Feasibility of a Geriatric Assessment to Detect and Quantify Sarcopenia and Physical Functioning in German Nursing Home Residents—A Pilot Study
Abstract
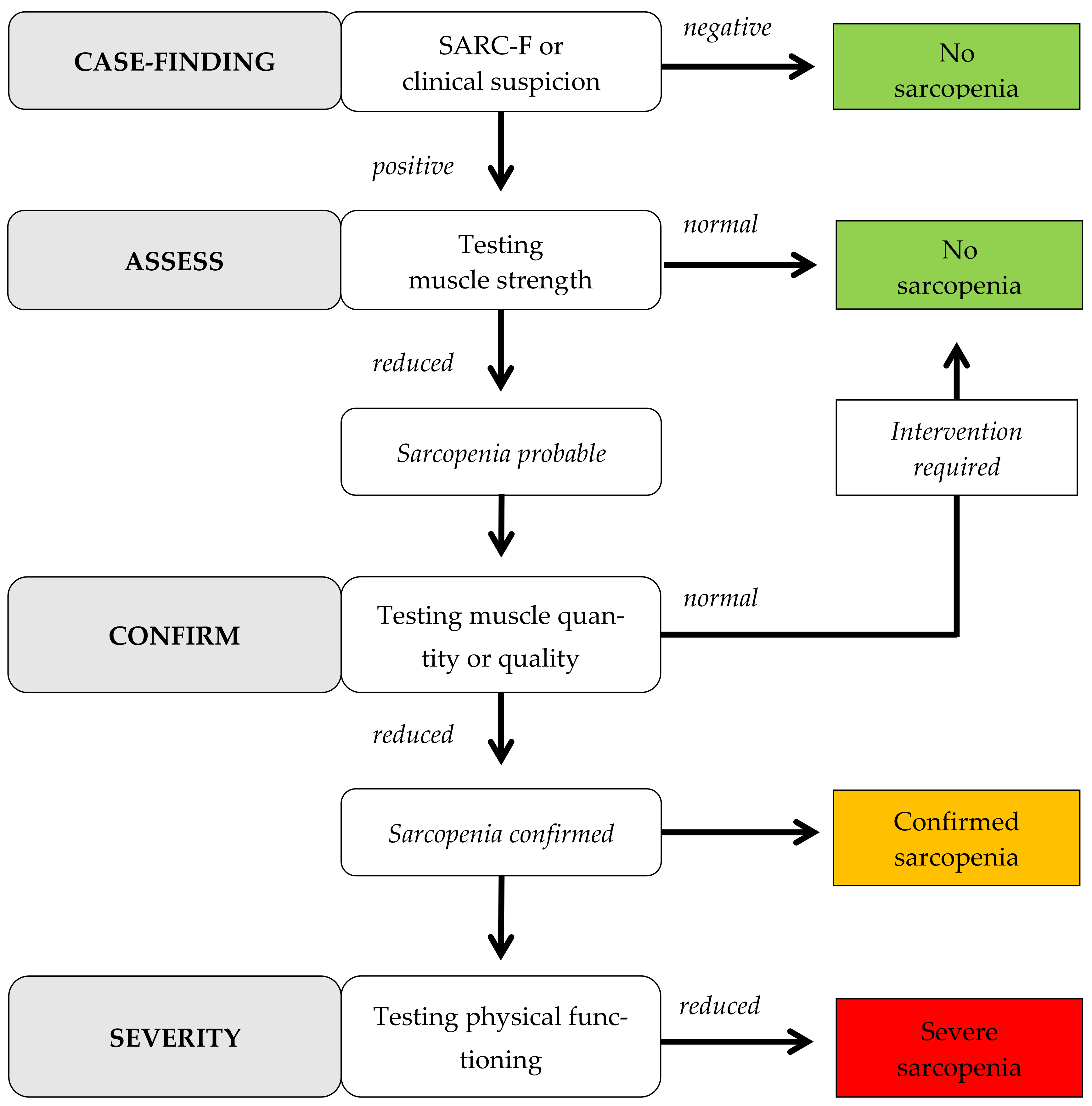
:1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Inclusion and Exclusion Criteria
2.3. Instruments
3. Results
3.1. Demographic Data
3.2. Geriatric Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bundesministerium für Gesundheit-Zahlen und Fakten zur Pflegeversicherung. Available online: https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/Statistiken/Pflegeversicherung/Zahlen_und_Fakten/Zahlen_und_Fakten_der_SPV_17.Februar_2020_barr.pdf (accessed on 11 May 2020).
