Frailty Prevalence and Association with Clinical Outcomes in Interstitial Lung Disease, Asthma, and Pleural Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Data Extraction and Quality Assessment
2.3. Outcomes
2.4. Key Exposure of Frailty
2.5. Data Analysis
2.6. Assessment of Subgroups and Statistical Heterogeneity
3. Results
3.1. Interstitial Lung Disease
3.1.1. Search Results and Quality Assessment on ILD Studies
3.1.2. Characteristics of Studies on ILD Included
3.1.3. Frailty Prevalence in ILD
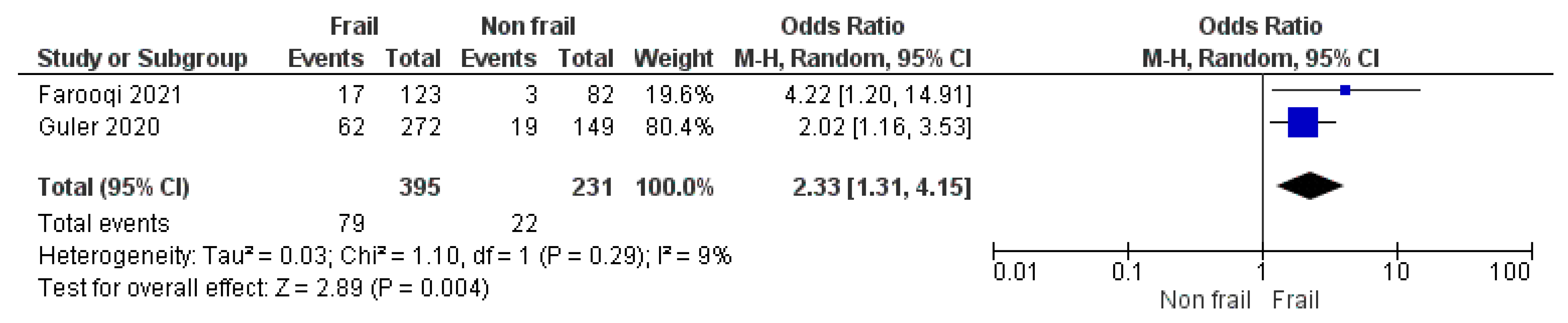
3.1.4. Frailty Score as a Predictor of Morbidity and Mortality in Patients with ILD
Long-Term Mortality
Number of Hospitalizations
3.2. Asthma
3.2.1. Search Results and Quality Assessment on Asthma Studies
3.2.2. Characteristics of Included Studies on Asthma
3.2.3. Frailty Prevalence in Asthma
3.3. Pleural Disease
Search Results on Pleural Disease Studies
4. Discussion
4.1. ILD
4.2. Asthma
4.3. Pleural Disease
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef]
- Buta, B.J.; Walston, J.D.; Godino, J.G.; Park, M.; Kalyani, R.R.; Xue, Q.-L.; Bandeen-Roche, K.; Varadhan, R. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res. Rev. 2016, 26, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Song, X.; Mitnitski, A. Changes in relative fitness and frailty across the adult lifespan: Evidence from the Canadian National Population Health Survey. CMAJ 2011, 183, E487–E494. [Google Scholar] [CrossRef]
- Smart, R.; Carter, B.; McGovern, J.; Luckman, S.; Connelly, A.; Hewitt, J.; Quasim, T.; Moug, S. Frailty exists in younger adults admitted as surgical emergency leading to adverse outcomes. J. Frailty Aging 2017, 6, 219–223. [Google Scholar] [CrossRef]
- Hewitt, J.; Carter, B.; Vilches-Moraga, A.; Quinn, T.J.; Braude, P.; Verduri, A.; Pearce, L.; Stechman, M.; Short, R.; Price, A.; et al. The effect of frailty on survival in patients with COVID-19 (COPE): A multicentre, European, observational cohort study. Lancet Public Health 2020, 5, e444–e451. [Google Scholar] [CrossRef]
- Mitnitski, A.; Mogilner, A.J.; Rockwood, K. Accumulation of deficit as a proxy measure of aging. Sci. World J. 2001, 1, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Angulo, J.; El Assar, M.; Rodriguez-Manas, L. Frailty and sarcopenia as the basis for the phenotypic manifestation of chronic diseases in older adults. Mol. Asp. Med. 2016, 50, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, P.; Nicholl, B.I.; Jani, B.D.; Lee, D.; McQueenie, R.; Mair, F.S. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: A prospective analysis of 493 737 UK Biobank participants. Lancet Public Health 2018, 3, e323–e332. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G.; Iliffe, S.; Walters, K. Frailty index as a predictor of mortality: A systematic review and meta-analysis. Age Ageing 2018, 47, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Onder, G.; Vetrano, D.L.; Marengoni, A.; Bell, J.S.; Johnell, K.; Palmer, K. Optimising Pharmacotherapy through Pharmacoepidemiology Network (OPPEN). Accounting for frailty when treating chronic diseases. Eur. J. Intern. Med. 2018, 56, 49–52. [Google Scholar] [CrossRef]
