Safety and Efficacy of a First-Line Chemotherapy Tailored by G8 Score in Elderly Metastatic or Locally Advanced Gastric and Gastro-Esophageal Cancer Patients: A Real-World Analysis
Abstract
:1. Introduction
2. Material and Methods
3. Results
4. Safety Outcome: Adverse Events
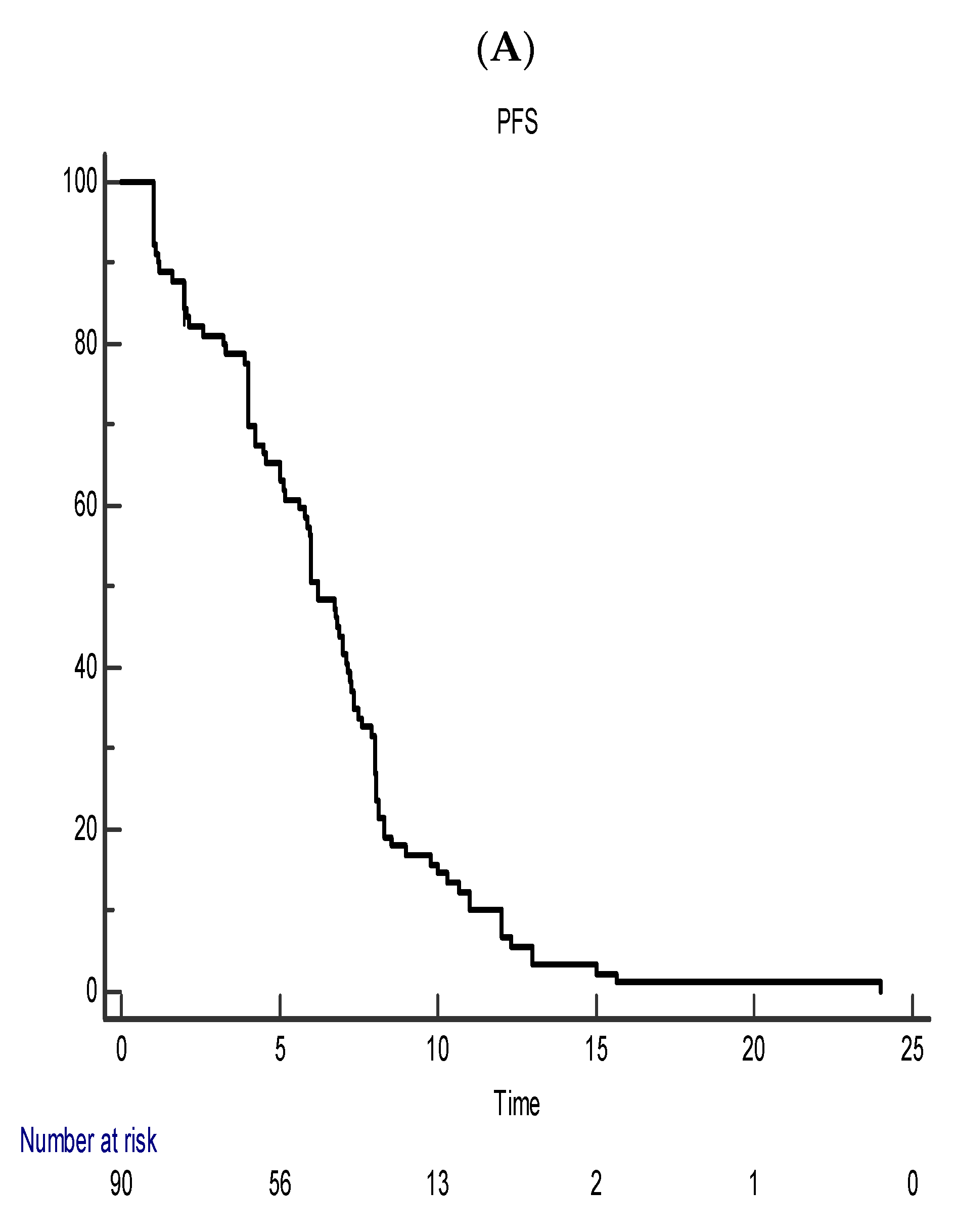
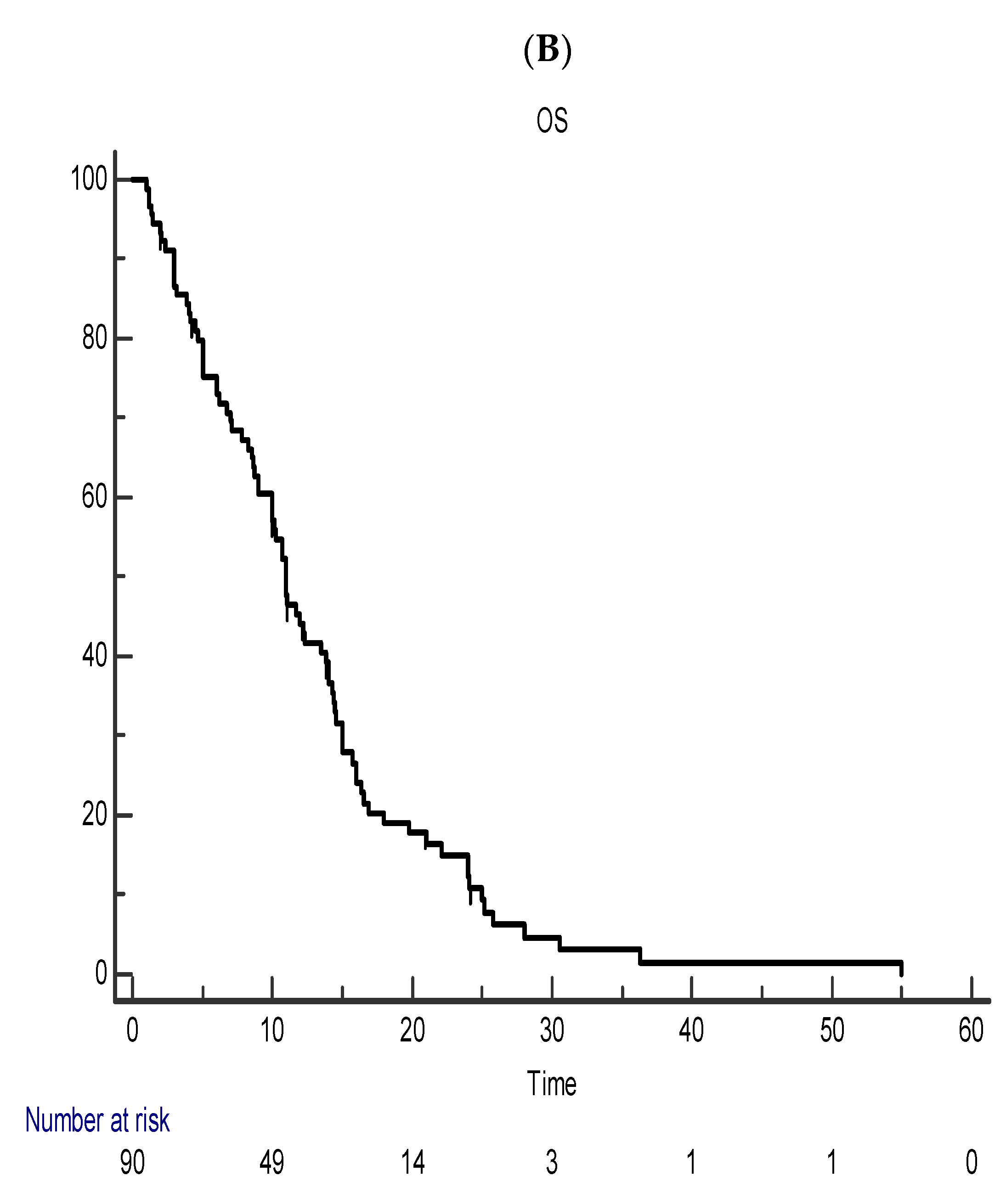
5. Efficacy Outcome
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Kelley, J.R.; Duggan, J.M. Gastric cancer epidemiology and risk factors. J. Clin. Epidemiol. 2003, 56, 1–9. [Google Scholar] [CrossRef]
- van Gestel, Y.R.; Lemmens, V.E.; de Hingh, I.H.; Steevens, J.; Rutten, H.J.; Nieuwenhuijzen, G.A.; van Dam, R.M.; Siersema, P.D. Influence of comorbidity and age on 1-, 2-, and 3-month postoperative mortality rates in gastrointestinal cancer patients. Ann. Surg. Oncol. 2013, 20, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Stober, J.; Hong, H.G.; Czado, C.; Ghosh, P. Comorbidity of chronic diseases in the elderly: Patterns identified by a copula design for mixed responses. Comput. Stat. Data Anal. 2015, 88, 28–39. [Google Scholar] [CrossRef]
- Hutchins, L.F.; Unger, J.M.; Crowley, J.J.; Coltman, C.A.; Albain, K.S. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N. Engl. J. Med. 1999, 341, 2061–2067. [Google Scholar] [CrossRef]
- Lewis, J.H.; Kilgore, M.L.; Goldman, D.P.; Trimble, E.L.; Kaplan, R.; Montello, M.J.; Housman, M.G.; Escarce, J.J. Participation of patients 65 years of age or older in cancer clinical trials. J. Clin. Oncol. 2003, 21, 1383–1389. [Google Scholar] [CrossRef]
- Murthy, V.H.; Krumholz, H.M.; Gross, C.P. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA 2004, 291, 2720–2726. [Google Scholar] [CrossRef]
- Talarico, L.; Chen, G.; Pazdur, R. Enrollment of elderly patients in clinical trials for cancer drug registration: A 7-year experience by the US Food and Drug Administration. J. Clin. Oncol. 2004, 22, 4626–4631. [Google Scholar] [CrossRef]
- Aapro, M.S.; Kohne, C.H.; Cohen, H.J.; Extermann, M. Never too old? Age should not be a barrier to enrollment in cancer clinical trials. Oncologist 2005, 10, 198–204. [Google Scholar] [CrossRef]
