The Clinical Variables Predicting the Acquisition of Independent Ambulation in the Acute Phase of Stroke: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Data
2.2. FAC
2.3. mRS
2.4. NIHSS
2.5. SIAS
2.6. FIM
2.7. ABMS2
2.8. Statistical Analysis
3. Results
3.1. Univariate Logistic Regression Analysis
3.2. ROC Curve Analysis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, B.C.V.; Khatri, P. Stroke. Lancet 2020, 396, 129–142. [Google Scholar] [CrossRef]
- Lee, H.J.; Lim, Y.C.; Lee, Y.S.; Kwon, S.; Lee, Y.J.; Ha, I.H. Analysis of medical service utilization for post-stroke sequelae in Korea between 2016 and 2018: A cross-sectional study. Sci. Rep. 2022, 12, 20501. [Google Scholar] [CrossRef] [PubMed]
- Craig, L.E.; Bernhardt, J.; Langhorne, P.; Wu, O. Early mobilization after stroke: An example of an individual patient data meta-analysis of a complex intervention. Stroke 2010, 41, 2632–2636. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, J.; Langhorne, P.; Lindley, R.I.; Thrift, A.G.; Ellery, F.; Collier, J.; Churilov, L.; Moodie, M.; Dewey, H.; Donnan, G. Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): A randomised controlled trial. Lancet 2015, 386, 30, Erratum in Lancet 2017, 389, 1884. [Google Scholar] [CrossRef][Green Version]
- Peurala, S.H.; Karttunen, A.H.; Sjögren, T.; Paltamaa, J.; Heinonen, A. Evidence for the effectiveness of walking training on walking and self-care after stroke: A systematic review and meta-analysis of randomized controlled trials. J. Rehabil. Med. 2014, 46, 387–399. [Google Scholar] [CrossRef]
- Pollock, A.; Baer, G.; Campbell, P.; Choo, P.L.; Forster, A.; Morris, J.; Pomeroy, V.M.; Langhorne, P. Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Database Syst. Rev. 2014, 2014, CD001920. [Google Scholar] [CrossRef]
- Perry, J.; Garrett, M.; Gronley, J.K.; Mulroy, S.J. Classification of walking handicap in the stroke population. Stroke 1995, 26, 982–989. [Google Scholar] [CrossRef]
- Duarte, E.; Marco, E.; Muniesa, J.M.; Belmonte, R.; Aguilar, J.J.; Escalada, F. Early detection of non-ambulatory survivors six months after stroke. NeuroRehabilitation 2010, 26, 317–323. [Google Scholar] [CrossRef]
- Kennedy, C.; Bernhardt, J.; Churilov, L.; Collier, J.M.; Ellery, F.; Rethnam, V.; Carvalho, L.B.; Donnan, G.A.; Hayward, K.S. Factors associated with time to independent walking recovery post-stroke. J. Neurol. Neurosurg. Psychiatry 2021, 92, 702–708. [Google Scholar] [CrossRef]
- Kollen, B.; van de Port, I.; Lindeman, E.; Twisk, J.; Kwakkel, G. Predicting improvement in gait after stroke: A longitudinal prospective study. Stroke 2005, 36, 2676–2680. [Google Scholar] [CrossRef]
