A Molecular and Epidemiological Investigation of a Large SARS-CoV-2 Outbreak in a Long-Term Care Facility in Luxembourg, 2021
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Setting and Testing
2.3. Sequencing
2.4. Phylogeny
2.5. Serosurvey
2.6. Statistical Analyzes
3. Results
3.1. Demographic and Epidemiological Characteristics
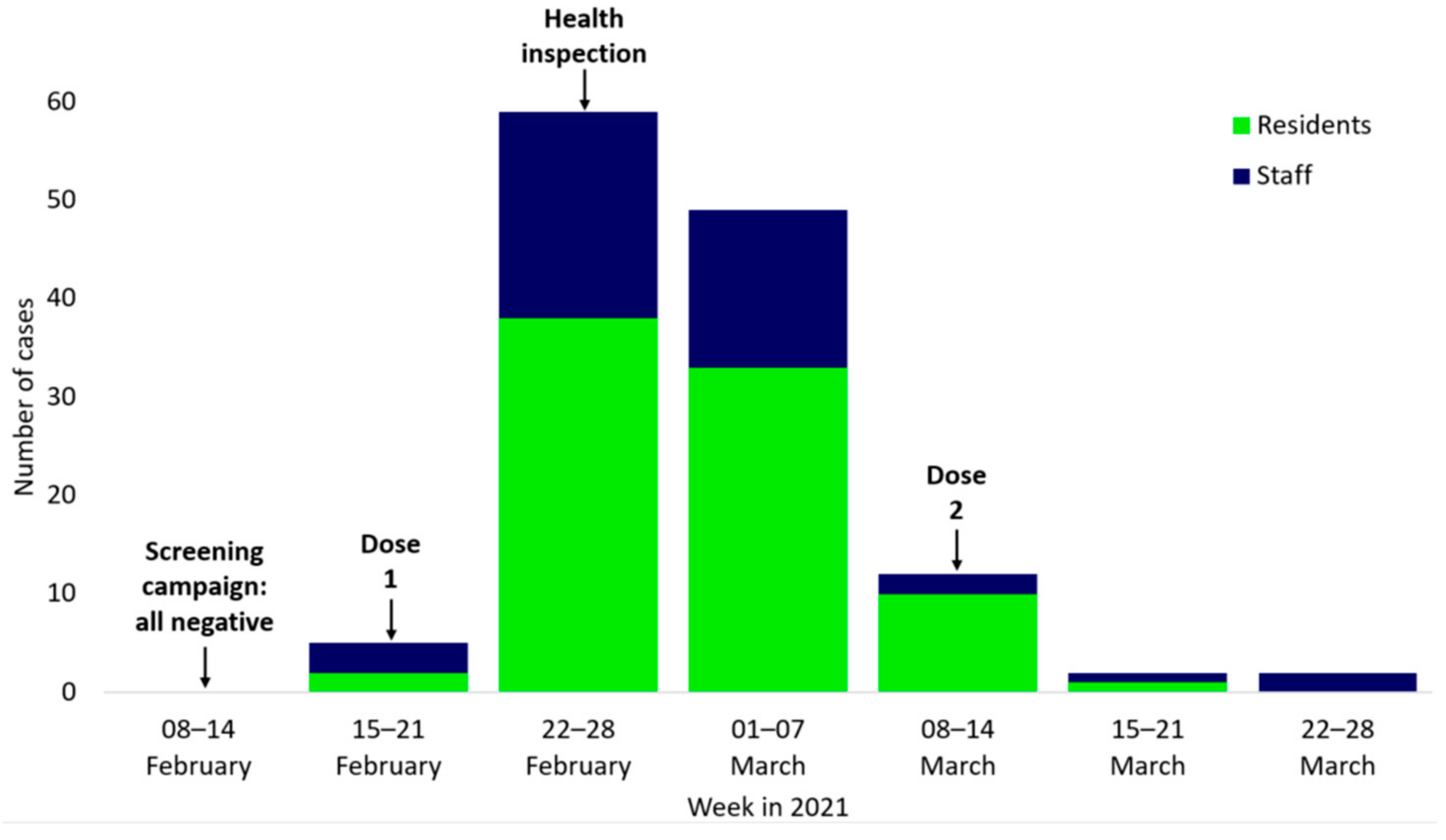
3.2. Chronological Description of the Outbreak
3.3. Variants
3.4. Reinfections
3.5. Seroprevalence
3.6. Outbreak Control Measures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bialek, S.; Boundy, E.; Bowen, V.; Chow, N.; Cohn, A.; Dowling, N.; Ellington, S.; Gierke, R.; Hall, A.; MacNeil, J.; et al. Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 343–346. [Google Scholar] [CrossRef]
- Graham, N.S.N.; Junghans, C.; Downes, R.; Sendall, C.; Lai, H.; McKirdy, A.; Elliott, P.; Howard, R.; Wingfield, D.; Priestman, M.; et al. SARS-CoV-2 Infection, Clinical Features and Outcome of COVID-19 in United Kingdom Nursing Homes. J. Infect. 2020, 81, 411–419. [Google Scholar] [CrossRef]
- Burton, J.K.; Bayne, G.; Evans, C.; Garbe, F.; Gorman, D.; Honhold, N.; McCormick, D.; Othieno, R.; Stevenson, J.E.; Swietlik, S.; et al. Evolution and Effects of COVID-19 Outbreaks in Care Homes: A Population Analysis in 189 Care Homes in One Geographical Region of the UK. Lancet Healthy Longev. 2020, 1, e21–e31. [Google Scholar] [CrossRef]
- Patel, M.C.; Chaisson, L.H.; Borgetti, S.; Burdsall, D.; Chugh, R.K.; Hoff, C.R.; Murphy, E.B.; Murskyj, E.A.; Wilson, S.; Ramos, J.; et al. Asymptomatic SARS-CoV-2 Infection and COVID-19 Mortality During an Outbreak Investigation in a Skilled Nursing Facility. Clin. Infect. Dis. 2020, 71, 2920–2926. [Google Scholar] [CrossRef]
- Krone, M.; Noffz, A.; Richter, E.; Vogel, U.; Schwab, M. Control of a COVID-19 Outbreak in a Nursing Home by General Screening and Cohort Isolation in Germany, March to May 2020. Eurosurveillance 2021, 26. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control Data on SARS-CoV-2 Variants in the EU/EEA. Available online: https://www.ecdc.europa.eu/en/publications-data/data-virus-variants-covid-19-eueea (accessed on 8 December 2022).