- Koizumi, W.; Narahara, H.; Hara, T.; Takagane, A.; Akiya, T.; Takagi, M.; Miyashita, K.; Nishizaki, T.; Kobayashi, O.; Takiyama, W.; et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet Oncol. 2008, 9, 215–221. [Google Scholar] [CrossRef]
- Trumper, M.; Ross, P.J.; Cunningham, D.; Norman, A.R.; Hawkins, R.; Seymour, M.; Harper, P.; Iveson, T.; Nicolson, M.; Hickish, T. Efficacy and tolerability of chemotherapy in elderly patients with advanced oesophago-gastric cancer: A pooled analysis of three clinical trials. Eur. J. Cancer 2006, 42, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; on behalf of the EGC; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27 (Suppl. S5), v38–v49. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Moiseyenko, V.M.; Tjulandin, S.; Majlis, A.; Constenla, M.; Boni, C.; Rodrigues, A.; Fodor, M.; Chao, Y.; Voznyi, E. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 Study Group. J. Clin. Oncol. 2006, 24, 4991–4997. [Google Scholar] [CrossRef]
- Hwang, I.G.; Ji, J.H.; Kang, J.H.; Lee, H.R.; Lee, H.; Chi, K.; Park, S.W.; Lee, S.J.; Kim, S.T.; Lee, J.; et al. A multi-center, open-label, randomized phase III trial of first-line chemotherapy with capecitabine monotherapy versus capecitabine plus oxaliplatin in elderly patients with advanced gastric cancer. J. Geriatr. Oncol. 2017, 8, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Wildiers, H.; Heeren, P.; Puts, M.; Topinkova, E.; Janssen-Heijnen, M.L.G.; Extermann, M.; Falandry, C.; Artz, A.; Brain, E.; Colloca, G.; et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J. Clin. Oncol. 2014, 32, 2595–2603. [Google Scholar] [CrossRef] [PubMed]
- Kenis, C.; Decoster, L.; van Puyvelde, K.; de Grève, J.; Conings, G.; Milisen, K.; Flamaing, J.; Lobelle, J.; Wildiers, H. Performance of two geriatric screening tools in older patients with cancer. JCO 2014, 32, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Kenis, C.; Bron, D.; Libert, Y.; Decoster, L.; van Puyvelde, K.; Scalliet, P.; Cornette, P.; Pepersack, T.; Luce, S.; Langenaeken, C.; et al. Relevance of a systematic geriatric screening and assessment in older patients with cancer: Results of a prospective multicentric study. Ann. Oncol. 2013, 24, 1306–1312. [Google Scholar] [CrossRef]
