Virtual Reality Therapy for Depression and Mood in Long-Term Care Facilities
Abstract
1. Overview
2. Place Attachment in LTCFs
3. Depression in LTCFs
3.1. Causation and Neural Mechanisms of Depression in LTCFs
3.2. Implications of Depression in LTCFs
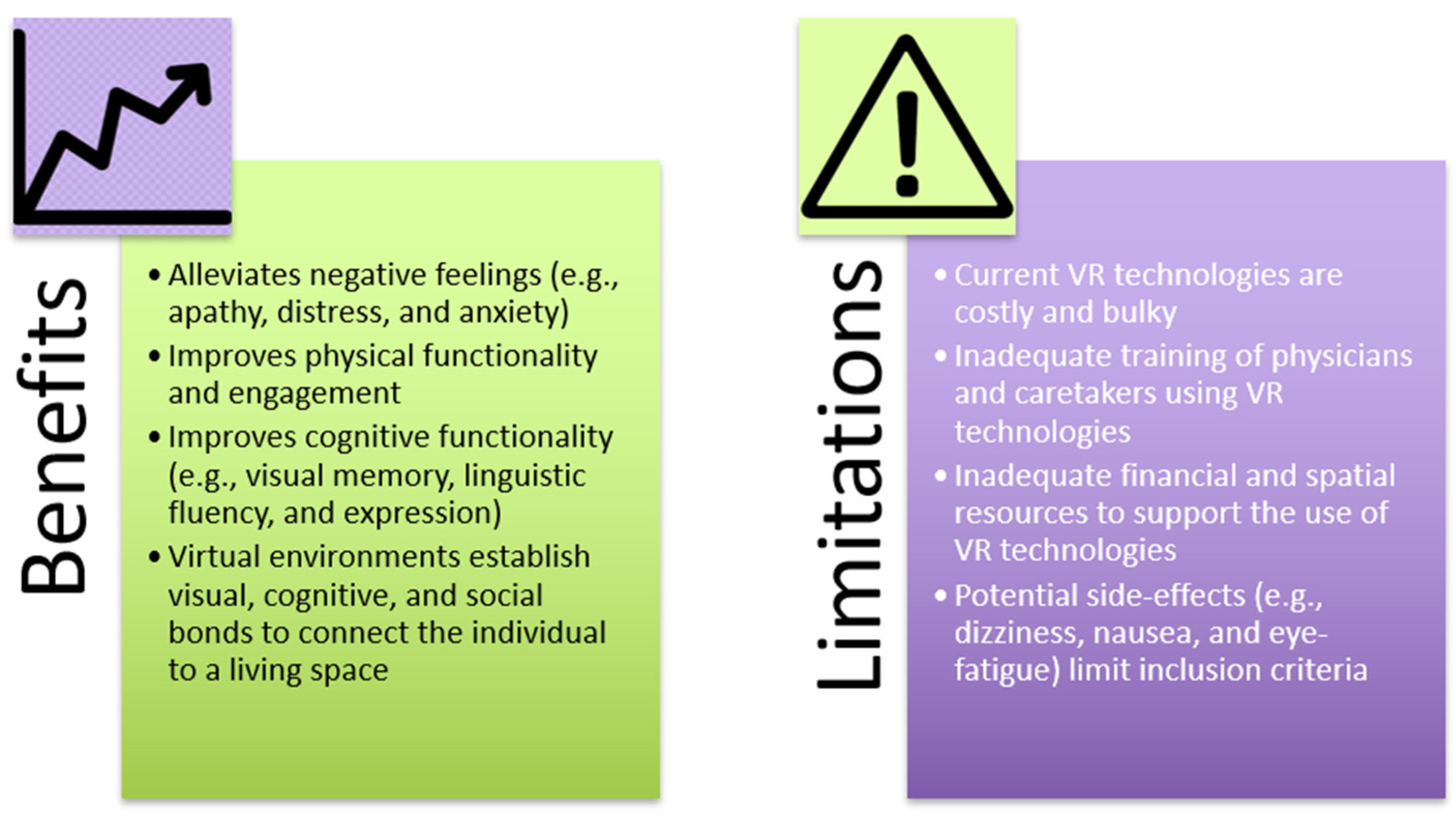
4. VR’s Effect on Depression and Mood in Older People
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, A.; Montano, Z.; Chen, V.J.; Gold, J.I. Virtual reality and pain management: Current trends and future directions. Pain Manag. 2011, 1, 147–157. [Google Scholar] [CrossRef]
- Bouchard, S.; Rizzo, A. Applications of Virtual Reality in Clinical Psychology and Clinical Cognitive Neuroscience–An Introduction. In Virtual Reality for Psychological and Neurocognitive Interventions; Rizzo, A., Bouchard, S., Eds.; Springer: New York, NY, USA, 2019; pp. 1–13. [Google Scholar] [CrossRef]
- Valmaggia, L.R.; Latif, L.; Kempton, M.J.; Rus-Calafell, M. Virtual reality in the psychological treatment for mental health problems: An systematic review of recent evidence. Psychiatry Res. 2016, 236, 189–195. [Google Scholar] [CrossRef]
- Norcross, J.C.; Pfund, R.A.; Prochaska, J.O. Psychotherapy in 2022: A Delphi poll on its future. Prof. Psychol. Res. Pract. 2013, 44, 363–370. [Google Scholar] [CrossRef]
- Rothbaum, B.O.; Hodges, L.F.; Kooper, R.; Opdyke, D.; Williford, J.S.; North, M. Effectiveness of computer-generated (virtual reality) graded exposure in the treatment of acrophobia. Am. J. Psychiatry 1995, 152, 626–628. [Google Scholar] [CrossRef]
- Park, M.J.; Kim, D.J.; Lee, U.; Na, E.J.; Jeon, H.J. A Literature Overview of Virtual Reality (VR) in Treatment of Psychiatric Disorders: Recent Advances and Limitations. Front. Psychiatry 2019, 10, 505. [Google Scholar] [CrossRef] [PubMed]
- Aging, Autonomy, and Architecture: Advances in Assisted Living; Schwarz, B., Brent, R., Eds.; The Johns Hopkins University Press: Baltimore, MD, USA, 1999. [Google Scholar]
- Kopec, D. Environmental Psychology for Design, 1st ed.; Fairchild Books: New York, NY, USA, 2012. [Google Scholar]
- Ortman, J.M.; Velkoff, V.A.; Hogan, H. An Aging Nation: The Older Population in the United States; US Census Bureau: Suitland, MD, USA, 2014. [Google Scholar]
- Choi, N.G.; Ransom, S.; Wyllie, R.J. Depression in older nursing home residents: The influence of nursing home environmental stressors, coping, and acceptance of group and individual therapy. Aging Ment. Health 2008, 12, 536–547. [Google Scholar] [CrossRef] [PubMed]
- De Mendonça Lima, C.A.; Ivbijaro, G. Mental health and wellbeing of older people: Opportunities and challenges. Ment. Health Fam. Med. 2013, 10, 125–127. [Google Scholar]
- Roy, N.; Dube, R.; Despres, C.; Freitas, A.; Legare, F. Choosing between staying at home or moving: A systematic review of factors influencing housing decisions among frail older adults. PLoS ONE 2018, 13, e0189266. [Google Scholar] [CrossRef]
- Dictionary.com. Nostalgia. Available online: https://www.dictionary.com/browse/nostalgia#:~:text=a%20wistful%20desire%20to%20return,that%20elicits%20or%20displays%20nostalgia (accessed on 28 February 2021).
- Environmental Gerontology: Making Meaningful Places in Old Age; Rowles, G.D., Bernard, M., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Place Attachment: Advances in Theory, Methods and Applications; Manzo, L.C., Devine-Wright, P., Eds.; Routledge Inc.: London, UK, 2014. [Google Scholar]