- Meijer, R.; Ihnenfeldt, D.S.; de Groot, I.J.; van Limbeek, J.; Vermeulen, M.; de Haan, R.J. Prognostic factors for ambulation and activities of daily living in the subacute phase after stroke. A systematic review of the literature. Clin. Rehabil. 2003, 17, 119–129. [Google Scholar] [CrossRef]
- Broderick, J.P.; Adeoye, O.; Elm, J. Evolution of the modified Rankin scale and its use in future stroke trials. Stroke 2017, 48, 2007–2012. [Google Scholar] [CrossRef] [PubMed]
- Holden, M.K.; Gill, K.M.; Magliozzi, M.R. Gait assessment for neurologically impaired patients. Standards for outcome assessment. Phys. Ther. 1986, 66, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Holden, M.K.; Gill, K.M.; Magliozzi, M.R.; Nathan, J.; Piehl-Baker, L. Clinical gait assessment in the neurologically impaired. Reliability and meaningfulness. Phys. Ther. 1984, 64, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Kwah, L.K.; Diong, J. National Institutes of Health Stroke Scale (NIHSS). J. Physiother. 2014, 60, 61. [Google Scholar] [CrossRef] [PubMed]
- Lyden, P.D.; Lu, M.; Levine, S.R.; Brott, T.G.; Broderick, J.; NINDS rtPA Stroke Study Group. A modified National Institutes of Health stroke scale for use in stroke clinical trials: Preliminary reliability and validity. Stroke 2001, 32, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Hashimoto, K.; Kobayashi, K.; Sugawara, H.; Abo, M. Revised version of the Ability for Basic Movement Scale (ABMS II) as an early predictor of functioning related to activities of daily living in patients after stroke. J. Rehabil. Med. 2010, 42, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Chumney, D.; Nollinger, K.; Shesko, K.; Skop, K.; Spencer, M.; Newton, R.A. Ability of functional independence measure to accurately predict functional outcome of stroke-specific population: Systematic review. J. Rehabil. Res. Dev. 2010, 47, 17–29. [Google Scholar] [CrossRef]
- van der Putten, J.J.; Hobart, J.C.; Freeman, J.A.; Thompson, A.J. Measuring change in disability after inpatient rehabilitation: Comparison of the responsiveness of the Barthel index and the Functional Independence Measure. J. Neurol. Neurosurg. Psychiatry 1999, 66, 480–484. [Google Scholar] [CrossRef]
- Chino, N.; Sonoda, S.; Domen, K.; Saitoh, E.; Kimura, A. Stroke Impairment Assessment Set (SIAS). A new evaluation instrument for stroke patients. Jpn. J. Rehabil. Med. 1994, 31, 119–125. [Google Scholar] [CrossRef]