- European Centre for Disease Prevention and Control. Overview of the Implementation of COVID-19 Vaccination Strategies and Vaccine Deployment Plans in the EU/EEA; European Centre for Disease Prevention and Control: Solna, Sweden, 2021; p. 28. [Google Scholar]
- Conseil Superieur des Maladies Infectieuses Recommandation Générales Du Conseil Superieur Des Maladies Infectieuses, Concernant La Stratégie Vaccinale Contre La COVID-19. Available online: https://sante.public.lu/dam-assets/fr/espace-professionnel/recommandations/conseil-maladies-infectieuses/covid-19/covid-19-annexes/recommandation-csmi-strategie-vaccinale-contre-la-covid.pdf (accessed on 8 December 2022).
- European Centre for Disease Prevention and Control COVID-19 Vaccine Tracker | European Centre for Disease Prevention and Control. Available online: https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#uptake-tab (accessed on 8 December 2022).
- Abdelrahman, T.; Aguayo, G.; Bausch, M.; Hanquet, G.; Kerger, A.; Rolland, Y.; Mossong, J.; Waringo, J. Rapport Du Groupe de Travail; Luxembourg. 2021, p. 28. Available online: https://gouvernement.lu/dam-assets/fr/publications/rapport-etude-analyse/rapport-special/Rapport-au-sujet-des-clusters-observes-dans-certaines-structures-hebergement-pour-personnes-agees.pdf (accessed on 13 December 2022).
- Loi Du 15 Juillet 2021 Portant Modification: 1° de La Loi Modifiée Du 17 Juillet 2020 Sur Les Mesures de Lutte Contre La Pandémie Covid-19; 2° de La Loi Modifiée Du 25 Novembre 1975 Concernant La Délivrance Au Public Des Médicaments; 3° de La Loi Modifiée Du 22 Janvier 2021 Portant: 1° Modification Des Articles L. 234-51, L. 234-52 et L. 234-53 Du Code Du Travail; ; 2° Dérogation Temporaire Aux Dispositions Des Articles L. 234-51, L. 234-52 et L. 234-53 Du Code Du Travail. 2021. Available online: https://legilux.public.lu/eli/etat/leg/loi/2021/07/15/a536/jo (accessed on 23 September 2021).
