Diffusion MRI Fiber Tractography and Benzodiazepine SPECT Imaging for Assessing Neural Damage to the Language Centers in an Elderly Patient after Successful Reperfusion Therapy
Abstract
1. Introduction
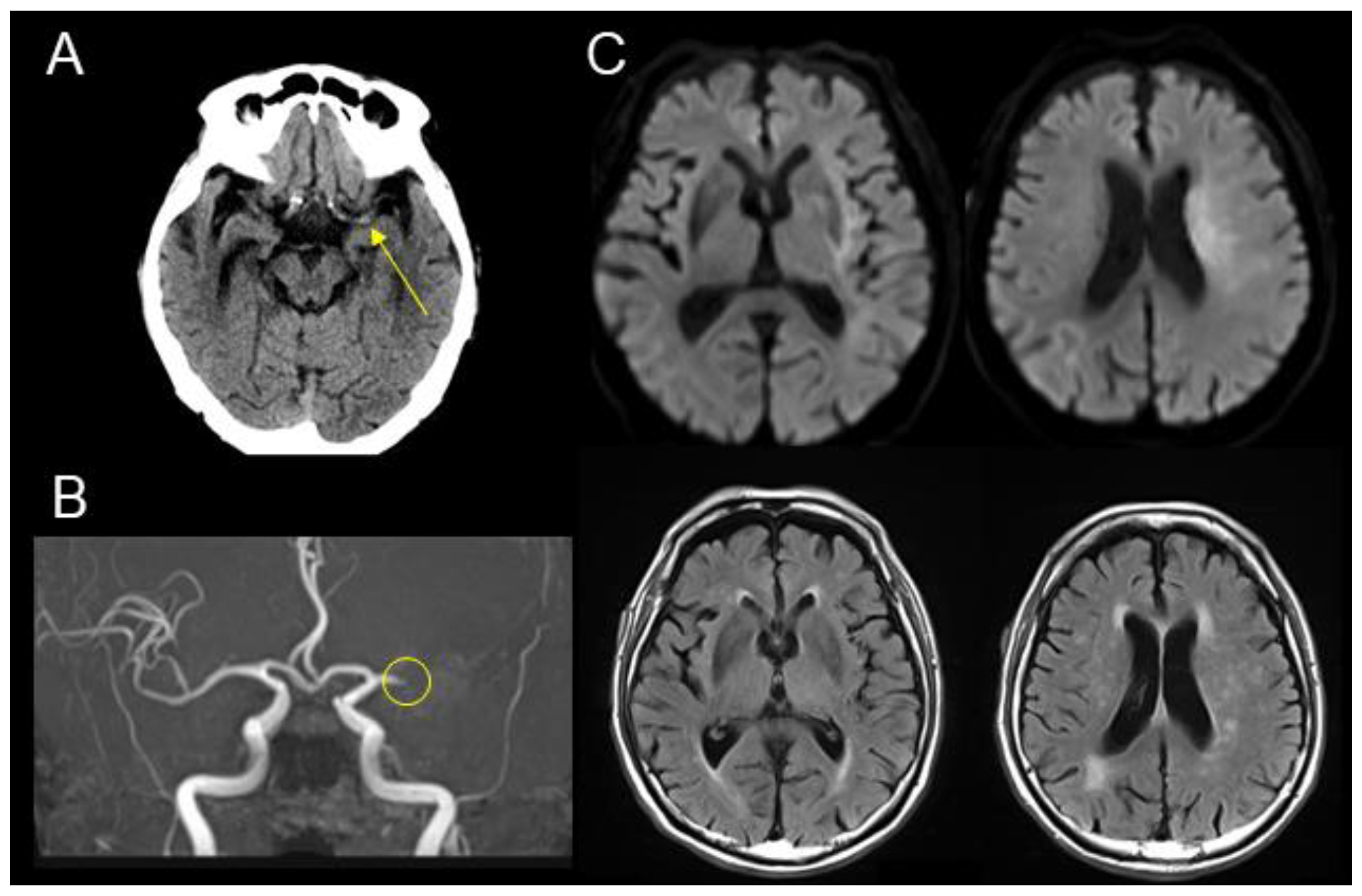
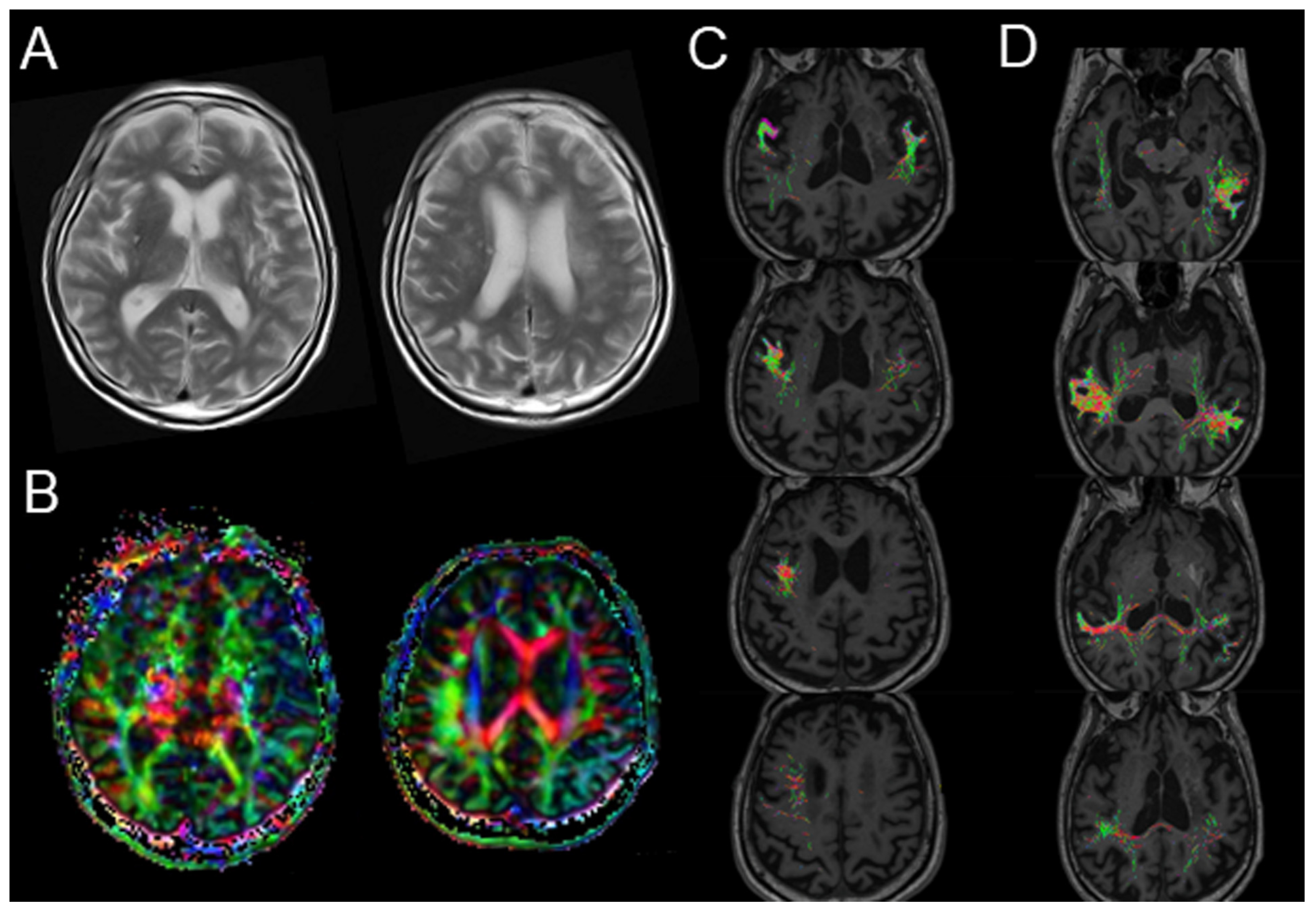
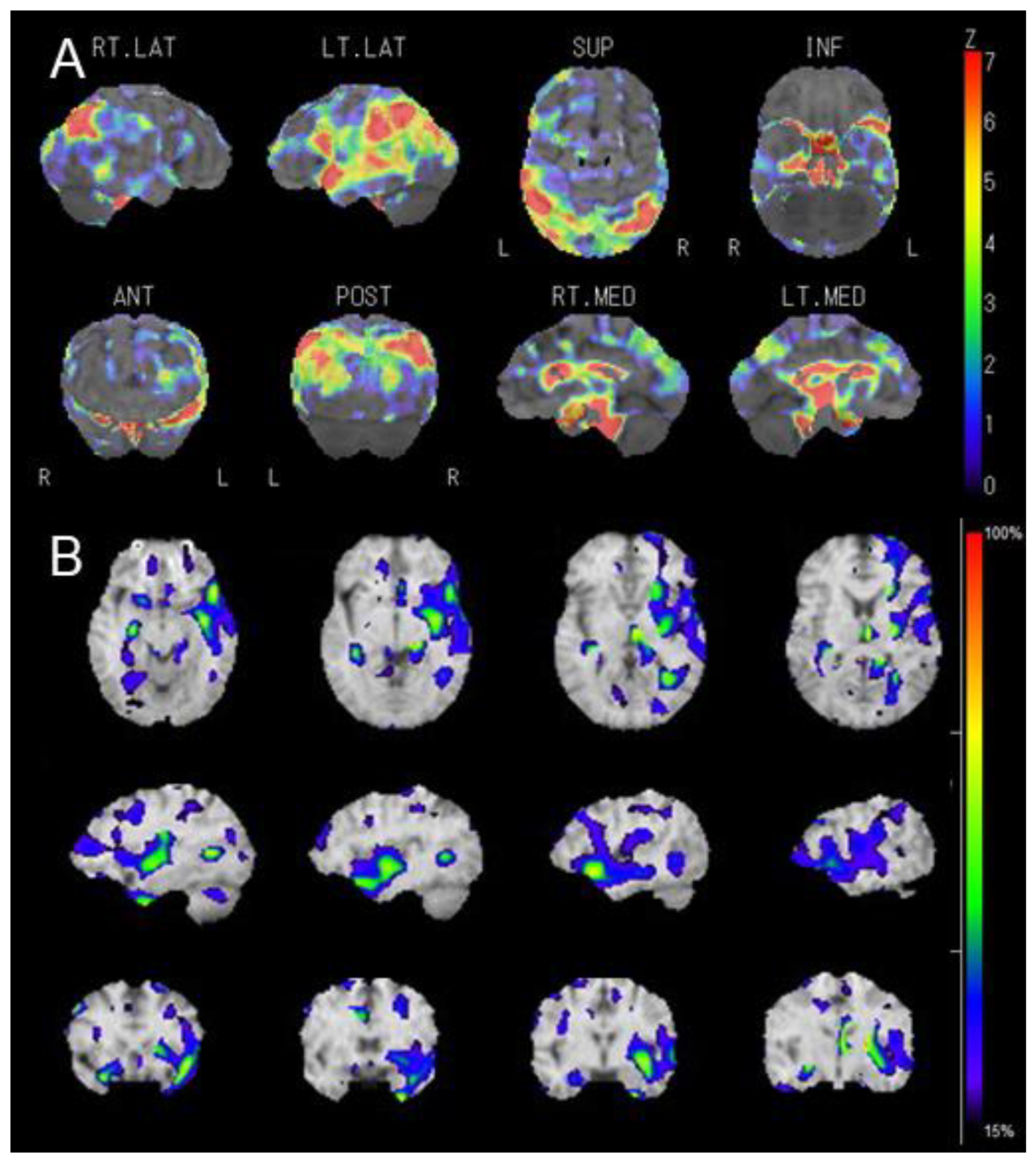
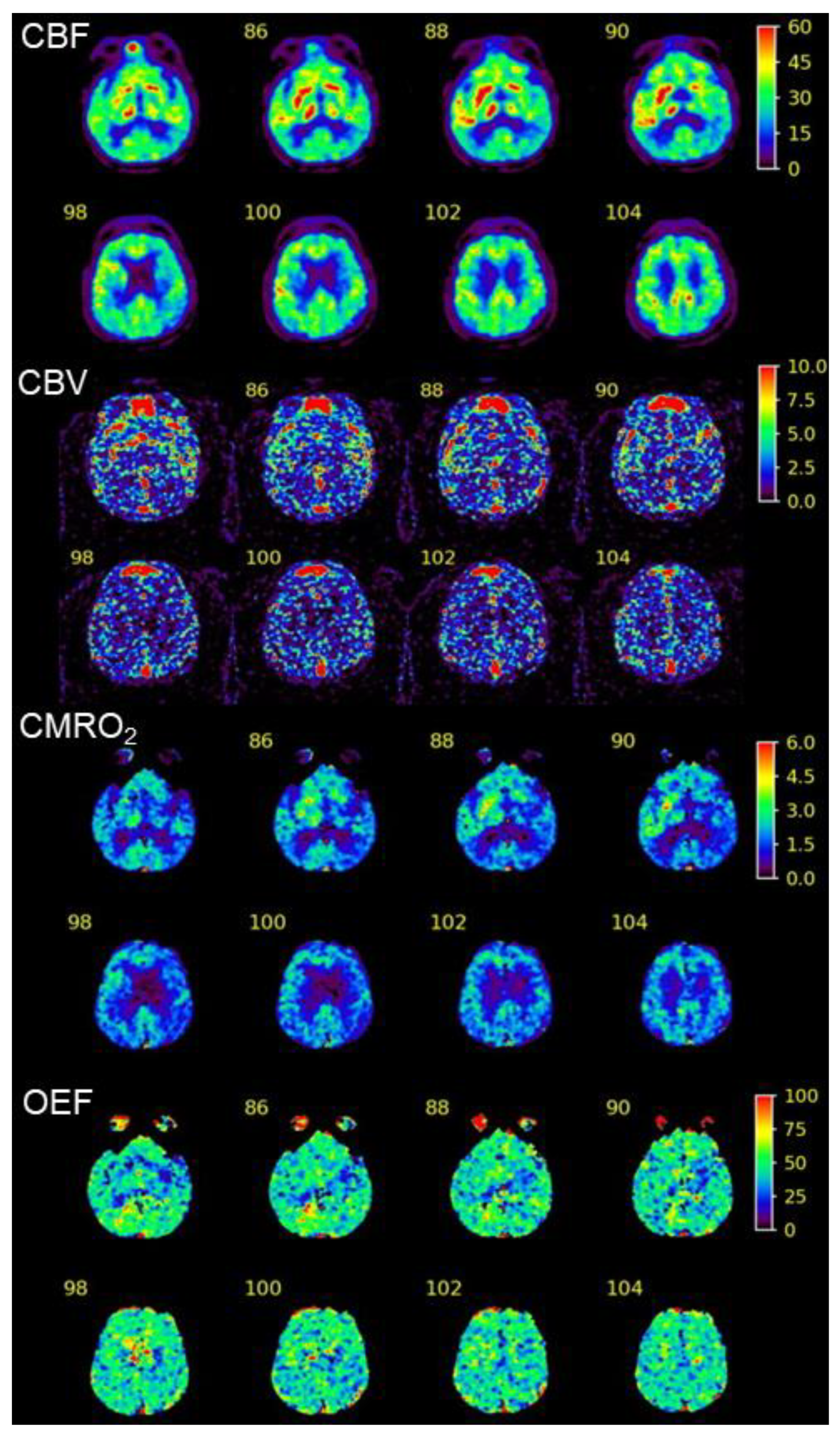
2. Case Presentation
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoshimura, S.; Sakai, N.; Yamagami, H.; Uchida, K.; Beppu, M.; Toyoda, K.; Matsumaru, Y.; Matsumoto, Y.; Kimura, K.; Takeuchi, M.; et al. Endovascular therapy for acute stroke with a large ischemic region. N. Engl. J. Med. 2022, 386, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Widimsky, P.; Snyder, K.; Sulzenko, J.; Hopkins, L.N.; Stetkarova, I. Acute ischaemic stroke: Recent advances in reperfusion treatment. Eur. Heart J. 2022, 44, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, B.J.; Han, M.K.; Park, T.H.; Lee, K.B.; Lee, B.C.; Yu, K.H.; Oh, M.S.; Cha, J.K.; Kim, D.H.; et al. Futile reperfusion and predicted therapeutic benefits after successful endovascular treatment according to initial stroke severity. BMC Neurol. 2019, 19, 11. [Google Scholar] [CrossRef]
- Rabinstein, A.A.; Albers, G.W.; Brinjikji, W.; Koch, S. Factors that may contribute to poor outcome despite good reperfusion after acute endovascular stroke therapy. Int. J. Stroke 2019, 14, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Cui, T.; Wang, C.; Zhu, Q.; Zhang, X.; Li, S.; Yang, Y.; Shang, W.; Wu, B. Prognostic