Eight Orthostatic Haemodynamic Patterns in The Irish Longitudinal Study on Ageing (TILDA): Stability and Clinical Associations after 4 Years
Abstract
1. Introduction
2. Methods
2.1. Sample
2.2. Active Stand Protocol
2.3. Longitudinal Variables
2.4. Clinical Characterisation Variables
2.5. Statistical Analyses
2.6. Ethics
3. Results
3.1. Morphological Pattern Stability, Clinical Characteristics, and Longitudinal Associations
3.2. Attrition Characteristics
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Freeman, R.; Wieling, W.; Axelrod, F.B.; Benditt, D.G.; Benarroch, E.; Biaggioni, I.; Cheshire, W.P.; Chelimsky, T.; Cortelli, P.; Gibbons, C.H.; et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin. Auton. Res. 2011, 21, 69–72. [Google Scholar] [CrossRef]
- Naschitz, J.E.; Rosner, I. Orthostatic hypotension: Framework of the syndrome. Postgrad. Med J. 2007, 83, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.; Kenny, R.A. Reproducibility of orthostatic hypotension in symptomatic elderly. Am. J. Med. 1996, 100, 418–422. [Google Scholar] [CrossRef]
- Ooi, W.L.; Barrett, S.; Hossain, M.; Kelley-Gagnon, M.; Lipsitz, L.A. Patterns of orthostatic blood pressure change and their clinical correlates in a frail, elderly population. JAMA 1997, 277, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Grossman, E.; Beloosesky, Y.; Grinblat, J. Orthostatic hypotension in acute geriatric ward: Is it a consistent finding? Arch. Intern. Med. 2002, 162, 2369–2374. [Google Scholar] [CrossRef] [PubMed]
- Belmin, J.; Abderrhamane, M.; Medjahed, S.; Sibony-Prat, J.; Bruhat, A.; Bojic, N.; Marquet, T. Variability of Blood Pressure Response to Orthostatism and Reproducibility of the Diagnosis of Orthostatic Hypotension in Elderly Subjects. J. Gerontol. Ser. A 2000, 55, M667–M671. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Beloosesky, Y.; Grinblat, J.; Grossman, E. Seasonal changes in orthostatic hypotension among elderly admitted patients. Aging Clin. Exp. Res. 2006, 18, 20–24. [Google Scholar] [CrossRef]
- Finucane, C.; Savva, G.M.; Kenny, R.A. Reliability of orthostatic beat-to-beat blood pressure tests: Implications for population and clinical studies. Clin. Auton. Res. 2017, 27, 31–39. [Google Scholar] [CrossRef]
- Stewart, J.M. Transient orthostatic hypotension is common in adolescents. J. Pediatr. 2002, 140, 418–424. [Google Scholar] [CrossRef]
- Molnar, F.; Frank, C.C. Determining the causes of postural hypotension. Can. Fam. Physician 2018, 64, 40. [Google Scholar]
- Kleipool, E.E.F.; Trappenburg, M.C.; Rhodius-Meester, H.F.M.; Lemstra, A.W.; van der Flier, W.M.; Peters, M.J.L.; Muller, M. Orthostatic Hypotension: An Important Risk Factor for Clinical Progression to Mild Cognitive Impairment or Dementia. The Amsterdam Dementia Cohort. J. Alzheimer’s Dis. JAD 2019, 71, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Wolters, F.J.; Mattace-Raso, F.U.; Koudstaal, P.J.; Hofman, A.; Ikram, M.A. Orthostatic Hypotension and the Long-Term Risk of Dementia: A Population-Based Study. PLoS Med. 2016, 13, e1002143. [Google Scholar] [CrossRef] [PubMed]
- Moloney, D.; O’Connor, J.; Newman, L.; Scarlett, S.; Hernandez, B.; Kenny, R.A.; Romero-Ortuno, R. Clinical clustering of eight orthostatic haemodynamic patterns in The Irish Longitudinal Study on Ageing (TILDA). Age Ageing 2020, 50, 854–860. [Google Scholar] [CrossRef]
- Whelan, B.J.; Savva, G.M. Design and methodology of the Irish Longitudinal Study on Ageing. J. Am. Geriatr. Soc. 2013, 61 (Suppl. 2), S265–S268. [Google Scholar] [CrossRef]
- Whelan, B. RANSAM: A random sample design for Ireland. J. Econ. Soc. Rev. 1979, 10, 169–174. [Google Scholar]
- TILDA. The Design of the Irish Longitudinal Study on Ageing. Available online: https://tilda.tcd.ie/publications/reports/pdf/Report_DesignReport.pdf (accessed on 20 April 2021).