- Nowossadeck, S. Demografischer Wandel, Pflegebedürftige und der künftige Bedarf an Pflegekräften. Bundesgesundheitsblatt 2013, 56, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Hagg-Grün, U.; Nikolaus, T.; Zeyfang, A. Mobilität, Immobilität, Stürze und Folgen. In Basiswissen Medizin des Alterns und des Alten Menschen, 3rd ed.; Zeyfang, A., Denkinger, M., Haag-Grün, U., Eds.; Springer: Berlin, Germany, 2018; pp. 39–50. [Google Scholar]
- Kalinowski, S.; Kuhnert, R.; Kölzsch, M. Schmerzen, Sturzangst und funktionelle Fähigkeiten von Menschen in Pflegeheimen–eine Querschnittsstudie. Pflege 2012, 25, 411–425. [Google Scholar] [CrossRef]
- Füzeki, E.; Banzer, W. Bewegung und Gesundheit im Alter. In Körperliche Aktivität und Gesundheit, 1st ed.; Banzer, W., Ed.; Springer: Berlin, Germany, 2017; pp. 139–152. [Google Scholar]
- Zhang, X.; Wang, C.; Dou, Q.; Zhang, W.; Yang, Y.; Xie, X. Sarcopenia as a predictor of all-cause mortality among older nursing home residents: A systematic review and metaanalysis. BMJ Open 2018, 8, e021252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landi, F.; Liperoti, R.; Fusco, D.; Mastropaolo, S.; Quattrociocchi, D.; Proia, A.; Russo, A.; Bernabei, R.; Onder, G. Prevalence and risk factors of sarcopenia among nursing home older residents. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 48–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senior, H.E.; Henwood, T.R.; Beller, E.M.; Mitchell, G.K.; Keogh, J.W. Prevalence and risk factors of sarcopenia among adults living in nursing homes. Maturitas 2015, 82, 418–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckinx, F.; Reginster, J.Y.; Brunois, T.; Lenaerts, C.; Beaudart, C.; Croisier, J.L.; Petermans, J.; Bruyère, O. Prevalence of sarcopenia in a population of nursing home residents according to their frailty status: Results of the SENIOR cohort. J. Musculoskelet. Neuronal Interact. 2017, 17, 209–217. [Google Scholar] [PubMed]
- Yalcin, A.; Aras, S.; Atmis, V.; Cengiz, O.K.; Cinar, E.; Atli, T.; Varli, M. Sarcopenia and mortality in older people living in a nursing home in Turkey. Geriatr. Gerontol. Int. 2017, 17, 1118–1124. [Google Scholar] [CrossRef]
- Bravo-José, P.; Moreno, E.; Espert, M.; Romeu, M.; Martínez, P.; Navarro, C. Prevalence of sarcopenia and associated factors in institutionalised older adult patients. Clin. Nutr. ESPEN 2018, 27, 113–119. [Google Scholar] [CrossRef]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef] [Green Version]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Malmstrom, T.K.; Morley, J.E. SARC-F: A simple questionnaire to rapidly diagnose sarcopenia. J. Am. Med. Dir. Assoc. 2013, 14, 531–532. [Google Scholar] [CrossRef]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef] [Green Version]