significance of admission glucose combined with hemoglobin A1c in acute ischemic stroke patients with reperfusion therapy. Brain Sci. 2022, 12, 294. [Google Scholar] [CrossRef]
- Tipirneni, S.; Stanwell, P.; Weissert, R.; Bhaskar, S.M.M. Prevalence and impact of cerebral microbleeds on clinical and safety outcomes in acute ischaemic stroke patients receiving reperfusion therapy: A systematic review and meta-analysis. Biomedicines 2023, 11, 2865. [Google Scholar] [CrossRef]
- Mitsuhashi, T.; Teranishi, K.; Tokugawa, J.; Mitsuhashi, T.; Hishii, M.; Oishi, H. Prognostic determinants of anterior large vessel occlusion in acute stroke in elderly patients. Geriatrics 2024, 9, 13. [Google Scholar] [CrossRef]
- Shen, H.; Killingsworth, M.C.; Bhaskar, S.M.M. Comprehensive meta-analysis of futile recanalization in acute ischemic stroke patients undergoing endovascular thrombectomy: Prevalence, factors, and clinical outcomes. Life 2023, 13, 1965. [Google Scholar] [CrossRef]
- Jones, T.A. Motor compensation and its effects on neural reorganization after stroke. Nat. Rev. Neurosci. 2017, 18, 267–280. [Google Scholar] [CrossRef]
- Cuthbert, S.C.; Goodheart, G.J., Jr. On the reliability and validity of manual muscle testing: A literature review. Chiropr. Osteopat. 2007, 15, 4. [Google Scholar] [CrossRef]