- Bellera, C.A.; Rainfray, M.; Mathoulin-Pélissier, S.; Mertens, C.; Delva, F.; Fonck, M.; Soubeyran, P.L. Screening older cancer patients: First evaluation of the G-8 screening tool. Ann. Oncol. 2012, 23, 2166–2172. [Google Scholar] [CrossRef]
- Decoster, L.; Van Puyvelde, K.; Mohile, S.; Wedding, U.; Basso, U.; Colloca, G.; Rostoft, S.; Overcash, J.; Wildiers, H.; Steer, C.; et al. Screening tools for multi-dimension al health problems warranting a geriatric assessment in older cancer patients: An update on SIOG recommendations dagger. Ann. Oncol. 2015, 26, 288–300. [Google Scholar] [CrossRef]
- Hamaker, M.E.; Jonker, J.M.; de Rooij, S.E.; Vos, A.G.; Smorenburg, C.H.; van Munster, B.C. Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: A systematic review. Lancet Oncol. 2012, 13, e437–e444. [Google Scholar] [CrossRef]
- Hurria, A.; Cirrincione, C.T.; Muss, H.B.; Kornblith, A.B.; Barry, W.; Artz, A.S.; Schmieder, L.; Ansari, R.; Tew, W.P.; Weckstein, D.; et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. JCO 2011, 29, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Hurria, A.; Gupta, S.; Zauderer, M.; Zuckerman, E.L.; Cohen, H.J.; Muss, H.; Rodin, M.; Panageas, K.S.; Holland, J.C.; Saltz, L.; et al. Developing a cancer- specific geriatric assessment: A feasibility study. Cancer 2005, 104, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Brink, T.L.; Yesavage, J.A.; Lum, O.; Heersema, P.H.; Adey, M.; Rose, T.L. Screening tests for geriatric depression. Clin. Gerontol. 1982, 1, 37–44. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Minimental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiat. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Repetto, L.; Fratino, L.; Audisio, R.A.; Venturino, A.; Gianni, W.; Vercelli, M.; Parodi, S.; Lago, D.D.; Gioia, F.; Monfardini, S.; et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: An Italian Group for Geriatric Oncology Study. J. Clin. Oncol. 2002, 20, 494–502. [Google Scholar] [CrossRef]