- Wijsenbeek, M.; Cottin, V. Spectrum of fibrotic lung diseases. New Engl. J. Med. 2020, 383, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an update) and Progressive Pulmonary Fibrosis in adults. An official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef]
- Guler, S.A.; Ryerson, C.J. Frailty in patients with interstitial lung disease. Curr. Opin. Pulm. Med. 2020, 26, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Reddel, H.K.; Bacharier, L.B.; Bateman, E.D.; Brightling, C.E.; Brusselle, G.G.; Buhl, R.; Cruz, A.A.; Duijts, L.; Drazen, J.M.; FitzGerald, J.M.; et al. Global Initiative for Asthma Strategy 2021: Executive summary and rationale for key changes. Eur. Respir. J. 2021, 59, 2102730. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Asthma. Available online: https://ginasthma.org/ (accessed on 18 July 2023).
- Bodtger, U.; Hallifax, R.J. Epidemiology: Why is pleural disease becoming more common? In Pleural Disease; Maskell, N.A., Laursen, C.B., Lee, Y.C.G., Rahman, N.M., Eds.; European Respiratory Society: Sheffield, UK, 2020; pp. 1–12. [Google Scholar]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Aguayo, G.A.; Donneau, A.-F.; Vaillant, M.T.; Schritz, A.; Franco, O.H.; Stranges, S.; Malisoux, L.; Guillaume, M.; Witte, D.R. Agreement between 35 published frailty scores in the general population. Am. J. Epidemiol. 2017, 186, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Ogawa, K.; Fujiwara, Y.; Yoshida, H.; Nishi, M.; Fukaya, T.; Kim, M.; Amano, H.; Lee, S.; Watanabe, N.; Shinkai, S. The validity of the “Kihon Check-list” as an index of frailty and its biomarkers and inflammatory markers in elderly people. Nihon Ronen Igakkai Zasshi Jpn. J. Geriatr. 2011, 48, 545–552. [Google Scholar] [CrossRef]
- Farooqi, M.A.M.; O’Hoski, S.; Goodwin, S.; Makhdami, N.; Aziz, A.; Cox, G.; Wald, J.; Ryerson, C.J.; Beauchamp, M.K.; Hambly, N.; et al. Prevalence and prognostic impact of physical frailty in interstitial lung disease: A prospective cohort study. Respirology 2021, 26, 683–689. [Google Scholar] [CrossRef]
- Guler, S.A.; Kwan, J.M.; Leung, J.M.; Khalil, N.; Wilcox, P.G.; Ryerson, C.J. Functional ageing in fibrotic interstitial lung disease: The impact of frailty on adverse health outcomes. Eur. Respir. J. 2020, 55, 1900647. [Google Scholar] [CrossRef] [PubMed]
- Labrecque, P.-F.; Dion, G.; Saey, D. Functional clinical impairments and frailty in interstitial lung disease patients. ERJ Open Res. 2022, 8, 00144–2022. [Google Scholar]
- Sheth, J.S.; Xia, M.; Murray, S.; Martinez, C.H.; Meldrum, C.A.; Belloli, E.A.; Salisbury, M.L.; White, E.S.; Holtze, C.H.; Flaherty, K.R. Frailty and geriatric conditions in older patients with idiopathic pulmonary fibrosis. Respir. Med. 2019, 148, 6–12. [Google Scholar] [CrossRef]
- Guler, S.A.; Kwan, J.M.; Winstone, T.A.; Milne, K.M.; Dunne, J.V.; Wilcox, P.G.; Ryerson, C.J. Severity and features of frailty in systemic sclerosis-associated interstitial lung disease. Respir. Med. 2017, 129, 1–7. [Google Scholar] [CrossRef]
- Milne, K.M.; Kwan, J.M.; Guler, S.; Winstone, T.A.; Le, A.; Khalil, N.; Camp, P.G.; Wilcox, P.G.; Ryerson, C.J. Frailty is common and strongly associated with dyspnea severity in fibrotic interstitial lung disease. Respirology 2017, 22, 728–734. [Google Scholar] [CrossRef]