- Thomalla, G.; Cheng, B.; Ebinger, M.; Hao, Q.; Tourdias, T.; Wu, O.; Kim, J.S.; Breuer, L.; Singer, O.C.; Warach, S.; et al. DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4·5 h of symptom onset (PRE-FLAIR): A multicentre observational study. Lancet Neurol. 2011, 10, 978–986. [Google Scholar] [CrossRef]
- Sugimoto, H.; Otake-Matsuura, M. Tract-based spatial statistics analysis of diffusion tensor imaging in older adults after the PICMOR intervention program: A pilot study. Front. Aging Neurosci. 2022, 14, 867417. [Google Scholar] [CrossRef] [PubMed]
- Nakagawara, J.; Kamiyama, K.; Takahashi, M.; Nakamura, H. Cortical neuron loss in post-traumatic higher brain dysfunction using 123I-iomazenil SPECT. Acta Neurochir. Suppl. 2013, 118, 245–250. [Google Scholar]
- Kato, H.; Isohashi, K.; Shimosegawa, E.; Hatazawa, J. Increase in extraction of I-123 iomazenil in patients with chronic cerebral ischemia. PLoS ONE 2018, 13, e0190720. [Google Scholar] [CrossRef] [PubMed]
- Grönberg, A.; Henriksson, I.; Stenman, M.; Lindgren, A.G. Incidence of Aphasia in Ischemic Stroke. Neuroepidemiology 2022, 56, 174–182. [Google Scholar] [CrossRef]
- Wu, C.; Qin, Y.; Lin, Z.; Yi, X.; Wei, X.; Ruan, Y.; He, J. Prevalence and impact of aphasia among patients admitted with acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 104764. [Google Scholar] [CrossRef]
- Pedersen, P.M.; Jørgensen, H.S.; Nakayama, H.; Raaschou, H.O.; Olsen, T.S. Aphasia in acute stroke: Incidence, determinants, and recovery. Ann. Neurol. 1995, 38, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Jang, S.H. Prediction of aphasia outcome using diffusion tensor tractography for arcuate fasciculus in stroke. AJNR Am. J. Neuroradiol. 2013, 34, 785–790. [Google Scholar] [CrossRef]