- Scannell, L.; Gifford, R. Defining place attachment: A tripartite organizing framework. J. Environ. Psychol. 2010, 30, 1–10. [Google Scholar] [CrossRef]
- Wright, C.J. Homelike Variables and Rates of Depression among Assisted Living Facility Residents; St. Catherine University: St. Paul, MN, USA, 2014. [Google Scholar]
- Schumacher, K.L.; Jones, P.S.; Meleis, A.I. Helping Elderly Persons in Transition: A Framework for Research and Practice. In Life Transitions in the Older Adult: Issues for Nurses and Other Health Professionals; Swanson, E.A., Tripp-Reimer, T., Eds.; Springer: New York, NY, USA, 1999; pp. 1–26. [Google Scholar]
- Stevens, A.K.; Raphael, H.; Green, S.M. A qualitative study of older people with minimal care needs experiences of their admission to a nursing home with Registered Nurse care. Qual. Ageing Older Adults 2015, 16, 94–105. [Google Scholar] [CrossRef]
- Scannell, L.; Gifford, R. The experienced psychological benefits of place attachment. J. Environ. Psychol. 2017, 51, 256–269. [Google Scholar] [CrossRef]
- Abela, J.R.; D’Alessandro, D.U. Beck’s cognitive theory of depression: A test of the diathesis-stress and causal mediation components. Br. J. Clin. Psychol. 2002, 41, 111–128. [Google Scholar] [CrossRef]
- Singh, M.K.; Gotlib, I.H. The neuroscience of depression: Implications for assessment and intervention. Behav. Res. Ther. 2014, 62, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Kendler, K.S.; Karkowski, L.M.; Prescott, C.A. Causal relationship between stressful life events and the onset of major depression. Am. J. Psychiatry 1999, 156, 837–841. [Google Scholar] [CrossRef]
- Pariante, C.M.; Lightman, S.L. The HPA axis in major depression: Classical theories and new developments. Trends Neurosci. 2008, 31, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Cowen, P.J. Not fade away: The HPA axis and depression. Psychol. Med. 2010, 40, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Nutt, D.; Demyttenaere, K.; Janka, Z.; Aarre, T.; Bourin, M.; Canonico, P.L.; Carrasco, J.L.; Stahl, S. The other face of depression, reduced positive affect: The role of catecholamines in causation and cure. J. Psychopharmacol. 2007, 21, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.S.; Oeltzschner, G.; Gould, N.F.; Leoutsakos, J.S.; Nassery, N.; Joo, J.H.; Kraut, M.A.; Edden, R.A.E.; Barker, P.B.; Wijtenburg, S.A.; et al. Neurotransmitters and Neurometabolites in Late-Life Depression: A Preliminary Magnetic Resonance Spectroscopy Study at 7T. J. Affect. Disord. 2021, 279, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Vreeburg, S.A.; Hoogendijk, W.J.; van Pelt, J.; Derijk, R.H.; Verhagen, J.C.; van Dyck, R.; Smit, J.H.; Zitman, F.G.; Penninx, B.W. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: Results from a large cohort study. Arch. Gen. Psychiatry 2009, 66, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Holsen, L.M.; Lancaster, K.; Klibanski, A.; Whitfield-Gabrieli, S.; Cherkerzian, S.; Buka, S.; Goldstein, J.M. HPA-axis hormone modulation of stress response circuitry activity in women with remitted major depression. Neuroscience 2013, 250, 733–742. [Google Scholar] [CrossRef]