- Sergi, G.; De Rui, M.; Veronese, N.; Bolzetta, F.; Berton, L.; Carraro, S.; Bano, G.; Coin, A.; Manzato, E.; Perissinotto, E. Assessing appendicular skeletal muscle mass with bioelectrical impedance analysis in free-living Caucasian older adults. Clin. Nutr. 2015, 34, 667–673. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Paillard, T.; Noé, F. Techniques and Methods for Testing the Postural Function in Healthy and Pathological Subjects. Biomed Res. Int. 2015, 2015, 891390. [Google Scholar] [CrossRef] [Green Version]
- Büsching, G. Short Physical Performance Battery Test–Ein Muss in der Geriatrie. Physiopraxis 2015, 13, 42–43. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed "Up & Go": A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar]
- Beauchet, O.; Annweiler, C.; Assal, F.; Bridenbaugh, S.; Herrmann, F.R.; Kressig, R.W.; Allali, G. Imagined Timed Up & Go test: A new tool to assess higher-level gait and balance disorders in older adults? J. Neurol. Sci. 2010, 294, 102–106. [Google Scholar]
- Hofheinz, M.; Mibs, M. The Prognostic Validity of the Timed Up and Go Test With a Dual Task for Predicting the Risk of Falls in the Elderly. Gerontol. Geriatr. Med. 2016, 2, 2333721416637798. [Google Scholar] [CrossRef]
- Papadopoulou, S.K.; Tsintavis, P.; Potsaki, P.; Papandreou, D. Differences in the Prevalence of Sarcopenia in Community-Dwelling, Nursing Home and Hospitalized Individuals. A Systematic Review and Meta-Analysis. J. Nutr. Health Aging 2020, 24, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Masanés, F.; Rojano I Luque, X.; Salvà, A.; Serra-Rexach, J.A.; Artaza, I.; Formiga, F.; Cuesta, F.; López Soto, A.; Ruiz, D.; Cruz-Jentoft, A.J. Cut-off points for muscle mass—not grip strength or gait speed—determine variations in sarcopenia prevalence. J. Nutr. Health Aging 2017, 21, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Perkisas, S.; De Cock, A.M.; Vandewoude, M.; Verhoeven, V. Prevalence of sarcopenia and 9-year mortality in nursing home residents. Aging Clin. Exp. Res. 2019, 31, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Johansson, J.; Strand, B.H.; Morseth, B.; Hopstock, L.A.; Grimsgaard, S. Differences in sarcopenia prevalence between upper-body and lower-body based EWGSOP2 muscle strength criteria: The Tromsø study 2015–2016. BMC Geriatr. 2020, 20, 461. [Google Scholar] [CrossRef] [PubMed]
- Reiss, J.; Iglseder, B.; Alzner, R.; Mayr-Pirker, B.; Pirich, C.; Kässmann, H.; Kreutzer, M.; Dovjak, P.; Reiter, R. Consequences of applying the new EWGSOP2 guideline instead of the former EWGSOP guideline for sarcopenia case finding in older patients. Age Ageing 2019, 48, 719–724. [Google Scholar] [CrossRef] [PubMed]
| Assessment | Basic Data |
|---|---|
| Demographic data | Age, sex, degree of care (inspection of patient files) |
| MMSE [15] | Recording of cognitive function |
| BI [16] | Recording of activities of daily living |