- TILDA. Cohort Maintenance Strategies Used by The Irish Longitudinal Study on Ageing (TILDA). Available online: https://tilda.tcd.ie/publications/reports/pdf/Report_CohortMaintenance.pdf (accessed on 20 April 2021).
- Donoghue, O.A.; McGarrigle, C.A.; Foley, M.; Fagan, A.; Meaney, J.; Kenny, R.A. Cohort Profile Update: The Irish Longitudinal Study on Ageing (TILDA). Int. J. Epidemiol. 2018, 47, 1398–1398l. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.D.; O’Connell, M.D.L.; Nolan, H.; Newman, L.; Knight, S.P.; Kenny, R.A. Impact of Standing Speed on the Peripheral and Central Hemodynamic Response to Orthostasis: Evidence From the Irish Longitudinal Study on Ageing. Hypertension 2020, 75, 524–531. [Google Scholar] [CrossRef]
- Andrews, J.S.; Desai, U.; Kirson, N.Y.; Zichlin, M.L.; Ball, D.E.; Matthews, B.R. Disease severity and minimal clinically important differences in clinical outcome assessments for Alzheimer’s disease clinical trials. Alzheimer’s Dement. 2019, 5, 354–363. [Google Scholar] [CrossRef]
- Ward, M.; May, P.; Briggs, R.; McNicholas, T.; Normand, C.; Kenny, R.A.; Nolan, A. Linking death registration and survey data: Procedures and cohort profile for The Irish Longitudinal Study on Ageing. HRB Open Res. 2020, 3, 43. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Kantor, E.D.; Rehm, C.D.; Haas, J.S.; Chan, A.T.; Giovannucci, E.L. Trends in Prescription Drug Use Among Adults in the United States From 1999-2012. JAMA 2015, 314, 1818–1831. [Google Scholar] [CrossRef] [PubMed]
- Divo, M.J.; Martinez, C.H.; Mannino, D.M. Ageing and the epidemiology of multimorbidity. Eur. Respir. J. 2014, 44, 1055–1068. [Google Scholar] [CrossRef]