- Krogh, J.; Videbech, P.; Renvillard, S.G.; Garde, A.H.; Jorgensen, M.B.; Nordentoft, M. Cognition and HPA axis reactivity in mildly to moderately depressed outpatients: A case-control study. Nord. J. Psychiatry 2012, 66, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.; Gallagher, P.; Del-Estal, D.; Hearn, A.; Ferrier, I.N.; Young, A.H. Hypothalamic-pituitary-adrenal axis function in patients with chronic depression. Psychol. Med. 2002, 32, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Stetler, C.; Miller, G.E. Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosom. Med. 2011, 73, 114–126. [Google Scholar] [CrossRef]
- Nandam, L.S.; Brazel, M.; Zhou, M.; Jhaveri, D.J. Cortisol and Major Depressive Disorder-Translating Findings from Humans to Animal Models and Back. Front. Psychiatry 2019, 10, 974. [Google Scholar] [CrossRef] [PubMed]
- Peacock, B.N.; Scheiderer, D.J.; Kellermann, G.H. Biomolecular aspects of depression: A retrospective analysis. Compr. Psychiatry 2017, 73, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Strawbridge, R.; Young, A.H.; Cleare, A.J. Biomarkers for depression: Recent insights, current challenges and future prospects. Neuropsychiatr. Dis. Treat. 2017, 13, 1245–1262. [Google Scholar] [CrossRef] [PubMed]
- Maurer, D.M.; Raymond, T.J.; Davis, B.N. Depression: Screening and diagnosis. Am. Fam. Physician 2018, 98, 508–515. [Google Scholar] [PubMed]
- Eaton, W.W.; Neufeld, K.; Chen, L.S.; Cai, G. A comparison of self-report and clinical diagnostic interviews for depression: Diagnostic interview schedule and schedules for clinical assessment in neuropsychiatry in the Baltimore epidemiologic catchment area follow-up. Arch. Gen. Psychiatry 2000, 57, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Chun, A.; Reinhardt, J.P.; Ramirez, M.; Ellis, J.M.; Silver, S.; Burack, O.; Eimicke, J.P.; Cimarolli, V.; Teresi, J.A. Depression recognition and capacity for self-report among ethnically diverse nursing homes residents: Evidence of disparities in screening. J. Clin. Nurs. 2017, 26, 4915–4926. [Google Scholar] [CrossRef]
- Goodarzi, Z.S.; Mele, B.S.; Roberts, D.J.; Holroyd-Leduc, J. Depression Case Finding in Individuals with Dementia: A Systematic Review and Meta-Analysis. J. Am. Geriatr. Soc. 2017, 65, 937–948. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Alexopoulos, G.S.; Abrams, R.C.; Young, R.C.; Shamoian, C.A. Cornell scale for depression in dementia. Biol. Psychiatry 1988, 23, 271–284. [Google Scholar] [CrossRef]
- Balsamo, M.; Cataldi, F.; Carlucci, L.; Padulo, C.; Fairfield, B. Assessment of late-life depression via self-report measures: A review. Clin. Interv. Aging 2018, 13, 2021–2044. [Google Scholar] [CrossRef] [PubMed]
- Most, E.I.; Scheltens, P.; Van Someren, E.J. Prevention of depression and sleep disturbances in elderly with memory-problems by activation of the biological clock with light—A randomized clinical trial. Trials 2010, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Drageset, J.; Eide, G.E.; Ranhoff, A.H. Anxiety and depression among nursing home residents without cognitive impairment. Scand. J. Caring Sci. 2013, 27, 872–881. [Google Scholar] [CrossRef]
- Conejero, I.; Olie, E.; Courtet, P.; Calati, R. Suicide in older adults: Current perspectives. Clin. Interv. Aging 2018, 13, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Schimmele, C.M.; Chappell, N.L. Aging and late-life depression. J. Aging Health 2012, 24, 3–28. [Google Scholar] [CrossRef]