- Liu, M.; Chino, N.; Tuji, T.; Masakado, Y.; Hase, K.; Kimura, A. Psychometric properties of the Stroke Impairment Assessment Set (SIAS). Neurorehabil. Neural Repair 2002, 16, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Liu, M.; Sonoda, S.; Domen, K.; Chino, N. The Stroke Impairment Assessment Set: Its internal consistency and predictive validity. Arch. Phys. Med. Rehabil. 2000, 81, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Abo, M.; Okamoto, T.; Tanaka, N. Utility of the revised version of the Ability for Basic Movement Scale in predicting ambulation during rehabilitation in poststroke patients. J. Stroke Cerebrovasc. Dis. 2017, 26, 1663–1669. [Google Scholar] [CrossRef]
- Mehrholz, J.; Wagner, K.; Rutte, K.; Meissner, D.; Pohl, M. Predictive validity and responsiveness of the functional ambulation category in hemiparetic patients after stroke. Arch. Phys. Med. Rehabil. 2007, 88, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Cinnera, A.M.; Marrano, S.; De Bartolo, D.; Iosa, M.; Bisirri, A.; Leone, E.; Stefani, A.; Koch, G.; Ciancarelli, I.; Paolucci, S.; et al. Convergent validity of the timed walking tests with functional ambulatory category in subacute stroke. Brain Sci. 2023, 13, 1089. [Google Scholar] [CrossRef]
- Masiero, S.; Avesani, R.; Armani, M.; Verena, P.; Ermani, M. Predictive factors for ambulation in stroke patients in the rehabilitation setting: A multivariate analysis. Clin. Neurol. Neurosurg. 2007, 109, 763–769. [Google Scholar] [CrossRef]
- Uwatoko, H.; Nakamori, M.; Imamura, E.; Imura, T.; Okada, K.; Matsumae, Y.; Okamoto, H.; Wakabayashi, S. Prediction of independent gait in acute stroke patients with hemiplegia using the Ability for Basic Movement Scale II Score. Eur. Neurol. 2020, 83, 49–55. [Google Scholar] [CrossRef]
- Yang, G.; Gu, R.; Sato, S.; Zheng, F.; Sano, M.; Yashima, C.; Eguchi, J.; Ishida, T.; Kawaguchi, M.; Kubo, J.; et al. The Ability for Basic Movement Scale II can predict functional outcome and discharge destination in stroke patients. J. Stroke Cerebrovasc. Dis. 2020, 29, 104484. [Google Scholar] [CrossRef]
- Domen, K. Reliability and validity of Stroke Impairment Assessment Set (SIAS) (1): Items of affected-side motor function, muscle tone, deep tendon reflex, and unaffected-side function. Jpn. J. Rehabil. Med. 1995, 32, 113–122. [Google Scholar] [CrossRef]
- Sonoda, S. Reliability and validity of Stroke Impairment Assessment Set (SIAS). (2): The items comprise the trunk, higher cortical function, and sensory function and effectiveess as outcome predictor. Jpn. J. Rehabil. Med. 1995, 32, 123–132. [Google Scholar] [CrossRef]
- Ishiwatari, M.; Tani, M.; Isayama, R.; Honaga, K.; Hayakawa, M.; Takakura, T.; Tanuma, A.; Kurosu, A.; Hatori, K.; Wada, F.; et al. Prediction of gait independence using the Trunk Impairment Scale in patients with acute stroke. Ther. Adv. Neurol. Disord. 2022, 15, 17562864221140180. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, K.; Tamiya, T.; Matsuoka, S.; Kimura, K. Factors of gait independence at 15 days after stroke onset and their interrelationships in StrokDecision Tree Analysise patients. Rigakuryoho Kagaku 2021, 36, 361–367. [Google Scholar] [CrossRef]
