.; Bello, H.J.; Pons-Biescas, A.; Roche, E.; Córdova-Martínez, A. L-citrulline supplementation and exercise in the management of sarcopenia. Nutrients 2021, 13, 3133. [Google Scholar] [CrossRef]
- De Liao, C.; Chen, H.-C.; Huang, S.W.; Liou, T.H. The Role of Muscle Mass Gain Following Protein Supplementation Plus Exercise Therapy in Older Adults with Sarcopenia and Frailty Risks: A Systematic Review and Meta-Regression Analysis of Randomized Trials. Nutrients 2019, 11, 1713. [Google Scholar] [CrossRef]
- Gielen, E.; Beckwée, D.; Delaere, A.; De Breucker, S.; Vandewoude, M.; Bautmans, I. Nutritional Interventions to Improve Muscle Mass, Muscle Strength, and Physical Performance in Older People: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutr. Rev. 2021, 79, 121–147. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; D’angelo, E.; Sisto, A.; Marzetti, E. Protein Intake and Muscle Health in Old Age: From Biological Plausibility to Clinical Evidence. Nutrients 2016, 8, 295. [Google Scholar] [CrossRef]
- Hearris, M.A.; Hammond, K.M.; Fell, J.W.; Morton, J.P. Regulation of Muscle Glycogen Metabolism During Exercise: Implications for Endurance Performance and Training Adaptations. Nutrients 2018, 10, 298. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.P.; Bauer, J.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein Intake and Exercise for Optimal Muscle Function with Aging: Recommendations From the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Arnau, F.M.; Fonfría-Vivas, R.; Cauli, O. Beneficial Effects of Leucine Supplementation on Criteria for Sarcopenia: A Systematic Review. Nutrients 2019, 11, 2504. [Google Scholar] [CrossRef] [PubMed]
- Traylor, D.A.; Gorissen, S.H.M.; Phillips, S.M. Perspective: Protein Requirements and Optimal Intakes in Aging: Are We Ready to Recommend More Than the Recommended Daily Allowance? Adv. Nutr. 2018, 9, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, Y.; Zhao, Y. Efficacy of Exercise on Muscle Function and Physical Performance in Older Adults with Sarcopenia: An Updated Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 8212. [Google Scholar] [CrossRef] [PubMed]
- Hanach, N.; McCullough, F.; Avery, A. The Impact of Dairy Protein Intake on Muscle Mass, Muscle Strength, and Physical Performance in Middle-Aged to Older Adults with or without Existing Sarcopenia: A Systematic Review and Meta-Analysis. Adv. Nutr. 2019, 10, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Biswas, A.; Ma, W.; Kandpal, M.; Coker, C.; Grandgenett, P.M.; Hollingsworth, M.A.; Jain, R.; Tanji, K.; Lόpez-Pintado, S.; et al. Metastatic Cancers Promote Cachexia Through ZIP14 Upregulation in Skeletal Muscle. Nat. Med. 2018, 24, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Summermatter, S.; Bouzan, A.; Pierrel, E.; Melly, S.; Stauffer, D.; Gutzwiller, S.; Nolin, E.; Dornelas, C.; Fryer, C.; Leighton-Davies, J.; et al. Blockade of Metallothioneins 1 and 2 Increases Skeletal Muscle Mass and Strength. Mol. Cell. Biol. 2017, 37, e00305-16. [Google Scholar] [CrossRef]