- Landré, B.; Nadif, R.; Goldberg, M.; Gourmelen, J.; Zins, M.; Ankri, J.; Herr, M. Asthma is associated with frailty among community-dwelling adults: The GAZEL cohort. BMJ Open Respir. Res. 2020, 7, e000526. [Google Scholar] [CrossRef] [PubMed]
- Kusunose, M.; Sanda, R.; Mori, M.; Narita, A.; Nishimura, K. Are frailty and patient-reported outcomes independent in subjects with asthma? A cross-sectional observational study. Clin. Respir. J. 2021, 15, 216–224. [Google Scholar] [CrossRef]
- Saketkoo, L.A.; Obi, O.N.; Patterson, K.C.; Russell, A.-M. Ageing with Interstitial Lung Disease: Preserving health and well being. Curr. Opin. Pulm. Med. 2022, 28, 321–336. [Google Scholar] [CrossRef]
- Figueiredo, R.G.; Pinheiro, G.P.; Arata, V.; Leal, M.F.M.; Santana, C.V.N.; Tiraboschi, T.L.; Bessa, J., Jr.; Cruz, A. Impact of frailty in elderly patients with moderate to severe asthma. PLoS ONE 2022, 17, e0270921. [Google Scholar] [CrossRef]

| Author, Year | Country | Population Details/Study Design | Primary Outcomes | No. Patients | Age & | Sex (% Male) (M:F) | Frailty Scale | Frailty Prevalence | Pre-Frailty Prevalence |
|---|---|---|---|---|---|---|---|---|---|
| Farooqi MAM, 2021 [21] | Canada | Outpatients with ILDs, 183 patients with IPF; prospective cohort study | Prevalence of physical frailty, impact of frailty on time to death and on time to lung transplantation in ILD patients | 463 | No age cut off 68 ± 11 | 55% 257 M 45% 206 F | Fried Frailty Phenotype | 123 (26.5%) | 258 (56%) |
| Guler SA, 2020 [22] | Canada | Outpatients with ILDs, 100 patients with IPF; prospective cohort study | Impact of frailty on time to death or lung transplantation, hospitalisations, time to hospital discharge, and health-related quality of life in ILD patients | 540 | No age cut off 67.4 ± 10.2 | 43% 232 M 57% 308 F | Frailty Index including 42 deficits (19 comorbidities and 23 items related to independence and self-care); frailty ≥ 0.21 | 272 (50.3%) | 119 (22%) |
| Sheth JS, 2019 [24] | US | Outpatients with IPF; cross-sectional study | Prevalence of frailty and investigation of geriatric conditions and number of comorbidities in ILD patients | 50 | >65 yrs * 73.8 ± 5.4 | 66% 33 M 34% 17 F | Fried Frailty Phenotype | 24 (48%) | 20 (40%) |
| Guler SA, 2017 [25] | Canada | Outpatients with systemic sclerosis-associated ILD (SSc-ILD) and non-connective tissue disease fibrotic ILD (non-CT fibrotic ILD); cross-sectional study | Prevalence of frailty and comparison of the features of frailty between SSc-ILD and non-CT fibrotic ILD patients | 253 | No age cut off 60.5 ± 11.8 SSc-ILD 69.3 ± 9.9 Fibrotic ILD | 45.4% 115 M 54.6% 138 F | Frailty Index including 42 deficits (19 comorbidities and 23 items related to independence and self-care); frailty ≥ 0.21 | 55% in SSc-ILD 50% in non-CT fibrotic ILD | 21% in SSc-ILD 20% in non-CT fibrotic ILD |
| Milne KM, 2017 [26] | Canada | Outpatients with ILDs, 41 patients with IPF; cross-sectional study | Prevalence of frailty and investigation of predictors of frailty in ILD patients | 129 | >40 yrs * 69 ± 9.2 | 54.3% 70 M 45.7% 59 F | Frailty Index including 42 deficits (19 comorbidities and 23 items related to independence and self-care); frailty ≥ 0.21 | 64 (50%) | 31 (24%) |