- Saedon, N.I.; Frith, J.; Goh, C.H.; Ahmad, W.A.W.; Khor, H.M.; Tan, K.M.; Chin, A.V.; Kamaruzzaman, S.B.; Tan, M.P. Orthostatic blood pressure changes and physical, functional and cognitive performance: The MELoR study. Clin. Auton. Res. 2020, 30, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Puisieux, F.; Boumbar, Y.; Bulckaen, H.; Bonnin, E.; Houssin, F.; Dewailly, P. Intraindividual Variability in Orthostatic Blood Pressure Changes Among Older Adults: The Influence of Meals. J. Am. Geriatr. Soc. 1999, 47, 1332–1336. [Google Scholar] [CrossRef] [PubMed]
- Webber, B.J.; Cropper, T.L.; Federinko, S.P. Syncope among U.S. Air Force basic military trainees, August 2012–July 2013. MSMR 2013, 20, 2–4. [Google Scholar]
- Kamaruzzaman, S.; Watt, H.; Carson, C.; Ebrahim, S. The association between orthostatic hypotension and medication use in the British Women’s Heart and Health Study. Age Ageing 2010, 39, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Holm, H.; Nägga, K.; Nilsson, E.D.; Melander, O.; Minthon, L.; Bachus, E.; Fedorowski, A.; Magnusson, M. Longitudinal and postural changes of blood pressure predict dementia: The Malmö Preventive Project. Eur. J. Epidemiol. 2017, 32, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Cremer, A.; Soumaré, A.; Berr, C.; Dartigues, J.F.; Gabelle, A.; Gosse, P.; Tzourio, C. Orthostatic Hypotension and Risk of Incident Dementia: Results From a 12-Year Follow-Up of the Three-City Study Cohort. Hypertension 2017, 70, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Romero-Ortuno, R.; Cogan, L.; Foran, T.; Kenny, R.A.; Fan, C.W. Continuous noninvasive orthostatic blood pressure measurements and their relationship with orthostatic intolerance, falls, and frailty in older people. J. Am. Geriatr. Soc. 2011, 59, 655–665. [Google Scholar] [CrossRef]