- Hickman, S.E.; Barrick, A.L.; Williams, C.S.; Zimmerman, S.; Connell, B.R.; Preisser, J.S.; Mitchell, C.M.; Sloane, P.D. The effect of ambient bright light therapy on depressive symptoms in persons with dementia. J. Am. Geriatr. Soc. 2007, 55, 1817–1824. [Google Scholar] [CrossRef]
- Huang, Y.; Carpenter, I. Identifying elderly depression using the Depression Rating Scale as part of comprehensive standardised care assessment in nursing homes. Aging Ment. Health 2011, 15, 1045–1051. [Google Scholar] [CrossRef]
- Guinjoan, S.M.; Bernabo, J.L.; Cardinali, D.P. Cardiovascular tests of autonomic function and sympathetic skin responses in patients with major depression. J. Neurol. Neurosurg. Psychiatry 1995, 59, 299–302. [Google Scholar] [CrossRef][Green Version]
- Borrione, L.; Brunoni, A.R.; Sampaio-Junior, B.; Aparicio, L.M.; Kemp, A.H.; Bensenor, I.; Lotufo, P.A.; Fraguas, R. Associations between symptoms of depression and heart rate variability: An exploratory study. Psychiatry Res. 2018, 262, 482–487. [Google Scholar] [CrossRef]
- Dascal, J.; Reid, M.; Ishak, W.W.; Spiegel, B.; Recacho, J.; Rosen, B.; Danovitch, I. Virtual reality and medical inpatients: A systematic review of randomized, controlled trials. Innov. Clin. Neurosci. 2017, 14, 14–21. [Google Scholar]
- Madrigal, E.; Prajapati, S.; Hernandez-Prera, J.C. Introducing a Virtual Reality Experience in Anatomic Pathology Education. Am. J. Clin. Pathol. 2016, 146, 462–468. [Google Scholar] [CrossRef]
- Pottle, J. Virtual reality and the transformation of medical education. Future Healthc. J. 2019, 6, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Klein, P.; Uhlig, M.; Will, H. The Touch and Feel of the Past—Using Haptic and VR Artefacts to Enrich Reminiscence Therapy for People with Dementia. Technologies 2018, 6, 104. [Google Scholar] [CrossRef]
- Schultheis, M.T.; Rizzo, A.A. The application of virtual reality technology in rehabilitation. Rehabil. Psychol. 2001, 46, 296–311. [Google Scholar] [CrossRef]
- Yang, J.E.; Lee, T.Y.; Kim, J.K. The effect of a VR exercise program on falls and depression in the elderly with mild depression in the local community. J. Phys. Ther. Sci. 2017, 29, 2157–2159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chan, J.Y.C.; Chan, T.K.; Wong, M.P.F.; Cheung, R.S.M.; Yiu, K.K.L.; Tsoi, K.K.F. Effects of virtual reality on moods in community older adults. A multicenter randomized controlled trial. Int. J. Geriatr. Psychiatry 2020, 35, 926–933. [Google Scholar] [CrossRef]
- Graf, L.; Liszio, S.; Masuch, M. Playing in virtual nature: Improving mood of elderly people using VR technology. In Proceedings of the Conference on Mensch und Computer (MuC ’20), Munich, Germany, 6 September 2020; pp. 155–164. [Google Scholar]
- Baños, R.M.; Etchemendy, E.; Castilla, D.; García-Palacios, A.; Quero, S.; Botella, C. Positive mood induction procedures for virtual environments designed for elderly people. Interact. Comput. 2012, 24, 131–138. [Google Scholar] [CrossRef]
- Barsasella, D.; Liu, M.F.; Malwade, S.; Galvin, C.J.; Dhar, E.; Chang, C.C.; Li, Y.J.; Syed-Abdul, S. Effects of Virtual Reality Sessions on the Quality of Life, Happiness, and Functional Fitness among the Older People: A Randomized Controlled Trial from Taiwan. Comput. Methods Programs Biomed. 2020. [Google Scholar] [CrossRef]