- Banks, J.L.; Marotta, C.A. Outcomes validity and reliability of the modified Rankin scale: Implications for stroke clinical trials: A literature review and synthesis. Stroke 2007, 38, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Jijimol, G.; Fayaz, R.K.; Vijesh, P.V. Correlation of trunk impairment with balance in patients with chronic stroke. NeuroRehabilitation 2013, 32, 323–325. [Google Scholar] [CrossRef]
- Karthikbabu, S.; Nayak, A.; Vijayakumar, K.; Misri, Z.; Suresh, B.; Ganesan, S.; Joshua, A.M. Comparison of physio ball and plinth trunk exercises regimens on trunk control and functional balance in patients with acute stroke: A pilot randomized controlled trial. Clin. Rehabil. 2011, 25, 709–719. [Google Scholar] [CrossRef]
- Ajčević, M.; Furlanis, G.; Naccarato, M.; Miladinović, A.; Buoite Stella, A.; Caruso, P.; Cillotto, T.; Accardo, A.; Manganotti, P. Hyper-acute EEG alterations predict functional and morphological outcomes in thrombolysis-treated ischemic stroke: A wireless EEG study. Med. Biol. Eng. Comput. 2021, 59, 121–129. [Google Scholar] [CrossRef]
- Ajčević, M.; Furlanis, G.; Stella, A.B.; Cillotto, T.; Caruso, P.; Ridolfi, M.; Lugnan, C.; Miladinović, A.; Ukmar, M.; Cova, M.A.; et al. A CT perfusion based model predicts outcome in wake-up stroke patients treated with recombinant tissue plasminogen activator. Physiol. Meas. 2020, 41, 075011. [Google Scholar] [CrossRef]
| Independent Ambulation Group (n = 24) | Dependent Ambulation Group (n = 45) | p-Value | Power | |||||
|---|---|---|---|---|---|---|---|---|
| Age (y) ※ | 69.5 | ± | 13.9 | 77.6 | ± | 11.5 | 0.01 | 0.70 |
| Sex (male/female) (n) ※※ | 18/24 (75%) | / | 6/24 (25%) | 23/45 (51%) | / | 22/45 (49%) | 0.07 | 0.99 |
| Etiology (CI/ICH) (n)※※ | 20/24 (83%) | / | 4/24 (17%) | 39/45 (87%) | / | 6/45 (13%) | 0.73 | 0.14 |
| mRS pre-hospitalization (score) | 0.3 | ± | 0.6 | 1.3 | ± | 1.1 | <0.01 | 0.99 |
| Admission period (days) | 16.8 | ± | 7.6 | 23.9 | ± | 15.3 | 0.05 | 0.61 |
| Rehabilitation period (days) | 15.2 | ± | 7.9 | 22.3 | ± | 14.8 | 0.06 | 0.63 |
| mRS (score) | 3.4 | ± | 0.7 | 4.1 | ± | 0.8 | <0.01 | 0.94 |
| NIHSS (score) | 3.1 | ± | 4.8 | 5.8 | ± | 5.6 | <0.01 | 0.50 |
| SIAS-MLE (score) | 13.8 | ± | 3.1 | 11.2 | ± | 4.2 | <0.01 | 0.76 |
| FIM-motor (score) | 60.3 | ± | 14.2 | 39.0 | ± | 16.5 | <0.01 | 0.99 |
| FIM-cognitive (score) | 28.5 | ± | 8.8 | 20.4 | ± | 9.2 | <0.01 | 0.93 |
| ABMS2 (score) | 25.2 | ± | 5.0 | 18.7 | ± | 6.2 | <0.01 | 0.99 |