- Masud, T.; Morris, R.O. Epidemiology of falls. Age Ageing 2001, 30 (Suppl. 4), 3–7. [Google Scholar] [CrossRef]
- Graafmans, W.C.; Ooms, M.E.; Hofstee, H.M.; Bezemer, P.D.; Bouter, L.M.; Lips, P. Falls in the elderly: A prospective study of risk factors and risk profiles. Am. J. Epidemiol. 1996, 143, 1129–1136. [Google Scholar] [CrossRef]
- Juraschek Stephen, P.; Simpson Lara, M.; Davis Barry, R.; Beach Jennifer, L.; Ishak, A.; Mukamal Kenneth, J. Effects of Antihypertensive Class on Falls, Syncope, and Orthostatic Hypotension in Older Adults. Hypertension 2019, 74, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- de Vries, M.; Seppala, L.J.; Daams, J.G.; van de Glind, E.M.M.; Masud, T.; van der Velde, N.; Blain, H.; Bousquet, J.; Bucht, G.; Caballero-Mora, M.A.; et al. Fall-Risk-Increasing Drugs: A Systematic Review and Meta-Analysis: I. Cardiovascular Drugs. J. Am. Med. Dir. Assoc. 2018, 19, 371.e1–371.e9. [Google Scholar] [CrossRef]
- Juraschek, S.P.; Hu, J.-R.; Cluett, J.L.; Ishak, A.; Mita, C.; Lipsitz, L.A.; Appel, L.J.; Beckett, N.S.; Coleman, R.L.; Cushman, W.C.; et al. Effects of Intensive Blood Pressure Treatment on Orthostatic Hypotension. Ann. Intern. Med. 2020, 174, 58–68. [Google Scholar] [CrossRef]
- Ylitalo, A.; Airaksinen, K.E.J.; Sellin, L.; Huikuri, H.V. Effects of combination antihypertensive therapy on baroreflex sensitivity and heart rate variability in systemic hypertension. Am. J. Cardiol. 1999, 83, 885–889. [Google Scholar] [CrossRef]
- Kumagai, K.; Suzuki, H.; Ichikawa, M.; Jimbo, M.; Nishizawa, M.; Ryuzaki, M.; Saruta, T. Comparison of Early and Late Start of Antihypertensive Agents and Baroreceptor Reflexes. Hypertension 1996, 27, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Lipsitz Lewis, A.; Gagnon, M.; Vyas, M.; Iloputaife, I.; Kiely Dan, K.; Sorond, F.; Serrador, J.; Cheng Debbie, M.; Babikian, V.; Cupples, L.A. Antihypertensive Therapy Increases Cerebral Blood Flow and Carotid Distensibility in Hypertensive Elderly Subjects. Hypertension 2005, 45, 216–221. [Google Scholar] [CrossRef]