- Elbe, S.; Buckland-Merrett, G. Data, Disease and Diplomacy: GISAID’s Innovative Contribution to Global Health: Data, Disease and Diplomacy. Glob. Chall. 2017, 1, 33–46. [Google Scholar] [CrossRef]
- Shu, Y.; McCauley, J. GISAID: Global Initiative on Sharing All Influenza Data – from Vision to Reality. Eurosurveillance 2017, 22, 30494. [Google Scholar] [CrossRef]
- Khare, S.; Gurry, C.; Freitas, L.; B Schultz, M.; Bach, G.; Diallo, A.; Akite, N.; Ho, J.; TC Lee, R.; Yeo, W.; et al. GISAID’s Role in Pandemic Response. China CDC Wkly. 2021, 3, 1049–1051. [Google Scholar] [CrossRef]
- Li, H. Minimap2: Pairwise Alignment for Nucleotide Sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (ITOL) v5: An Online Tool for Phylogenetic Tree Display and Annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows; IBM Corp: Armonk, NY, USA, 2020. [Google Scholar]
- European Centre for Disease Prevention and Control Risk Factors and Risk Groups. Available online: https://www.ecdc.europa.eu/en/covid-19/latest-evidence/risk-factors-risk-groups (accessed on 6 April 2022).
- Williams, S.V.; Vusirikala, A.; Ladhani, S.N.; Fernandez Ruiz De Olano, E.; Iyanger, N.; Aiano, F.; Stoker, K.; Gopal Rao, G.; John, L.; Patel, B.; et al. An Outbreak Caused by the SARS-CoV-2 Delta (B.1.617.2) Variant in a Care Home after Partial Vaccination with a Single Dose of the COVID-19 Vaccine Vaxzevria, London, England, April 2021. Eurosurveillance 2021, 26, 2100626. [Google Scholar] [CrossRef]
- Tober-Lau, P.; Schwarz, T.; Hillus, D.; Spieckermann, J.; Helbig, E.T.; Lippert, L.J.; Thibeault, C.; Koch, W.; Bergfeld, L.; Niemeyer, D.; et al. Outbreak of SARS-CoV-2 B.1.1.7 Lineage after Vaccination in Long-Term Care Facility, Germany, February-March 2021. Emerg. Infect. Dis. 2021, 27, 2169–2173. [Google Scholar] [CrossRef]
- Cavanaugh, A.M.; Fortier, S.; Lewis, P.; Arora, V.; Johnson, M.; George, K.; Tobias, J.; Lunn, S.; Miller, T.; Thoroughman, D.; et al. COVID-19 Outbreak Associated with a SARS-CoV-2 R.1 Lineage Variant in a Skilled Nursing Facility After Vaccination Program — Kentucky, March 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 639–643. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Chodick, G.; Tene, L.; Patalon, T.; Gazit, S.; Ben Tov, A.; Cohen, D.; Muhsen, K. Assessment of Effectiveness of 1 Dose of BNT162b2 Vaccine for SARS-CoV-2 Infection 13 to 24 Days After Immunization. JAMA Netw. Open 2021, 4, e2115985. [Google Scholar] [CrossRef]
- Krutikov, M.; Stirrup, O.; Nacer-Laidi, H.; Azmi, B.; Fuller, C.; Tut, G.; Palmer, T.; Shrotri, M.; Irwin-Singer, A.; Baynton, V.; et al. Outcomes of SARS-CoV-2 Omicron Infection in Residents of Long-Term Care Facilities in England (VIVALDI): A Prospective, Cohort Study. Lancet Healthy Longev. 2022, 3, e347–e355. [Google Scholar] [CrossRef]
- Herold, M.; d’Hérouël, A.F.; May, P.; Delogu, F.; Wienecke-Baldacchino, A.; Tapp, J.; Walczak, C.; Wilmes, P.; Cauchie, H.-M.; Fournier, G.; et al. Genome Sequencing of SARS-CoV-2 Allows Monitoring of Variants of Concern through Wastewater. Water 2021, 13, 3018. [Google Scholar] [CrossRef]
- Gangavarapu, K.; Latif, A.A.; Mullen, J.; Alkuzweny, M.; Hufbauer, E.; Tsueng, G.; Haag, E.; Zeller, M.; Aceves, C.M.; Zaiets, K.; et al. B.1.1.420 Lineage Report. Available online: https://outbreak.info/ (accessed on 12 December 2022).