- Kuroda, H.; Yamamoto, D.; Koizumi, H.; Shimizu, S.; Kumabe, T. Cortical neural damage associated with cerebral hyperperfusion after reperfusion therapy for acute ischemic stroke: 123I-iomazenil single-photon emission computed tomography findings. NMC Case Rep. J. 2021, 8, 367–370. [Google Scholar] [CrossRef]
- Berndt, M.T.; Maegerlein, C.; Boeckh-Behrens, T.; Wunderlich, S.; Zimmer, C.; Wirth, S.; Muck, F.G.; Monch, S.; Friedrich, B.; Kaesmacher, J. Microstructural integrity of salvaged penumbra after mechanical thrombectomy. AJNR Am. J. Neuroradiol. 2020, 41, 79–85. [Google Scholar] [CrossRef]
- Jang, S.H. Diffusion tensor imaging studies on arcuate fasciculus in stroke patients: A review. Front. Hum. Neurosci. 2013, 7, 749. [Google Scholar] [CrossRef]
- Chen, X.; Chen, L.; Zheng, S.; Wang, H.; Dai, Y.; Chen, Z.; Huang, R. Disrupted brain connectivity networks in aphasia revealed by resting-state fMRI. Front. Aging Neurosci. 2021, 13, 666301. [Google Scholar] [PubMed]
- Yu, Q.; Jiang, Y.; Sun, Y.; Ju, X.; Ye, T.; Liu, N.; Qian, S.; Liu, K. Effects of damage to the integrity of the left dual-stream frontotemporal network mediated by the arcuate Ffasciculus and uncinate fasciculus on acute/subacute post-stroke aphasia. Brain Sci. 2023, 13, 1324. [Google Scholar] [CrossRef]