- Dent, E.; Martin, F.C.; Bergman, H.; Woo, J.; Romero-Ortuno, R.; Walston, J.D. Management of frailty: Opportunities, challenges, and future directions. Lancet 2019, 394, 1376–1386. [Google Scholar] [CrossRef]
- Winker, R.; Barth, A.; Bidmon, D.; Ponocny, I.; Weber, M.; Mayr, O.; Robertson, D.; Diedrich, A.; Maier, R.; Pilger, A.; et al. Endurance exercise training in orthostatic intolerance: A randomized, controlled trial. Hypertension 2005, 45, 391–398. [Google Scholar] [CrossRef]
- Alty, J.; Farrow, M.; Lawler, K. Exercise and dementia prevention. Pract. Neurol. 2020, 20, 234. [Google Scholar] [CrossRef] [PubMed]
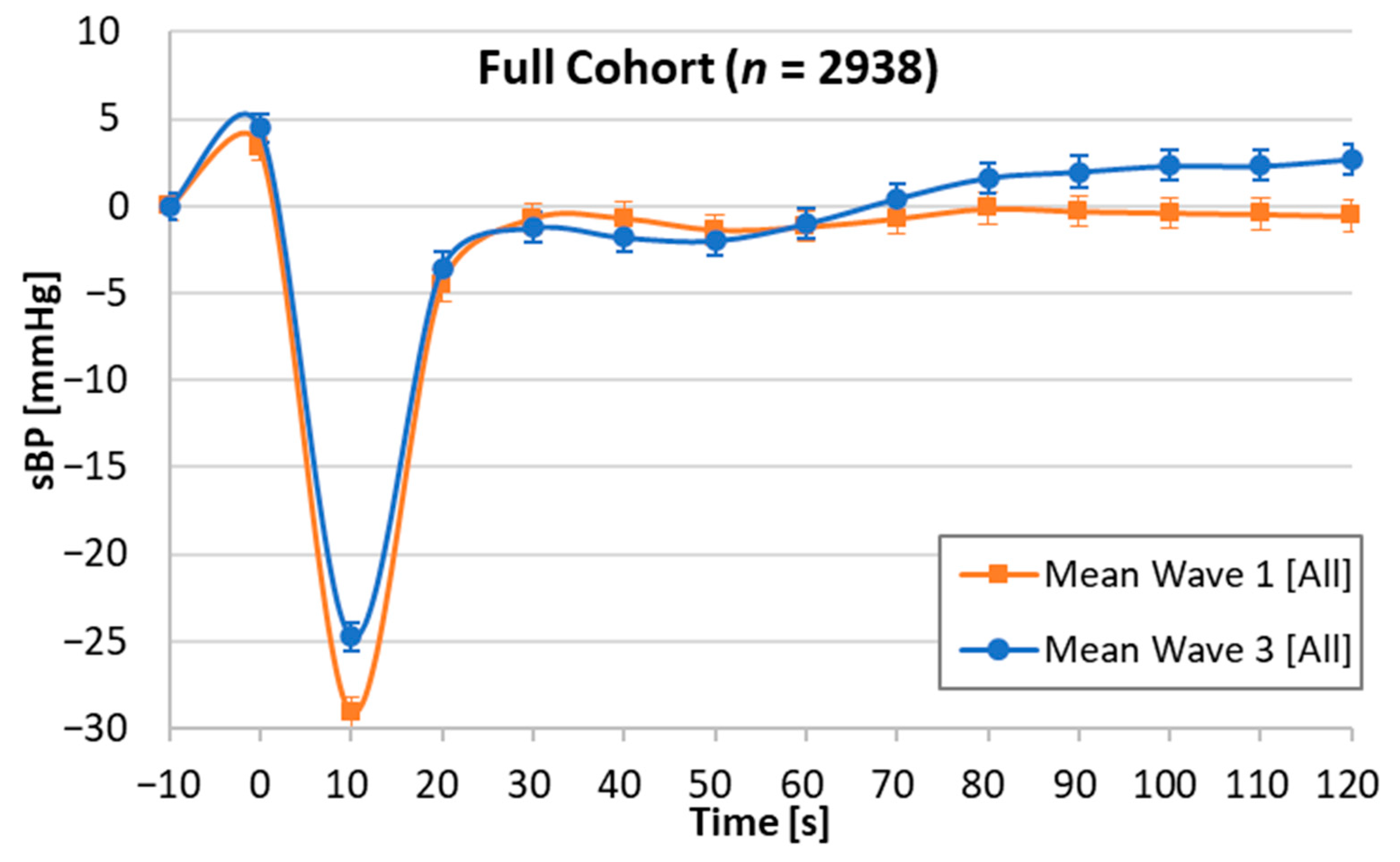
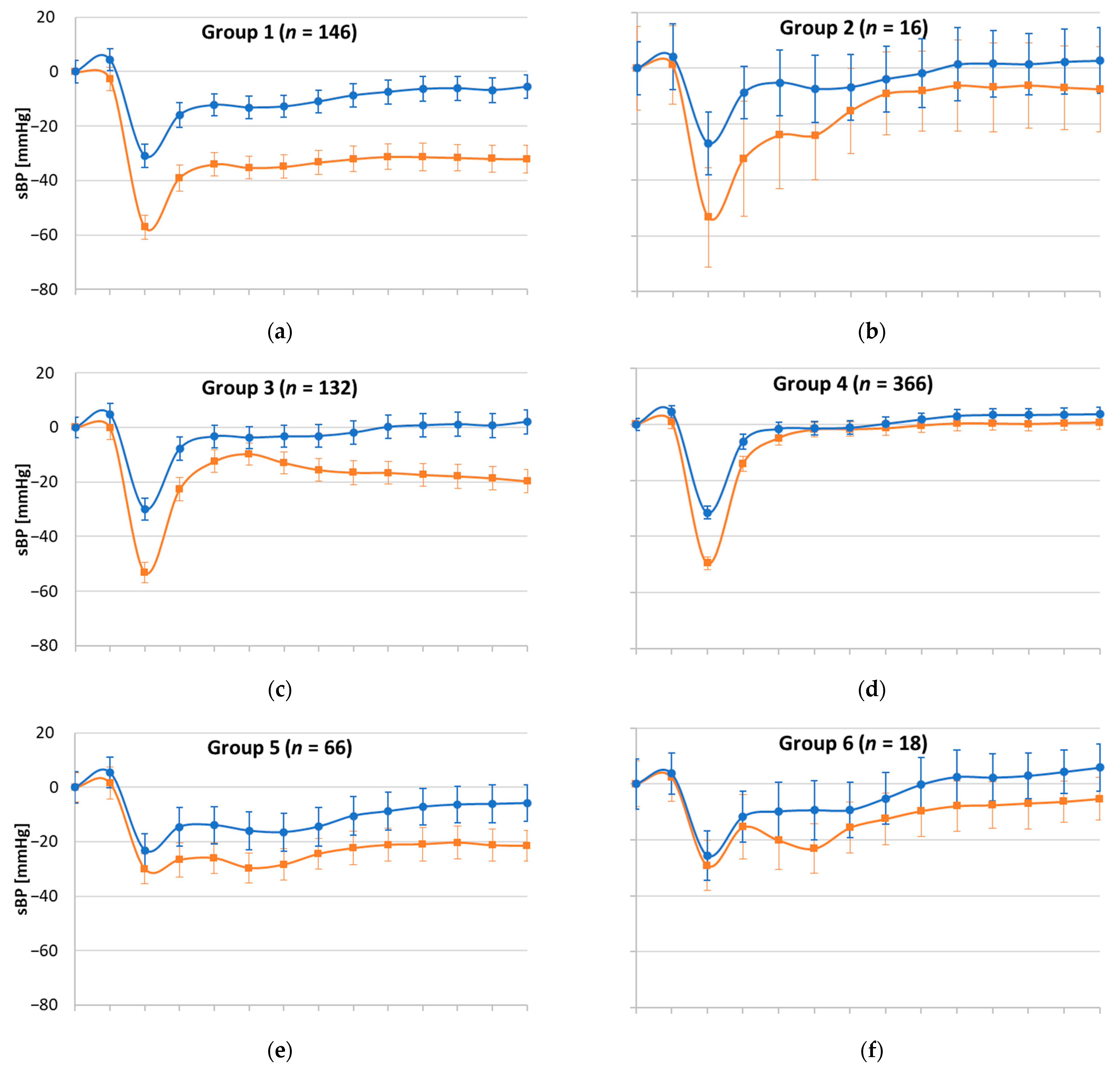
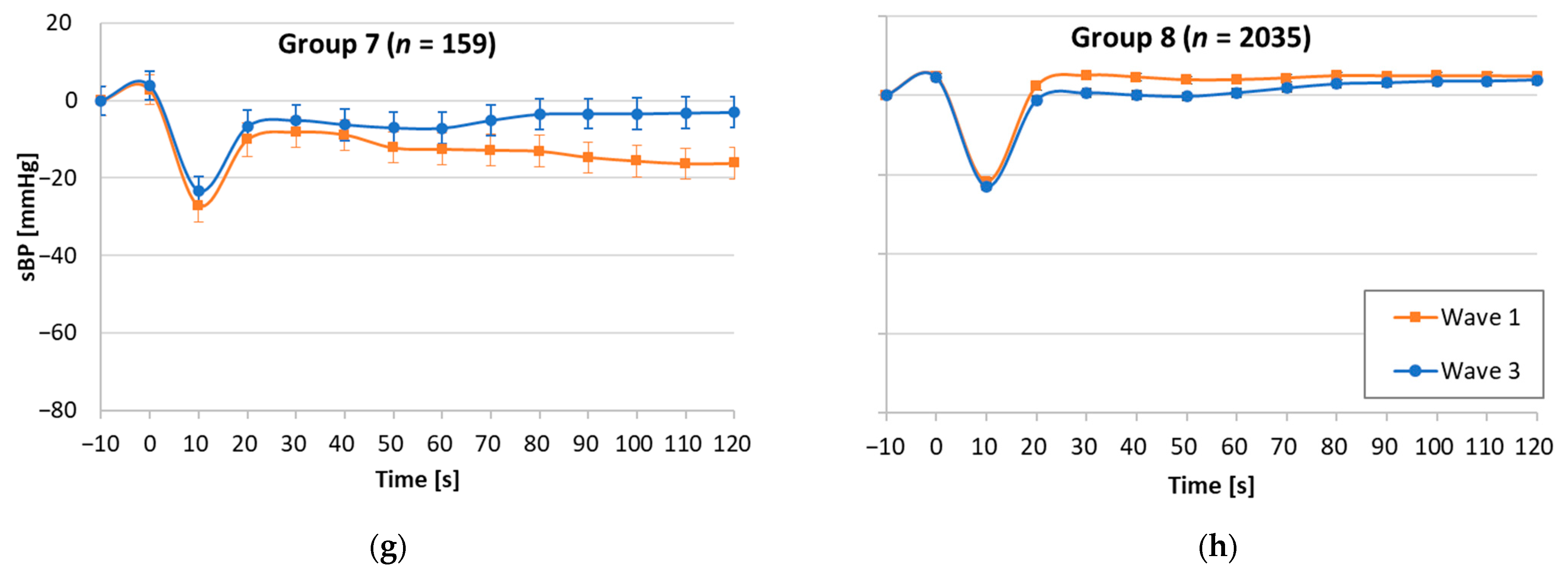
| W1 | W3 | W1 | W3 | W1 | W3 | W1 | W3 | W1 | W3 | W1 | W3 | W1 | W3 | W1 | W3 | W1 | W3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Entire Cohort (n = 2938) | Group 1 (n = 146) | Group 2 (n = 16) | Group 3 (n = 132) | Group 4 (n = 366) | Group 5 (n = 66) | Group 6 (n = 18) | Group 7 (n = 159) | Group 8 (n = 2035) | ||||||||||
| Mean Baseline SBP (SD) [mmHg] | 134.9 (21.9) | 140.7 (24.4) | 148.8 (25.5) | 146.9 (25.0) | 131.0 (30.8) | 141.7 (19.4) | 143.6 (22.6) | 141.3 (21.9) | 139.6 (22.3) | 143.6 (20.1) | 142.1 (22.1) | 141.9 (23.8) | 147.4 (17.9) | 154.5 (19.5) | 145.2 (24.7) | 146.6 (23.8) | 131.4 (20.1) | 139.1 (20.8) |
| % immediate deficit | 23.6 | 14.4 | 100 | 26.0 | 100.0 | 12.5 | 100.0 | 23.5 | 100.0 | 26.5 | 0 | 12.1 | 0 | 22.2 | 0 | 13.8 | 0 | 10.8 |