- Perez, L.J.; Orf, G.S.; Berg, M.G.; Rodgers, M.A.; Meyer, T.V.; Mohaimani, A.; Olivo, A.; Harris, B.; Mowerman, I.; Padane, A.; et al. The Early SARS-CoV-2 Epidemic in Senegal Was Driven by the Local Emergence of B.1.416 and the Introduction of B.1.1.420 from Europe. Virus Evol. 2022, 8, veac025. [Google Scholar] [CrossRef]
- Garcia, V.; Vig, V.; Peillard, L.; Ramdani, A.; Mohamed, S.; Halfon, P. First Description of Two Immune Escape Indian B.1.1.420 and B.1.617.1 SARS-CoV2 Variants in France. bioRxiv 2021, 2021.05.12.443357. [Google Scholar] [CrossRef]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; COVID-19 Genomics UK (COG-UK) Consortium; et al. SARS-CoV-2 Variants, Spike Mutations and Immune Escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control SARS-CoV-2 Variants of Concern as of 5 January 2022. Available online: https://www.ecdc.europa.eu/en/covid-19/variants-concern (accessed on 6 January 2022).
- Yurkovetskiy, L.; Wang, X.; Pascal, K.E.; Tomkins-Tinch, C.; Nyalile, T.P.; Wang, Y.; Baum, A.; Diehl, W.E.; Dauphin, A.; Carbone, C.; et al. Structural and Functional Analysis of the D614G SARS-CoV-2 Spike Protein Variant. Cell 2020, 183, 739–751.e8. [Google Scholar] [CrossRef]
- McCallum, M.; De Marco, A.; Lempp, F.A.; Tortorici, M.A.; Pinto, D.; Walls, A.C.; Beltramello, M.; Chen, A.; Liu, Z.; Zatta, F.; et al. N-Terminal Domain Antigenic Mapping Reveals a Site of Vulnerability for SARS-CoV-2. Cell 2021, 184, 2332–2347.e16. [Google Scholar] [CrossRef]
- Cavanaugh, A.M.; Thoroughman, D.; Miranda, H.; Spicer, K. Suspected Recurrent SARS-CoV-2 Infections Among Residents of a Skilled Nursing Facility During a Second COVID-19 Outbreak — Kentucky, July–November 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 273–277. [Google Scholar] [CrossRef]
- Blain, H.; Tuaillon, E.; Gamon, L.; Pisoni, A.; Miot, S.; Rolland, Y.; Picot, M.; Bousquet, J. Antibody Response after One and Two Jabs of the BNT162b2 Vaccine in Nursing Home Residents: The CONsort-19 Study. Allergy 2022, 77, 271–281. [Google Scholar] [CrossRef]
- Costa, A.P.; Manis, D.R.; Jones, A.; Stall, N.M.; Brown, K.A.; Boscart, V.; Castellino, A.; Heckman, G.A.; Hillmer, M.P.; Ma, C.; et al. Risk Factors for Outbreaks of SARS-CoV-2 Infection at Retirement Homes in Ontario, Canada: A Population-Level Cohort Study. Can. Med. Assoc. J. 2021, 193, E672–E680. [Google Scholar] [CrossRef]
- Morciano, M.; Stokes, J.; Kontopantelis, E.; Hall, I.; Turner, A.J. Excess Mortality for Care Home Residents during the First 23 Weeks of the COVID-19 Pandemic in England: A National Cohort Study. BMC Med. 2021, 19, 71. [Google Scholar] [CrossRef]
- Suetens, C.; Kinross, P.; Gallego Berciano, P.; Arroyo Nebreda, V.; Hassan, E.; Calba, C.; Fernandes, E.; Peralta-Santos, A.; Casaca, P.; Shodu, N.; et al. Increasing Risk of Breakthrough COVID-19 in Outbreaks with High Attack Rates in European Long-Term Care Facilities, July to October 2021. Eurosurveillance 2021, 26, 2101070. [Google Scholar] [CrossRef]