- Miller, M.D.; Towers, A. A Manual of Guidelines for Scoring the Cumulative Illness Rating Scale for Geriatricians (CIRS-G); University of Pittsburg: Pittsburg, PA, USA, 1991; pp. 1–12. [Google Scholar]
- Corsi, D.C.; Turriziani, A.; Cavanna, L.; Morino, P.; Ribecco, A.S.; Ciaparrone, M.; Lanzetta, G.; Pinto, C.; Zagonel, V. Consensus document of the Italian Association of Medical Oncology and the Italian Society of Palliative Care on early palliative care. Tumori J. 2019, 105, 103–112. [Google Scholar] [CrossRef]
- Moghadamyeghaneh, Z.; Hwang, G.; Hanna, M.H.; Phelan, M.J.; Carmichael, J.C.; Mills, S.D.; Pigazzi, A.; Dolich, M.O.; Stamos, M.J. Even modest hypoalbuminemia affects outcomes of colorectal surgery patients. Am. J. Surg. 2015, 210, 276–284. [Google Scholar] [CrossRef]
- Robinson, R. Low serum albumin and total lymphocyte count as predictors of 30 day hospital readmission in patients 65 years of age or older. Peer J. 2015, 3, e1181. [Google Scholar] [CrossRef]
- Paillaud, E.; Liuu, E.; Laurent, M.; le Thuaut, A.; Vincent, H.; Raynaud-Simon, A.; Bastuji-Garin, S.; Tournigand, C.; Caillet, P.; Canoui-Poitrine, F.; et al. Geriatric syndromes increased the nutritional risk in elderly cancer patients independently from tumour site and metastatic status. The ELCAPA-05 cohort study. Clin. Nutr. 2014, 33, 330–335. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Pozzo, C.; Strippoli, A.; Bria, E.; Tortora, G.; Gasbarrini, A.; Mele, M.C. Effects of nutritional interventions on nutritional status in patients with gastric cancer: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2020, 38, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Pozzo, C.; Strippoli, A.; Bria, E.; Tortora, G.; Gasbarrini, A.; Mele, M.C. Muscle mass, assessed at diagnosis by L3-CT scan as a prognostic marker of clinical outcomes in patients with gastric cancer: A systematic review and meta-analysis. Clin. Nutr. 2020, 39, 2045–2054. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, J.H.; Kim, J.W.; Kim, B.C. Chemotherapy in Elderly Patients with Gastric Cancer. J. Cancer 2016, 7, 88–94. [Google Scholar] [CrossRef]