- Gamito, P.; Oliveira, J.; Alves, C.; Santos, N.; Coelho, C.; Brito, R. Virtual Reality-Based Cognitive Stimulation to Improve Cognitive Functioning in Community Elderly: A Controlled Study. Cyberpsychol. Behav. Soc. Netw. 2020, 23, 150–156. [Google Scholar] [CrossRef]
- Brimelow, R.E.; Dawe, B.; Dissanayaka, N. Preliminary Research: Virtual Reality in Residential Aged Care to Reduce Apathy and Improve Mood. Cyberpsychol. Behav. Soc. Netw. 2020, 23, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Saredakis, D.; Keage, H.A.; Corlis, M.; Loetscher, T. Using Virtual Reality to Improve Apathy in Residential Aged Care: Mixed Methods Study. J. Med. Internet Res. 2020, 22, e17632. [Google Scholar] [CrossRef] [PubMed]
- D’Cunha, N.M.; Isbel, S.T.; Frost, J.; Fearon, A.; McKune, A.J.; Naumovski, N.; Kellett, J. Effects of a virtual group cycling experience on people living with dementia: A mixed method pilot study. Dement. Lond. 2020. [Google Scholar] [CrossRef]
- Lavoie, R.; Main, K.; King, C.; King, D. Virtual experience, real consequences: The potential negative emotional consequences of virtual reality gameplay. Virtual Real. 2020, 25, 69–81. [Google Scholar] [CrossRef]
- Garrett, B.; Taverner, T.; Gromala, D.; Tao, G.; Cordingley, E.; Sun, C. Virtual Reality Clinical Research: Promises and Challenges. JMIR Serious Games 2018, 6, e10839. [Google Scholar] [CrossRef]
- Baniasadi, T.; Ayyoubzadeh, S.M.; Mohammadzadeh, N. Challenges and Practical Considerations in Applying Virtual Reality in Medical Education and Treatment. Oman Med. J. 2020, 35, e125. [Google Scholar] [CrossRef]
- Wiederhold, B.K.; Riva, G. Virtual Reality Therapy: Emerging Topics and Future Challenges. Cyberpsychol. Behav. Soc. Netw. 2019, 22, 3–6. [Google Scholar] [CrossRef]
- Mesa-Gresa, P.; Gil-Gomez, H.; Lozano-Quilis, J.A.; Gil-Gomez, J.A. Effectiveness of Virtual Reality for Children and Adolescents with Autism Spectrum Disorder: An Evidence-Based Systematic Review. Sensors 2018, 18, 2486. [Google Scholar] [CrossRef]
- Klepeis, N.E.; Nelson, W.C.; Ott, W.R.; Robinson, J.P.; Tsang, A.M.; Switzer, P.; Behar, J.V.; Hern, S.C.; Engelmann, W.H. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Anal. Environ. Epidemiol. 2001, 11, 231–252. [Google Scholar] [CrossRef]
- Chiamulera, C.; Ferrandi, E.; Benvegnu, G.; Ferraro, S.; Tommasi, F.; Maris, B.; Zandonai, T.; Bosi, S. Virtual Reality for Neuroarchitecture: Cue Reactivity in Built Spaces. Front. Psychol. 2017, 8, 185. [Google Scholar] [CrossRef] [PubMed]

| Participants | VR Experience | Study Design | Findings | Source |
|---|---|---|---|---|
| RACF residents: 13 total (9 females and 4 males); 66–93 years old. | Smartphone-based VR experience with relaxing videos (e.g., nature). | All participants exposed once to the VR experience. Measurements taken before and after. | ↓ total apathy ↑ facial expression, eye contact, physical engagement, verbal tone, verbal expression. | Brimelow et al., 2020 [62] |
| Members of 19 community centers: 236 total (180 females and 56 males); 60+ years old. | VR cognitive stimulation experience: virtual tour of Hong Kong’s famous sites. | Trial group exposed once to the VR experience. Control group exposed to paper-and-pencil cognitive stimulation activity. | ↑ total positive affect (e.g., interested, excited, strong, alert, determined) ↓ total negative affect (e.g., distressed, upset, guilty, hostile). | Chan et al., 2020 [57] |