| Labrecque P-F, 2022 [23] | Canada | Outpatients with ILDs, 17 patients with IPF, and 15 control subjects; cross-sectional study | Prevalence of frailty and comparison with exercise capacity, functional mobility, muscle function and composition, and health-related quality of life between ILD patients and healthy subjects | 36 patients 15 control subjects | No age cut off 70 ± 7 ILD patients 69 ± 7 Control subjects | 78% 28 M 22% 8 F Control subjects 67% 10 M 33% 5 F | Fried Frailty Phenotype | 9 (25%) in ILD patients | 19 (53%) in ILD patients |
| Author | Country | Population Details/Study Design | Primary Outcomes | No. Patients | Age & | Sex (% Male) (M:F) | Asthma Diagnosis/Definition Criteria | Frailty Scale | Frailty Prevalence | Pre-Frailty Prevalence |
|---|---|---|---|---|---|---|---|---|---|---|
| Landre’ B, 2020 [27] | France | Community-dwelling adults; prospective analysis with 26 years follow up | Prevalence of frailty in subjects with or without current asthma | 12,345 Current asthma in 2015: 372 (3%) | 69.8 ± 3.5 | 67% 250 M 33% 122 F | Self reported diagnosis | Fried frailty phenotype | 13% (47/372) | 38% (142/372) |
| Kusunose M, 2021 [28] | Japan | Outpatients with asthma in stable condition | Prevalence of frailty and influence of frailty on asthma severity | 69 | No age cut off 69.4 ± 10.8 | 49.3% 34 M 50.7% 35 F | GINA guidelines | Kihon Checklist | 14.5% (10/69) | 30.4% (21/69) |
| Hanlon P, 2018 [9] | UK | Community-dwelling adults; prospective analysis with 7 years follow up | Prevalence of frailty, all-cause mortality at 7-year follow-up and influence of frailty and pre-frailty on mortality | 493,737 Asthma 57,169 (11.6%) | Age cut off 40–69 Range 37–73 | 46% 227,119 M 54% 266,618 F | Self reported diagnosis | Fried frailty phenotype | 6% (3358/ 57,169) | 42.1% (24,073/ 57,169) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verduri, A.; Carter, B.; Rice, C.; Laraman, J.; Barton, E.; Clini, E.; Maskell, N.A.; Hewitt, J. Frailty Prevalence and Association with Clinical Outcomes in Interstitial Lung Disease, Asthma, and Pleural Disease. Geriatrics 2023, 8, 82. https://doi.org/10.3390/geriatrics8040082
Verduri A, Carter B, Rice C, Laraman J, Barton E, Clini E, Maskell NA, Hewitt J. Frailty Prevalence and Association with Clinical Outcomes in Interstitial Lung Disease, Asthma, and Pleural Disease. Geriatrics. 2023; 8(4):82. https://doi.org/10.3390/geriatrics8040082
Chicago/Turabian StyleVerduri, Alessia, Ben Carter, Ceara Rice, James Laraman, Eleanor Barton, Enrico Clini, Nick A. Maskell, and Jonathan Hewitt. 2023. "Frailty Prevalence and Association with Clinical Outcomes in Interstitial Lung Disease, Asthma, and Pleural Disease" Geriatrics 8, no. 4: 82. https://doi.org/10.3390/geriatrics8040082
APA StyleVerduri, A., Carter, B., Rice, C., Laraman, J., Barton, E., Clini, E., Maskell, N. A., & Hewitt, J. (2023). Frailty Prevalence and Association with Clinical Outcomes in Interstitial Lung Disease, Asthma, and Pleural Disease. Geriatrics, 8(4), 82. https://doi.org/10.3390/geriatrics8040082