- Yu, Q.; Sun, Y.; Liao, X.; Qian, W.; Ye, T. Integrity of the left arcuate fasciculus segments significantly affects language performance in individuals with acute/subacute post-stroke aphasia: A cross-sectional diffusion tensor imaging study. Brain Sci. 2022, 12, 907. [Google Scholar] [CrossRef]
- Sul, B.; Lee, K.B.; Hong, B.Y.; Kim, J.S.; Kim, J.; Hwang, W.S.; Lim, S.H. Association of lesion location with long-term recovery in post-stroke aphasia and language deficits. Front. Neurol. 2019, 10, 776. [Google Scholar] [CrossRef]
- Suzuki, T.; Ogasawara, K.; Kuroda, H.; Chida, K.; Aso, K.; Kobayashi, M.; Fujiwara, S.; Yoshida, K.; Terasaki, K.; Ogawa, A. Comparison of early and late images on 123I-iomazenil SPECT with cerebral blood flow and oxygen extraction fraction images on PET in the cerebral cortex of patients with chronic unilateral major cerebral artery occlusive disease. Nucl. Med. Commun. 2012, 33, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Nakagawara, J.; Sperling, B.; Lassen, N.A. Incomplete brain infarction of reperfused cortex may be quantitated with iomazenil. Stroke 1997, 28, 124–132. [Google Scholar] [CrossRef]
- Hatazawa, J.; Satoh, T.; Shimosegawa, E.; Okudera, T.; Inugami, A.; Ogawa, T.; Fujita, H.; Noguchi, K.; Kanno, I.; Miura, S.; et al. Evaluation of cerebral infarction with iodine 123-iomazenil SPECT. J. Nucl. Med. 1995, 36, 2154–2161. [Google Scholar] [PubMed]
- Hatazawa, J.; Shimosegawa, E.; Satoh, T.; Kanno, I.; Uemura, K. Central benzodiazepine receptor distribution after subcortical hemorrhage evaluated by means of 123I iomazenil and SPECT. Stroke 1995, 26, 2267–2271. [Google Scholar] [CrossRef]