| % stabilisation deficit | 10.2 | 10.5 | 100 | 34.9 | 100 | 12.5 | 0 | 15.2 | 0 | 10.4 | 100 | 37.9 | 100 | 22.2 | 0 | 13.8 | 0 | 7.2 |
| % late deficit | 17.8 | 17.0 | 100 | 43.2 | 0 | 18.8 | 100 | 21.2 | 0 | 17.5 | 100 | 45.5 | 0 | 16.7 | 100 | 32.1 | 0 | 12.7 |
| Mean time to stand in seconds (SD) | 7.6 (3.0) | 7.2 (2.9) | 7.9 (2.7) | 7.4 (3.2) | 8.3 (3.5) | 7.1 (2.4) | 7.3 (2.3) | 7.2 (2.6) | 7.5 (2.8) | 7.1 (2.7) | 9.7 (4.5) | 8.3 (3.6) | 9.0 (3.8) | 8.9 (5.7) | 8.1 (3.2) | 7.5 (3.4) | 7.5 (2.9) | 7.2 (2.9) |
| Mean age in years (SD) | 59.8 (7.9) | 64.2 (7.9) | 62.8 (9.1) | 67.1 (9.1) | 65.4 (9.6) | 69.9 (9.7) | 59.6 (7.2) | 64.0 (7.2) | 60.5 (7.7) | 65.0 (7.6) | 64.9 (7.9) | 69.2 (7.9) | 64.9 (9.4) | 69.2 (9.6) | 60.1 (8.1) | 64.5 (8.1) | 59.2 (7.7) | 63.5 (7.7) |
| Sex (n (%)) [female] | 1609 (54.8) | 1609 (54.8) | 93 (63.7) | 93 (63.7) | 8 (50.0) | 8 (50.0) | 93 (70.4) | 93 (70.4) | 211 (57.7) | 211 (57.7) | 42 (63.6) | 42 (63.6) | 9 (50.0) | 9 (50.0) | 100 (62.9) | 100 (62.9) | 1053 (51.7) | 1053 (51.7) |
| Multimorbidity (n (%)) | 1239 (42.2) | 1302 (44.4) | 66 (45.2) | 80 (54.8) | 9 (56.3) | 11 (68.8) | 52 (39.4) | 62 (47.0) | 164 (44.8) | 173 (47.4) | 34 (51.5) | 38 (57.6) | 11 (61.1) | 9 (50.0) | 70 (44.0) | 74 (46.5) | 833 (40.9) | 855 (42.1) |
| No. of chronic conditions (mean (SD)) | 1.4 (1.1) | 1.5 (1.2) | 1.4 (1.1) | 1.8 (1.5) | 1.9 (1.2) | 2.0 (1.2) | 1.3 (1.1) | 1.5 (1.3) | 1.4 (1.0) | 1.6 (1.3) | 1.6 (1.2) | 1.9 (1.3) | 1.8 (1.1) | 1.6 (1.0) | 1.4 (1.1) | 1.6 (1.2) | 1.3 (1.1) | 1.4 (1.2) |
| No. of cardiovascular conditions (mean (SD)) | 0.1 (0.5) | 0.1 (0.3) | 0.2 (0.5) | 0.2 (0.4) | 0(0) | 0.2 (0.5) | 0.1 (0.4) | 0.1 (0.4) | 0.2 (0.5) | 0.1 (0.4) | 0.1 (0.4) | 0.1 (0.4) | 0.1 (0.3) | 0.1 (0.3) | 0.1 (0.5) | 0.1 (0.3) | 0.1 (0.4) | 0.1 (0.3) |