- Hall, P.S.; Swinson, D.; Cairns, D.A.; Waters, J.S.; Petty, R.; Allmark, C.; Ruddock, S.; Falk, S.; Wadsley, J.; Roy, R.; et al. Efficacy of Reduced-Intensity Chemotherapy With Oxaliplatin and Capecitabine on Quality of Life and Cancer Control Among Older and Frail Patients With Advanced Gastroesophageal Cancer: The GO2 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Extermann, M.; Hurria, A. Comprhensive Geriatric Assessment for Older Patients With Cancer. J. Clin. Oncol. 2007, 25, 1824–1831. [Google Scholar] [CrossRef]
| Overall N (%) | |
|---|---|
| Characteristic | 90 (100) |
| Age | |
| Median (years) Range (years) Elderly (≥80) | 78 75–87 20 (22.2) |
| Sex | |
| Male Female | 62 (68.9) 28 (31.1) |
| Comorbidities | |
| Hypertension Diabetes Atherosclerosis Osteoporosis Hypercholesterolemia Benign prostatic hyperplasia COPD | 45 (50) 25 (27.7) 23 (25.5) 20 (22.2) 10 (11.1) 25 (27.7) 18 (20) |
| Sidedness | |
| GC GEC | 72 (80) 18 (20) |
| Locally Advanced Metastatic | 10 (11.1) 80 (88.9) |
| ECOG-PS | |
| 0–1 2 | 52 (57.8) 38 (42.2) |
| G8 score | |
| ≤14 >15 | 66 (73.3) 24 (26.7) |
| Median weight loss | 6 kg (0–18) |
| Median number of comorbidities Range | 2 0–6 |
| Median number of drugs taken Range | 3 0–8 |
| Most frequent comorbidities | |
| Hypertension Diabetes Atherosclerosis Osteoporosis Benign prostatic hyperplasia COPD | 45 (50) 25 (27.7) 23 (25.5) 20 (22–2) 25 (27.7) 18 (20) |
| BM1 | |
| <19 20–25 >25 | 22 (24.4) 63 (70) 5 (5.6) |
| CT | |
| Trastuzumab-based CT Doublet-CT Mono-CT | 14 (15.5) 50 (55.5) 26 (28.8) |
| Median cycles administered Range | 7 1–17 |
| Second-line chemotherapy Third-line chemotherapy | 15 (21.4) 5 (5.5) |
| Overall N (90) | ||||
|---|---|---|---|---|
| Adverse Events (AE) | G-G2 N (%) 46 (51.1) | Mono/Doublet-CT | G3-G4 N (%) 28 (31.1) | Mono/Doublet-CT |
| Hematologic AE | ||||
| Neutropenia | 9 (10) | 5/9 | 12 (13.3) | 5/7 |
| Anemia | 12 (13.3) | 3/8 | 2 (2.2) | 0/2 |
| Thrombocytopenia | 6 (6.6) | 3/3 | 4 (4.4) | 3/4 |
| Non-hematological AE | ||||
| Asthenia | 23 (25.5) | 6/11 | 15 (16.6) | 3/5 |
| Diarrhea | 10 (11.1) | 4/6 | 7 (7.8) | 8/0 |
| Mucositis/stomatitis | 8 (8.8) | 2/6 | 9 (10) | 1/2 |
| Neuropathy | 8 (8.8) | 0/5 | 5 (5.5) | 0/5 |