- Theodorou, A.A.; Panayiotou, G.; Volaklis, K.A.; Douda, H.T.; Paschalis, V.; Nikolaidis, M.G.; Smilios, I.; Toubekis, A.; Kyprianou, D.; Papadopoulos, I.; et al. Aerobic, resistance and combined training and detraining on body composition, muscle strength, lipid profile and inflammation in coronary artery disease patients. Res. Sports Med. 2016, 24, 171–184. [Google Scholar] [CrossRef]
- Fry, C.S.; Noehren, B.; Mula, J.; Ubele, M.F.; Westgate, P.M.; Kern, P.A.; Peterson, C.A. Fibre type-specific satellite cell response to aerobic training in sedentary adults. J. Physiol. 2014, 592, 2625–2635. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, L.; Chen, S.T.; Bae, J.H.; Kim, D.Y.; Liu, X.; Song, W. Effects and Moderators of Exercise on Sarcopenic Components in Sarcopenic Elderly: A Systematic Review and Meta-Analysis. Front. Med. 2021, 19, 649748. [Google Scholar] [CrossRef] [PubMed]
- Bakar, M.R.A.; Kadir, A.A.; Wahab, S.Z.A.; Karim, A.H.A.; Hussain, N.H.N.; Noor, N.M.; Omar, J.; Bin Bai@ Bae, S.; Mahmood, W.H.W.; Razak, A.A.; et al. Randomized controlled trial on the effect of channa striatus extract on measurement of the uterus, pulsatility index, resistive index of uterine artery and superficial skin wound artery in post lower segment caesarean section women. PLoS ONE 2015, 10, e0133514. [Google Scholar] [CrossRef]
- Kadir, A.A.; Ab Wahab, S.Z.; Zulkifli, M.M.; Noor, N.M.; Baie, S.B.B.; Haron, J. The therapeutic effect of oral Channa striatus extract on primary knee osteoarthritis patients. Agro Food Industry Hi Tech 2014, 25, 44–48. [Google Scholar]
- Scarpignato, C.; Gatta, L.; Zullo, A.; Blandizzi, C. Effective and safe proton pump inhibitor therapy in acid-related diseases—A position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016, 14, 179. [Google Scholar] [CrossRef]
| Characteristics | Intervention Ophiocephalus striatus (n = 40) | Control Placebo (n = 38) | p-Value |
|---|---|---|---|
| Age, median (min–max) | 70 (62–85) | 71 (60–88) | 0.627 |
| 60–65 years | 9 (22.5) | 11 (28.9) | 0.137 |
| 66–70 years | 12 (30) | 5 (13.2) | |
| 71–75 years | 14 (35) | 10 (26.3) | |
| ≥76 years | 5 (12.5) | 12 (31.6) | |
| Sex, n (%) | 0.854 | ||
| Men | 15 (37.5) | 16 (42.1) | |
| Women | 25 (62.5) | 22 (57.9) | |
| Smoking status, n (%) | 0.431 | ||
| Yes | 16 (40) | 11 (28.9) | |
| No | 24 (60) | 27 (71.1) | |
| Nutrition status, n (%) | 0.799 | ||
| Normal | 7 (17.5) | 7 (18.4) | |
| At risk for malnutrition | 28 (70) | 28 (73.7) | |
| Malnutrition | 5 (12.5) | 3 (7.9) | |
| ASMI (kg/m2), median (min–max) | |||
| Men | 6.6 (5.86–6.90) | 6.57 (4.92–6.80) | 0.565 |
| Women | 5.60 (4.70–5.68) | 5.54 (5.00–5.68) | 0.716 |
| Handgrip strength (kg), mean ± SD | |||
| Men | 17.27 ± 5.26 | 21.38 ± 6.35 | 0.060 |
| Women | 17.48 ± 6.15 | 19.55 ± 6.86 | 0.282 |
| Gait speed (m/s), mean ± SD | 0.70 ± 0.20 | 0.69 ± 0.22 | 0.886 |
| Sarcopenia severity | 0.662 | ||
| Sarcopenia | 17 (42.5) | 19 (50) | |