| Hypertension (n (%)) | 1090 (37.2) | 1013 (34.5) | 60 (41.1) | 57 (39.0) | 6 (37.5) | 7 (43.8) | 60 (45.5) | 39 (29.6) | 169 (46.6) | 149 (40.8) | 27 (40.9) | 28 (42.4) | 8 (44.4) | 9 (50.0) | 75 (47.2) | 60 (37.7) | 685 (33.7) | 664 (32.7) |
| Diabetes (n (%)) | 147 (5.0) | 190 (6.5) | 10 (6.9) | 14 (9.6) | 1 (6.3) | 1 (6.3) | 5 (3.8) | 9 (6.8) | 16 (4.4) | 17 (4.6) | 3 (4.6) | 3 (4.6) | 2 (11.1) | 3 (16.7) | 6 (3.8) | 7 (4.4) | 104 (5.1) | 136 (6.7) |
| Parkinson’s (n (%)) | 6 (0.2) | 12 (0.4) | 0 (0) | 1 (0.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1.5) | 1 (1.5) | 0 (0) | 0 (0) | 2 (1.3) | 4 (2.5) | 3 (0.2) | 6 (0.3) |
| Binary Frailty (prefrail/frail) (n (%)) | 676 (23.4) | 799 (32.4) | 44 (31.0) | 39 (32.5) | 5 (31.3) | 4 (30.8) | 32 (24.2) | 35 (32.7) | 82 (22.8) | 89 (28.6) | 18 (27.3) | 24 (50.0) | 3 (16.7) | 3 (20.0) | 49 (31.8) | 45 (33.1) | 443 (22.1) | 560 (32.6) |
| Antihypertensives (n (%)) | 803 (27.3) | 1066 (36.3) | 50 (34.3) | 67 (45.9) | 6 (37.5) | 6 (37.5) | 32 (24.2) | 51 (38.6) | 105 (28.7) | 149 (40.7) | 27 (40.9) | 30 (45.5) | 6 (33.3) | 8 (44.4) | 39 (24.5) | 66 (41.5) | 538 (26.4) | 689 (33.9) |
| ACEI/ARB (n (%)) | 536 (18.3) | 747 (25.4) | 27 (18.6) | 39 (26.7) | 4 (25.0) | 4 (25.0) | 18 (13.6) | 37 (28.0) | 76 (20.8) | 105 (28.7) | 17 (26.2) | 25 (37.9) | 2 (11.8) | 4 (22.2) | 27 (17.1) | 51 (32.1) | 365 (18.0) | 482 (23.7) |
| Beta-blockers (n (%)) | 274 (9.4) | 369 (12.6) | 22 (15.2) | 28 (19.2) | 0 (0) | 0 (0) | 12 (9.1) | 14 (10.6) | 38 (10.4) | 60 (16.4) | 12 (18.5) | 11 (16.7) | 1 (5.9) | 2 (11.1) | 14 (8.9) | 23 (14.5) | 175 (8.6) | 231 (11.4) |