| Morbidity status | Recording of diseases in categories (inspection of patient files) |
| Assessment | Primary Outcomes |
| SARC-F [17] | Questionnaire for subjective self-assessment to determine sarcopenia |
| HFM [18] | Measurement of maximum hand force by isometric test (in kg) |
| BIA [19] | Measurement of ASMM (in kg) and SMI (in kg/m2) with BIA |
| 4MWST (SPPB) [20] | Measurement of walking speed over a 4-meter walking distance (in m/s) |
| Assessment | Secondary Outcomes |
| Balance test (SPPB) [20,21] | Measurement of standing time in three different stand variants (in s) and CoP measurement with a Kistler® force plate (in mm/m/s) |
| 5CRT (SPPB) [20] | Measurement of strength capacity of lower extremity by repetition method (in s) |
| TUGsingle task [23] | Recording risk of falls, gait and balance performance (in s) |
| TUGmotor imagery [24] | |
| TUGdual task [25] |
| Criteria of Sarcopenia | Cut-off-Value ♀ | Cut-off-Value ♂ |
|---|---|---|
| 1. Analysis of reduced muscle strength | ||
| HFM | <16 kg | <27 kg |
| 5CRT | >15 s | |
| 2. Analysis of reduced muscle quantity or quality | ||
| ASMM | <15 kg | <20 kg |
| SMI | <5.5 kg/m2 | <7.0 kg/m2 |
| 3. Analysis of reduced physical functioning | ||
| 4MWST | ≤0.8 m/s | |
| SPPB | ≤8 points | |
| TUGsingle task | ≥20 s | |
| ID | Age | Walking Aids | Degree of Care | MMSE | BI | Morbidities |
|---|---|---|---|---|---|---|
| P1 | 92 | rollator | 2. | 25 | 75 | none |
| P2 | 88 | rollator | 2. | 26 | 85 | 2|11 |
| P3 | 91 | rollator | 3. | 26 | 65 | 2|4|5|8|10b+ |
| P4 | 77 | none | 3. | 14 | 100 | 2|8|10b−|11 |
| P5 | 88 | rollator | 3. | 12 | 65 | 2|4|7|10b−|11 |
| P6 | 84 | rollator | 3. | 22 | 80 | 1|2|3|4|6|9II |
| P7 | 86 | rollator | 4. | 28 | 80 | 1|8 |
| P8 | 81 | rollator | 4. | n.p. | n.p. | 1|2|3|4|5|6|7|8|9II|10b+|11 |
| P9 | 91 | rollator | 2. | n.p. | n.p. | 2|4|7|8 |
| P10 | 97 | none | 2. | 24 | 80 | 2|3 |
| ID | BMI | SARC-F | HFM 1 | ASMM|SMI | 4MWT Habitual|Maximum |
|---|---|---|---|---|---|
| P1♀ | 18.0 kg/m2 | 4/10 * | 12 kg * | 8.80 kg *|4.9 kg/m2 * | 0.60 m/s *|0.62 m/s * |
| P2♀ | 32.0 kg/m2 | 6/10 * | 16 kg | 16.40 kg|8.2 kg/m2 | 0.47 m/s *|0.59 m/s * |
| P3♀ | 29.1 kg/m2 | 4/10 * | 18 kg | 21.10 kg|8.9 kg/m2 | 0.44 m/s *|0.53 m/s * |
| P4♀ | 16.1 kg/m2 | 2/10 | 18 kg | 10.01 kg *|5.3 kg/m2 * | 0.43 m/s *|0.67 m/s * |
| P5♀ | 25.4 kg/m2 | 7/10 * | 15 kg * | n.p.|n.p. | 0.38 m/s *|0.58 m/s * |
| P6♂ | 26.1 kg/m2 | 10/10 * | 30 kg | 20.40 kg|8.8 kg/m2 | 0.32 m/s *|n.a. |
| P7♀ | 34.9 kg/m2 | 6/10 * | 18 kg | 17.30 kg|7.6 kg/m2 | 0.47 m/s *|0.55 m/s * |
| P8♂ | 22.5 kg/m2 | 5/10 * | 29 kg | 19.80 kg *|8.2 kg/m2 | 0.22 m/s *|n.a. |
| P9♀ | 27.0 kg/m2 | n.p. | 12 kg * | 12.10 kg *|7.3 kg/m2 | 0.59 m/s *|0.54 m/s * |
| P10♀ | 27.4 kg/m2 | 1/10 | 20 kg | 15.40 kg|5.7 kg/m2 | 0.40 m/s *|0.81 m/s |
| ID | 5CRT | SPPB | TUGsingle task | TUGmotor imagery 1 | TUGdual task 1 | CoP 1,2 |