| Severe sarcopenia | 23 (57.5) | 19 (50) | |
| Functional status, n (%) | 0.898 | ||
| Independent | 31 (77.5) | 28 (73.7) | |
| Mildly dependent | 9 (22.5) | 10 (26.3) | |
| Comorbid, n (%) | |||
| Hypertension | 25 (62.5) | 20 (52.6) | 0.38 |
| Diabetes melitus | 4 (10) | 4 (10.5) | 0.94 |
| Osteoarthritis | 10 (25) | 8 (21.1) | 0.68 |
| Osteoporosis | 6 (15) | 10 (26.3) | 0.21 |
| COPD | 6 (15) | 9 (23.7) | 0.33 |
| Cerebrovascular disease | 4 (10) | 2 (5.3) | 0.43 |
| Cognitive function, n (%) | 0.246 | ||
| Normal | 35 (87.5) | 37 (97.4) | |
| Moderate cognitive impairment | 4 (10) | 1 (2.6) | |
| Severe cognitive impairment | 1 (2.5) | 0 (0) | |
| Mental status, n (%) | |||
| Normal | 36 (90) | 33 (86.8) | 0.935 |
| Mild depression | 4 (10) | 5 (13.2) | |
| PASE | 0.82 | ||
| Men | 34.8 ± 33.82 | 53.8 ± 38.03 | |
| Women | 57 ± 27.28 | 51.3 ± 31.78 | |
| Laboratorium | |||
| Hb, g/dL | 12.52 ± 1.27 | 12.45 ± 1.43 | 0.820 |
| Blood sugar, mg/dL | 84.50 (46.0–330.0) | 86.00 (58.0–408.0) | 0.779 |
| Urea, mg/dL | 30 (17.0–77.0) | 31.0 (15.0–62.0) | 0.685 |
| Creatinine, mg/dL | 0.79 (0.52–1.77) | 0.79 (0.54–1.28) | 0.964 |
| eGFR, mL/min/1.73 m2 | 78.35 ± 21.70 | 79.50 ± 16.64 | 0.795 |
| Albumin, g/dL | 4.35 (3.6–4.9) | 4.3 (3.1–4.8) | 0.537 |
| IGF-1, ng/dL | 77.08 ± 20.9 | 78.92 ± 21.7 | 0.705 |
| IL-6, pg/dL | 44.51 ± 23.57 | 36.47 ± 17.45 | 0.092 |
| Anthropometric | |||
| Body weight (kg), mean ± SD | 42.87 ± 9.04 | 44.75 ± 7.93 | 0.332 |
| Body Mass Index, mean ± SD | 17.62 ± 2.71 | 17.71 ± 2.72 | 0.807 |
| Upper arm circumference, mean ± SD | 21.74 ± 3.08 | 22.63 ± 2.87 | 0.190 |
| Waist circumference, mean ± SD | 77.16 ± 10.47 | 75.62 ± 8.78 | 0.484 |
| Calf circumference, mean ± SD | 27.03 ± 2.40 | 27.96 ± 2.29 | 0.083 |
| Intake by food recall | |||
| Calorie (kcal/day) | 1428.48 ± 379.82 | 1605.29 ± 363.63 | 0.039 * |
| Men | 1478.5 ± 405.91 | 1818.53 ± 334.1 | 0.016 * |
| Women | 1398.47 ± 368.53 | 1450.20 ± 305.61 | 0.606 |
| Protein (g/day) | 52.29 ± 15.72 | 57.73 ± 16.54 | 0.140 |
| Men | 49.28 ± 14.56 | 61.64 ± 16.16 | 0.034 * |
| Women | 54.09 ± 16.39 | 54.88 ± 16.58 | 0.870 |
| Carbohydrate (g/day) | 188.0 ± 52.37 | 217 ± 63.78 | 0.031 * |
| Men | 204.94 ± 46.94 | 266.57 ± 58.34 | 0.003 * |
| Women | 177.85 ± 53.71 | 181.06 ± 38.85 | 0.818 |
| Fat (g/day) | 48.74 ± 21.98 | 53.13 ± 26.32 | 0.425 |
| Men | 42.41 ± 19.93 | 55.46 ± 32.2 | 0.189 |
| Women | 52.55 ± 22.66 | 51.44 ± 21.75 | 0.866 |
| Zinc (mg/day) | 4.32 ± 1.41 | 5.33 ± 1.93 | 0.01 * |
| Men | 4.11 ± 1.53 | 5.27 ± 1.41 | 0.036 * |
| Women | 4.45 ± 1.35 | 5.39 ± 2.27 | 0.088 |
| Variable | Intervention Group | Placebo Group | Intervention Effect | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Day 14 | p-Value | Baseline | Day-14 | p-Value | Mean Difference (95% CI) | p-Value | |
| Calf circumference, cm | 27.03 ± 2.4 | 27.76 ± 2.51 | 0.02 | 27.96 ± 2.29 | 28.16 ± 2.49 | 0.37 | 0.53 (−0.22–1.3) | 0.16 |
| Men | 28.3 ± 1.8 | 28.7 ± 1.16 | 0.38 | 28.7 ± 2.29 | 29 ± 2.48 | 0.41 | 0.1 (−0.96–1.32) | 0.74 |