| Anorexia | 11 (12.2) | 2/9 | 4 (4.4) | 1/3 |
| Dysgeusia | 12 (13.3) | 1/11 | - | - |
| Nausea | 9 (10) | 3/6 | - | - |
| Vomiting | 7 (7.7) | 1/6 | ||
| Univariate Analysis | ||
|---|---|---|
| PFS | OS | |
| Variable | HR (95% CI); p-Value | HR (95% CI); p-Value |
| Albumin count | ||
| <3.5 vs. >3.5 g/dL | 1.3 (0.79–2.20); p = 0.234 | 1.12 (0.66–1.88); p = 0.64 |
| BMI | ||
| <20 vs. >20 kg/m2 | 1.10 (0.67–1.81); p = 0.67 | 0.94 (0.57–1.56); p = 0.82 |
| Weight loss | ||
| <10 vs. >10% | 1.93 (1.26–2.96); p = 0.0008 | 1.47 (0.95–2.28); p = 0.06 |
| Gender | ||
| Female vs. Male | 0.88 (0.55–1.40); p = 0.57 | 0.89 (0.55–1.45); p = 0.64 |
| PS ECOG | ||
| 0–1 vs. 2 | 0.31 (0.17–0.51); p < 0.0001 | 0.28 (0.16–0.49); p < 0.0001 |
| G8 score | ||
| Normal vs. Abnormal | 1.01 (0.5–1.8); p < 0.0001 | 1.80 (1.15–2.81); p = 0.0151 |
| T location Upper vs. middle/lower | 1.36(0.76–2.43); p = 0.22 | 1.58 (0.96–2.59); p = 0.081 |
| Liver metastasis | ||
| yes vs. no | 0.8 (0.58–1.34); p = 0.56 | 0.95 (0.54–1.67); p = 0.86 |
| Peritoneal metastasis yes vs. no | 0.99 (0.64–1.55); p = 0.99 | 0.89 (0.56–1.40); p = 0.62 |
| Chemotherapy | ||
| Doublet-CT vs. Mono-CT | 0.48 (0.28–0.85); p = 0.0010 | 0.49 (0.27–0.88); p = 0.0021 |
| Toxicity AE G3/G4 yes vs. no | 0.99 (0.64–1.53); p = 0.97 | 0.77 (0.49–1.22); p = 0.29 |
| Drug dose reduction yes. vs. no | 0.68 (0.38–1.22); p = 0.14 | 0.60 (0.32–1.10); p = 0.06 |
| Median drugs taken <3 vs. >3 | 1.22 (0.73–2.17); p = 0.89 | 0.99 (0.57–1.69); p = 0.75 |
| Multivariate Analysis | ||
| PFS | OS | |
| Variable | HR (95% CI); p-Value | HR (95% CI); p-Value |
| PS ECOG | ||
| 0-1 vs. 2 | 6.31 (2.70–14.77); p < 0.0001 | 9.8 (3.88–24.88); p < 0.0001 |
| Albumin count | ||
| <3.5 vs. >3.5 g/dL | 0.97 (0.52–1.79); p = 0.92 | 2.12 (1.027–4.39); p = 0.043 |
| Chemotherapy Doublet-CT vs. Mono-CT | 0.89 (0.50–1.60); p = 0.72 | 1.46 (0.67–3.19); p = 0.33 |
| G8 score Normal vs. abnormal | 2.88 (1.52–5.44); p = 0.0012 | 1.5 (0.83–2.77); p = 0.036. |
| Weight loss <10 vs. >10% | 1.21 (0.71–2.04); p = 0.46 | 0.97 (0.58–1.62); p = 0.93 |
| G8 Normal | G8 Abnormal | p-Value | |
|---|---|---|---|
| Albumin count | |||