| Unadjusted Logistic Regression | Adjusted Logistic Regression | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | Wald | df | OR | 95% CI | p-Value | Power | B | SE | Wald | df | OR | 95% CI | p-Value | Power | |||||
| mRS | −1.311 | 0.412 | 10.142 | 1 | 0.270 | 0.120 | – | 0.604 | 0.001 | 0.215 | −1.127 | 0.441 | 6.525 | 1 | 0.324 | 0.136 | – | 0.769 | 0.011 | 0.208 |
| NIHSS | −0.162 | 0.093 | 3.013 | 1 | 0.851 | 0.709 | – | 1.021 | 0.083 | 0.922 | −0.120 | 0.084 | 2.046 | 1 | 0.887 | 0.752 | – | 1.045 | 0.153 | 0.653 |
| SIAS-MLE | 0.264 | 0.119 | 4.933 | 1 | 1.301 | 1.031 | – | 1.642 | 0.026 | 0.587 | 0.338 | 0.138 | 6.020 | 1 | 1.403 | 1.070 | – | 1.838 | 0.014 | 0.438 |
| FIM-motor | 0.102 | 0.028 | 12.897 | 1 | 1.107 | 1.047 | – | 1.170 | 0.000 | 0.722 | 0.092 | 0.032 | 8.152 | 1 | 1.096 | 1.029 | – | 1.167 | 0.004 | 0.604 |
| FIM-cognitive | 0.102 | 0.033 | 9.510 | 1 | 1.107 | 1.038 | – | 1.181 | 0.002 | 0.762 | 0.062 | 0.040 | 2.405 | 1 | 1.064 | 0.984 | – | 1.150 | 0.121 | 0.490 |
| ABMS2 | 0.261 | 0.078 | 11.300 | 1 | 1.298 | 1.115 | – | 1.512 | 0.001 | 0.399 | 0.256 | 0.098 | 6.792 | 1 | 1.292 | 1.066 | – | 1.566 | 0.009 | 0.318 |
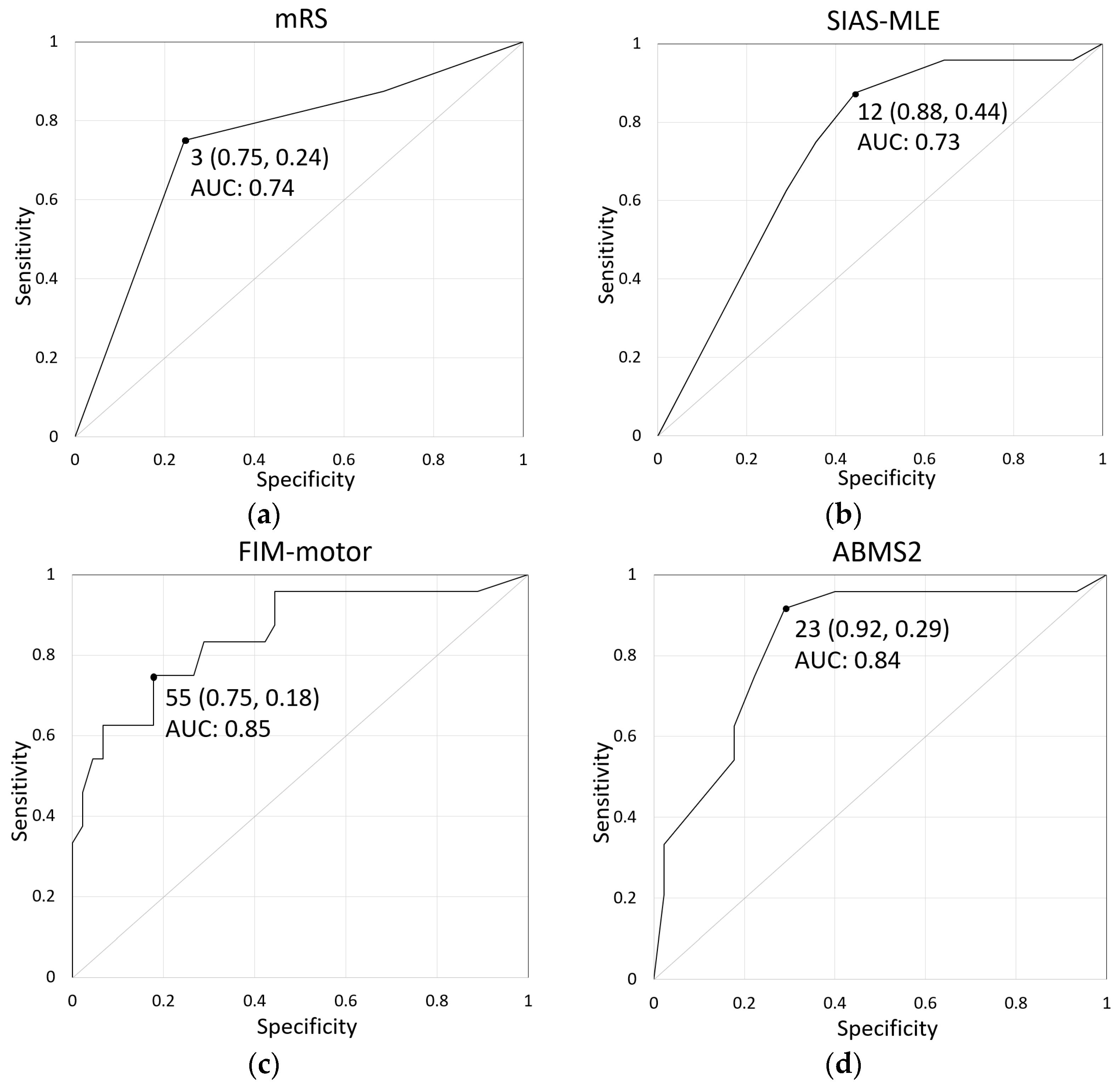
| Cutoff | Sensitivity | 1—Specificity | AUC | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|---|---|
| mRS | 3 | 0.75 | 0.24 | 0.74 | 0.62–0.87 | <0.01 |
| SIAS-MLE | 12 | 0.88 | 0.44 | 0.73 | 0.61–0.86 | <0.01 |
| FIM-motor | 55 | 0.75 | 0.18 | 0.85 | 0.75–0.95 | <0.01 |
| ABMS2 | 23 | 0.92 | 0.29 | 0.84 | 0.73–0.94 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koumo, M.; Maki, Y.; Goda, A.; Uchida, K.; Ogawa, S.; Matsui, T.; Hidemura, N.; Adachi, T. The Clinical Variables Predicting the Acquisition of Independent Ambulation in the Acute Phase of Stroke: A Retrospective Study. Geriatrics 2023, 8, 80. https://doi.org/10.3390/geriatrics8040080
Koumo M, Maki Y, Goda A, Uchida K, Ogawa S, Matsui T, Hidemura N, Adachi T. The Clinical Variables Predicting the Acquisition of Independent Ambulation in the Acute Phase of Stroke: A Retrospective Study. Geriatrics. 2023; 8(4):80. https://doi.org/10.3390/geriatrics8040080
Chicago/Turabian StyleKoumo, Masatoshi, Yoshinori Maki, Akio Goda, Kensaku Uchida, Shohei Ogawa, Tatsumi Matsui, Nozomu Hidemura, and Tomohiro Adachi. 2023. "The Clinical Variables Predicting the Acquisition of Independent Ambulation in the Acute Phase of Stroke: A Retrospective Study" Geriatrics 8, no. 4: 80. https://doi.org/10.3390/geriatrics8040080
APA StyleKoumo, M., Maki, Y., Goda, A., Uchida, K., Ogawa, S., Matsui, T., Hidemura, N., & Adachi, T. (2023). The Clinical Variables Predicting the Acquisition of Independent Ambulation in the Acute Phase of Stroke: A Retrospective Study. Geriatrics, 8(4), 80. https://doi.org/10.3390/geriatrics8040080