| Women | 26.3 ± 2.42 | 27.2 ± 2.93 | 0.02 | 27.4 ± 2.17 | 27.6 ± 2.38 | 0.61 | 0.7 (−0.29–1.79) | 0.15 |
| IGF-1, ng/dL | 77.08 ± 20.9 | 81.27 ± 30.22 | 0.23 | 78.92 ± 21.7 | 77.79 ± 27.72 | 0.8 | 5.32 (−6.72–17.35) | 0.38 |
| Men | 82.65 ± 17.35 | 84.32 ± 29.61 | 0.42 | 70.89 ± 24.29 | 73.28 ± 28.75 | 0.4 | −0.7 (−22.72–24.12) | 0.95 |
| Women | 73.75 ± 22.43 | 79.45 ± 31.03 | 0.23 | 84.75 ± 17.97 | 81.06 ± 27.14 | 0.29 | 9.38 (−4.25–23.01) | 0.17 |
| IL-6, pg/dL | 44.51 ± 23.57 | 33.41 ± 17.83 | <0.001 * | 36.47 ± 17.45 | 61.39 ± 35.26 | 0.00 * | −36 (−49.2–(−22.67)) | <0.001 * |
| Men | 42.31 ± 22.09 | 27.98 ± 13.38 | 0.02 * | 41.06 ± 21.29 | 62.53 ± 28.04 | 0.01 * | −35.79 (−53.73–(−17.85)) | 0.00 * |
| Women | 45.83 ± 24.76 | 36.67 ± 19.56 | 0.07 | 33.14 ± 13.6 | 60.56 ± 40.34 | 0.002 * | −36.58 (−55.6–(−17.55)) | 0.00 * |
| ASMI, kg/m2 | ||||||||
| Men | 6.49 ± 0.39 | 6.56 ± 0.56 | 0.36 | 6.43 ± 0.46 | 6.58 ± 0.55 | 0.14 | −0.08 (−0.39–0.23) | 0.62 |
| Women | 5.43 ± 0.28 | 5.58 ± 0.42 | 0.03 * | 5.48 ± 0.2 | 5.59 ± 0.29 | 0.03 * | 0.04 (−0.12–0.21) | 0.62 |
| Handgrip strength, kg | ||||||||
| Men | 22.2 ± 5.48 | 23.2 ± 5.89 | 0.12 | 23.44 ± 6.62 | 25 ± 7.21 | 0.03 * | −0.56 (−2.41–1.28) | 0.54 |
| Women | 15.88 ± 5.54 | 17.48 ± 5.11 | <0.001 * | 16.5 ± 4.5 | 17.23 ± 4.58 | 0.18 | 0.87 (−0.64–2.38) | 0.25 |
| Gait speed, m/s | ||||||||
| Men | 0.72 ± 0.22 | 0.79 ± 0.21 | 0.02 * | 0.71 ± 0.22 | 0.77 ± 0.25 | 0.05 | 0.21 (−0.06–0.1) | 0.61 |
| Women | 0.69 ± 0.2 | 0.73 ± 0.26 | 0.16 | 0.68 ± 0.22 | 0.77 ± 0.25 | 0.11 | −0.05 (−0.13–0.08) | 0.64 |
| Creatinine, mg/dL | 0.87 ± 0.28 | 0.89 ± 0.34 | 0.41 | 0.83 ± 0.2 | 0.86 ± 0.26 | 0.39 | 0.01 (−0.08–0.07) | 0.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riviati, N.; Legiran, L.; Saleh, I.; Indrajaya, T.; Ali, Z.; Irfannuddin; Probosuseno. Ophiocephalus striatus Extract Supplementation Decreases Serum IL-6 Levels in Older People with Sarcopenia—A Single-Center Experience. Geriatrics 2024, 9, 35. https://doi.org/10.3390/geriatrics9020035
Riviati N, Legiran L, Saleh I, Indrajaya T, Ali Z, Irfannuddin, Probosuseno. Ophiocephalus striatus Extract Supplementation Decreases Serum IL-6 Levels in Older People with Sarcopenia—A Single-Center Experience. Geriatrics. 2024; 9(2):35. https://doi.org/10.3390/geriatrics9020035
Chicago/Turabian StyleRiviati, Nur, Legiran Legiran, Irsan Saleh, Taufik Indrajaya, Zulkhair Ali, Irfannuddin, and Probosuseno. 2024. "Ophiocephalus striatus Extract Supplementation Decreases Serum IL-6 Levels in Older People with Sarcopenia—A Single-Center Experience" Geriatrics 9, no. 2: 35. https://doi.org/10.3390/geriatrics9020035
APA StyleRiviati, N., Legiran, L., Saleh, I., Indrajaya, T., Ali, Z., Irfannuddin, & Probosuseno. (2024). Ophiocephalus striatus Extract Supplementation Decreases Serum IL-6 Levels in Older People with Sarcopenia—A Single-Center Experience. Geriatrics, 9(2), 35. https://doi.org/10.3390/geriatrics9020035