- Zhao, L.; Biesbroek, J.M.; Shi, L.; Liu, W.; Kuijf, H.J.; Chu, W.W.; Abrigo, J.M.; Lee, R.K.; Leung, T.W.; Lau, A.Y.; et al. Strategic infarct location for post-stroke cognitive impairment: A multivariate lesion-symptom mapping study. J. Cereb. Blood Flow. Metab. 2018, 38, 1299–1311. [Google Scholar] [CrossRef]
- Mandalaneni, K.; Rayi, A.; Jillella, D.V. Stroke Reperfusion Injury. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Turner, D.A.; Yang, W. Phase-specific manipulation of neuronal activity: A promising stroke therapy approach. Neural Regen. Res. 2021, 16, 1425–1426. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Douglas, A.; Gil, S.M.; Jabrah, D.; Pandit, A.; Gilvarry, M.; McCarthy, R.; Prendergast, J.; Jood, K.; Redfors, P.; et al. S100b in acute ischemic stroke clots is a biomarker for post-thrombectomy intracranial hemorrhages. Front. Neurol. 2022, 13, 1067215. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Chen, W.; Yan, E.; Deng, Y.; Xu, Z.; Wang, S.; Fu, X.; Wei, B.; Wang, M.; Hou, J.; et al. The relationship between neuron-specific enolase and clinical outcomes in patients undergoing mechanical thrombectomy. Neuropsychiatr. Dis. Treat. 2023, 19, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Su, P.; Lin, D.D.M.; Goldberg, E.B.; Walker, A.; Leigh, R.; Hillis, A.E.; Lu, H. Simultaneous hemodynamic and structural imaging of ischemic stroke with magnetic resonance fingerprinting arterial spin labeling. Stroke 2022, 53, 2016–2025. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutoh, T.; Yoshida, Y.; Tatewaki, Y.; Chin, H.; Tochinai, R.; Moroi, J.; Ishikawa, T. Diffusion MRI Fiber Tractography and Benzodiazepine SPECT Imaging for Assessing Neural Damage to the Language Centers in an Elderly Patient after Successful Reperfusion Therapy. Geriatrics 2024, 9, 30. https://doi.org/10.3390/geriatrics9020030
Mutoh T, Yoshida Y, Tatewaki Y, Chin H, Tochinai R, Moroi J, Ishikawa T. Diffusion MRI Fiber Tractography and Benzodiazepine SPECT Imaging for Assessing Neural Damage to the Language Centers in an Elderly Patient after Successful Reperfusion Therapy. Geriatrics. 2024; 9(2):30. https://doi.org/10.3390/geriatrics9020030
Chicago/Turabian StyleMutoh, Tatsushi, Yasuyuki Yoshida, Yasuko Tatewaki, Hongkun Chin, Ryota Tochinai, Junta Moroi, and Tatsuya Ishikawa. 2024. "Diffusion MRI Fiber Tractography and Benzodiazepine SPECT Imaging for Assessing Neural Damage to the Language Centers in an Elderly Patient after Successful Reperfusion Therapy" Geriatrics 9, no. 2: 30. https://doi.org/10.3390/geriatrics9020030
APA StyleMutoh, T., Yoshida, Y., Tatewaki, Y., Chin, H., Tochinai, R., Moroi, J., & Ishikawa, T. (2024). Diffusion MRI Fiber Tractography and Benzodiazepine SPECT Imaging for Assessing Neural Damage to the Language Centers in an Elderly Patient after Successful Reperfusion Therapy. Geriatrics, 9(2), 30. https://doi.org/10.3390/geriatrics9020030