| RACF residents: 10 total (8 females and 2 males); 75–94 years old. | VR immersive biking experience: included both a video and a stationary pedal system to follow along. | Trial group exposed once to the VR experience. Control group exposed to a standard occupational therapy activity. | No significant measured changes in mood or apathy. Subjective experience: most participants enjoyed the scenery and reminisced about previous biking memories. | D’Cunha et al., 2020 [64] |
| University Aging Center patients: 60 total (46 females and 14 males); 60–94 years old. | Headset-based VR experience with nine apps, each involving high, low, or very low intensity movement. | Trial group exposed to biweekly VR experiences for 6 weeks. Control group received no intervention. | ↑ happiness Greater EQ-5D improvement after VR therapy than in controls | Barsasella et al., 2020 [60] |
| Daycare center users: 43 total (34 females and 9 males); 67–87 years old | VR cognitive stimulation experience: increasingly complex attention, memory, and executive tasks in a virtual city environment. | Trial group exposed to biweekly VR experiences for 6 weeks. Control group exposed to weekly paper-and-pencil cognitive stimulation for 6 weeks. | ↑ general, visual memory ↑ attention | Gamito et al., 2020 [61] |
| Senior University participants: 18 total (14 females and 4 males); 58–79 years old | Two natural virtual environment (VE) experiences: (1) joy-inducing VE; (2) relaxation-inducing VE. | All participants exposed to one or both VEs; one to three total exposures. Measurements taken before and after. | For both VEs: ↑ joy, relaxation ↓ sadness, anxiety | Banos et al., 2012 [59] |
| Pensioners residing at home: 14 total (8 females and 6 males); 66–84 years old | Headset-based VR forest walk experience with embedded mini-games. | All participants exposed once to the VR experience. Measurements taken before and after. | ↑ total positive affect | Graf et al., 2020 [58] |
| RACF residents: 17 total (10 females and 7 males); 72–95 years old | Headset-based VR wandering experience based on Google Street View. | All participants exposed twice to the VR experience. Measurements taken before and after. | Correlation between ↓ apathy (after VR exposure) and ↑ semantic fluency | Saredakis et al., 2020 [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, K.; Dilawar, A.; Yousef, M.S.; Holroyd, S.; El-Hammali, H.; Abdelmonem, M. Virtual Reality Therapy for Depression and Mood in Long-Term Care Facilities. Geriatrics 2021, 6, 58. https://doi.org/10.3390/geriatrics6020058
Zhai K, Dilawar A, Yousef MS, Holroyd S, El-Hammali H, Abdelmonem M. Virtual Reality Therapy for Depression and Mood in Long-Term Care Facilities. Geriatrics. 2021; 6(2):58. https://doi.org/10.3390/geriatrics6020058
Chicago/Turabian StyleZhai, Kevin, Azwa Dilawar, Mohammad S. Yousef, Sean Holroyd, Haithem El-Hammali, and Marwa Abdelmonem. 2021. "Virtual Reality Therapy for Depression and Mood in Long-Term Care Facilities" Geriatrics 6, no. 2: 58. https://doi.org/10.3390/geriatrics6020058
APA StyleZhai, K., Dilawar, A., Yousef, M. S., Holroyd, S., El-Hammali, H., & Abdelmonem, M. (2021). Virtual Reality Therapy for Depression and Mood in Long-Term Care Facilities. Geriatrics, 6(2), 58. https://doi.org/10.3390/geriatrics6020058