|---|---|---|---|---|---|---|
| P1♀ | n.p. | n.p. | 21.19 s * | 6.50 s | n.p. | n.p. |
| P2♀ | n.p. | n.p. | n.p. | n.p. | n.p. | n.p. |
| P3♀ | n.p. | n.p. | n.p. | n.p. | n.p. | n.p. |
| P4♀ | 19.78 * | 8/12 * | 15.41 s | 10.60 s | 17.31 s | 119.3 mm (0.01 m/s) parallel 317.1 mm (0.03 m/s) semi-tandem 434.3 mm (0.04 m/s) tandem |
| P5♀ | n.p. | n.p. | 42.94 s * | n.a. | n.p. | n.p. |
| P6♂ | n.p. | n.p. | n.p. | n.p. | n.p. | n.p. |
| P7♀ | n.p. | n.p. | n.p. | n.p. | n.p. | n.p. |
| P8♂ | n.p. | n.p. | n.p. | n.p. | n.p. | n.p. |
| P9♀ | n.p. | n.p. | n.p. | n.p. | n.p. | n.p. |
| P10♀ | n.p. | n.p. | n.p. | n.p. | n.p. | n.p. |
| ID | Suspicion of Sarcopenia | Assessing the Suspicion | Confirmation of Sarcopenia | Physical Functioning | Severity of Sarcopenia |
|---|---|---|---|---|---|
| P1♀ | positive | probably | positive | limited | severe |
| P2♀ | positive | improbably | negative | limited | none |
| P3♀ | positive | improbably | negative | limited | none |
| P4♀ | negative | uncertain | positive | uncertain | uncertain |
| P5♀ | positive | probably | not feasible | limited | uncertain |
| P6♂ | positive | improbably | negative | limited | none |
| P7♀ | positive | improbably | negative | limited | none |
| P8♂ | not feasible | improbably | uncertain | limited | uncertain |
| P9♀ | positive | probably | uncertain | limited | uncertain |
| P10♀ | negative | improbably | negative | unlimited | none |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haigis, D.; Pomiersky, R.; Altmeier, D.; Frahsa, A.; Sudeck, G.; Thiel, A.; Eschweiler, G.; Nieß, A.M. Feasibility of a Geriatric Assessment to Detect and Quantify Sarcopenia and Physical Functioning in German Nursing Home Residents—A Pilot Study. Geriatrics 2021, 6, 69. https://doi.org/10.3390/geriatrics6030069
Haigis D, Pomiersky R, Altmeier D, Frahsa A, Sudeck G, Thiel A, Eschweiler G, Nieß AM. Feasibility of a Geriatric Assessment to Detect and Quantify Sarcopenia and Physical Functioning in German Nursing Home Residents—A Pilot Study. Geriatrics. 2021; 6(3):69. https://doi.org/10.3390/geriatrics6030069
Chicago/Turabian StyleHaigis, Daniel, Rebekka Pomiersky, Dorothée Altmeier, Annika Frahsa, Gorden Sudeck, Ansgar Thiel, Gerhard Eschweiler, and Andreas Michael Nieß. 2021. "Feasibility of a Geriatric Assessment to Detect and Quantify Sarcopenia and Physical Functioning in German Nursing Home Residents—A Pilot Study" Geriatrics 6, no. 3: 69. https://doi.org/10.3390/geriatrics6030069
APA StyleHaigis, D., Pomiersky, R., Altmeier, D., Frahsa, A., Sudeck, G., Thiel, A., Eschweiler, G., & Nieß, A. M. (2021). Feasibility of a Geriatric Assessment to Detect and Quantify Sarcopenia and Physical Functioning in German Nursing Home Residents—A Pilot Study. Geriatrics, 6(3), 69. https://doi.org/10.3390/geriatrics6030069