| >3.5 g/dL <3.5 g/dL | 23 1 | 44 22 | 0.0113 |
| BMI | |||
| High Low | 20 4 | 48 18 | 0.4484 |
| Weight-loss | |||
| <10% >10% | 16 8 | 25 41 | 0.0288 |
| Liver metastasis | |||
| Yes No | 11 13 | 34 32 | 0.8116 |
| Peritoneum | |||
| Yes No | 12 12 | 18 48 | 0.0768 |
| Toxicity | |||
| Yes No | 3 21 | 28 37 | 0.0148 |
| PS ECOG | |||
| 0–1 2 | 22 2 | 30 36 | 0.0002 |
| Tumor site | |||
| GC GEC | 20 4 | 52 14 | 0.8581 |
| Chemotherapy | |||
| Doublet-CT vs. Mono-CT | 22 2 | 42 24 | 0.0197 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zurlo, I.V.; Pozzo, C.; Strippoli, A.; Mignogna, S.; Basso, M.; Vivolo, R.; Trovato, G.; Ciaburri, M.; Morelli, F.; Bria, E.; et al. Safety and Efficacy of a First-Line Chemotherapy Tailored by G8 Score in Elderly Metastatic or Locally Advanced Gastric and Gastro-Esophageal Cancer Patients: A Real-World Analysis. Geriatrics 2022, 7, 107. https://doi.org/10.3390/geriatrics7050107
Zurlo IV, Pozzo C, Strippoli A, Mignogna S, Basso M, Vivolo R, Trovato G, Ciaburri M, Morelli F, Bria E, et al. Safety and Efficacy of a First-Line Chemotherapy Tailored by G8 Score in Elderly Metastatic or Locally Advanced Gastric and Gastro-Esophageal Cancer Patients: A Real-World Analysis. Geriatrics. 2022; 7(5):107. https://doi.org/10.3390/geriatrics7050107
Chicago/Turabian StyleZurlo, Ina Valeria, Carmelo Pozzo, Antonia Strippoli, Samantha Mignogna, Michele Basso, Raffaella Vivolo, Giovanni Trovato, Michele Ciaburri, Franco Morelli, Emilio Bria, and et al. 2022. "Safety and Efficacy of a First-Line Chemotherapy Tailored by G8 Score in Elderly Metastatic or Locally Advanced Gastric and Gastro-Esophageal Cancer Patients: A Real-World Analysis" Geriatrics 7, no. 5: 107. https://doi.org/10.3390/geriatrics7050107
APA StyleZurlo, I. V., Pozzo, C., Strippoli, A., Mignogna, S., Basso, M., Vivolo, R., Trovato, G., Ciaburri, M., Morelli, F., Bria, E., Leo, S., & Tortora, G. (2022). Safety and Efficacy of a First-Line Chemotherapy Tailored by G8 Score in Elderly Metastatic or Locally Advanced Gastric and Gastro-Esophageal Cancer Patients: A Real-World Analysis. Geriatrics, 7(5), 107. https://doi.org/10.3390/geriatrics7050107