- Vilches, T.N.; Nourbakhsh, S.; Zhang, K.; Juden-Kelly, L.; Cipriano, L.E.; Langley, J.M.; Sah, P.; Galvani, A.P.; Moghadas, S.M. Multifaceted Strategies for the Control of COVID-19 Outbreaks in Long-Term Care Facilities in Ontario, Canada. Prev. Med. 2021, 148, 106564. [Google Scholar] [CrossRef]
- Rios, P.; Radhakrishnan, A.; Williams, C.; Ramkissoon, N.; Pham, B.; Cormack, G.V.; Grossman, M.R.; Muller, M.P.; Straus, S.E.; Tricco, A.C. Preventing the Transmission of COVID-19 and Other Coronaviruses in Older Adults Aged 60 Years and above Living in Long-Term Care: A Rapid Review. Syst. Rev. 2020, 9, 218. [Google Scholar] [CrossRef]
- Gmehlin, C.G.; Munoz-Price, L.S. Coronavirus Disease 2019 (COVID-19) in Long-Term Care Facilities: A Review of Epidemiology, Clinical Presentations, and Containment Interventions. Infect. Control Hosp. Epidemiol. 2020, 43, 504–509. [Google Scholar] [CrossRef]

| Population | Cases before Spring Outbreak | Cases during Spring Outbreak | Hospitalized Spring Outbreak | Deaths Spring Outbreak | |
|---|---|---|---|---|---|
| N | N (AR 1) | N (AR) | N (HR 2) | N (CFR 3) | |
| Residents | 154 | 38 (24.7%) | 84 (54.5%) | 18 (21.4%) | 23 (27.4%) |
| Female | 110 | 25 (22.7%) | 61 (55.5%) | 11 (18%) | 15 (24.6%) |
| Male | 44 | 13 (29.5%) | 23 (52.3%) | 7 (30.4%) | 8 (34.8%) |
| Median age (IQR 4) | 87 (81–91) | 88 (82–93) | 88 (83–91) | 89.5 (82–90) | 89 (82–91) |
| Staff | 173 | 29 (16.8%) | 45 (26%) | 1 (2.2%) | 0 (%) |
| Female | 139 | 21 (15.1%) | 39 (28.1%) | 1 (2.6%) | 0 (%) |
| Male | 34 | 8 (23.5%) | 6 (17.6%) | 0 (%) | 0 (%) |
| Median age (IQR) | 40 (33–51) | 40 (32–50) | 39 (32–48) | 43 | NA |
| Total | 327 | 67 (20.5%) | 129 (39.4%) | 19 (14.7%) | 23 (17.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ernst, C.; Pires-Afonso, Y.; Bejko, D.; Huberty, C.; Dentzer, T.G.; Wienecke-Baldacchino, A.; Hugoson, E.; Alvarez, D.; Weydert, M.; Vergison, A.; et al. A Molecular and Epidemiological Investigation of a Large SARS-CoV-2 Outbreak in a Long-Term Care Facility in Luxembourg, 2021. Geriatrics 2023, 8, 19. https://doi.org/10.3390/geriatrics8010019
Ernst C, Pires-Afonso Y, Bejko D, Huberty C, Dentzer TG, Wienecke-Baldacchino A, Hugoson E, Alvarez D, Weydert M, Vergison A, et al. A Molecular and Epidemiological Investigation of a Large SARS-CoV-2 Outbreak in a Long-Term Care Facility in Luxembourg, 2021. Geriatrics. 2023; 8(1):19. https://doi.org/10.3390/geriatrics8010019
Chicago/Turabian StyleErnst, Corinna, Yolanda Pires-Afonso, Dritan Bejko, Conny Huberty, Thomas G. Dentzer, Anke Wienecke-Baldacchino, Eric Hugoson, Daniel Alvarez, Murielle Weydert, Anne Vergison, and et al. 2023. "A Molecular and Epidemiological Investigation of a Large SARS-CoV-2 Outbreak in a Long-Term Care Facility in Luxembourg, 2021" Geriatrics 8, no. 1: 19. https://doi.org/10.3390/geriatrics8010019
APA StyleErnst, C., Pires-Afonso, Y., Bejko, D., Huberty, C., Dentzer, T. G., Wienecke-Baldacchino, A., Hugoson, E., Alvarez, D., Weydert, M., Vergison, A., & Mossong, J. (2023). A Molecular and Epidemiological Investigation of a Large SARS-CoV-2 Outbreak in a Long-Term Care Facility in Luxembourg, 2021. Geriatrics, 8(1), 19. https://doi.org/10.3390/geriatrics8010019