| Diuretics (n (%)) | 127 (4.3) | 127 (4.3) | 9 (6.2) | 12 (8.2) | 1 (6.3) | 2 (12.5) | 5 (3.8) | 3 (2.3) | 11 (3.0) | 16 (4.4) | 5 (7.7) | 6 (9.1) | 1 (5.9) | 0 (0) | 8 (5.1) | 8 (5.1) | 87 (4.3) | 80 (3.9) |
| CCBs (n (%)) | 200 (6.8) | 293 (10.0) | 13 (9.0) | 20 (13.7) | 3 (18.8) | 4 (25.0) | 5 (3.8) | 16 (12.1) | 15 (4.1) | 44 (12.0) | 7 (10.8) | 9 (13.6) | 4 (23.5) | 3 (16.7) | 9 (5.7) | 13 (8.2) | 144 (7.1) | 184 (9.0) |
| Alpha blockers (n (%)) | 28 (1.0) | 30 (1.0) | 4 (2.8) | 3 (2.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (0.8) | 2 (0.6) | 1 (1.5) | 1 (1.5) | 0 (0) | 0 (0) | 2 (1.3) | 1 (0.6) | 18 (0.9) | 23 (1.1) |
| Psychotropics (n (%)) | 219 (7.5) | 300 (10.2) | 20 (13.7) | 24 (16.4) | 2 (12.5) | 3 (18.8) | 8 (6.1) | 9 (6.8) | 36 (9.8) | 46 (12.6) | 6 (9.1) | 10 (15.2) | 0 (0) | 2 (11.1) | 17 (10.7) | 20 (12.6) | 130 (6.4) | 186 (9.1) |
| Orthostatic intolerance (n (%)) | 1150 (39.2) | 853 (29.1) | 68 (46.6) | 51 (34.9) | 8 (50.0) | 5 (31.3) | 57 (43.2) | 33 (25.0) | 168 (45.9) | 126 (34.4) | 23 (34.9) | 19 (28.8) | 7 (38.9) | 5 (27.8) | 73 (45.9) | 48 (30.2) | 746 (36.7) | 566 (28.0) |
| GP visits in past year (mean (SD)) | 3.1 (4.4) | 3.2 (3.1) | 3.5 (3.5) | 3.5 (3.2) | 3.3 (3.1) | 3.0 (2.1) | 3.1 (3.3) | 3.1 (2.4) | 2.9 (3.3) | 3.3 (3.0) | 3.4 (3.8) | 3.3 (3.2) | 3.6 (2.3) | 3.0 (2.4) | 3.6 (3.6) | 3.7 (2.9) | 3.0 (4.8) | 3.1 (3.1) |
| GP visits in past year (median (IQR)) | 2 (3) | 2 (3) | 2.5 (3) | 3 (3) | 2 (2.5) | 2 (3.5) | 2 (3) | 2 (2) | 2 (3) | 2 (3) | 2.5 (3) | 2 (2) | 2.5 (3) | 3 (3) | 3 (3) | 3 (3) | 2 (3) | 2 (3) |
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Group 7 | Group 8 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | |
| New fall | 0.99 (0.68–1.42) | 0.936 | 1.63 (0.76–3.48) | 0.210 | 0.98 (0.64–1.50) | 0.942 | 1.36 (1.07–1.72) | 0.012 | 1.25 (0.79–1.96) | 0.339 | 0.87 (0.33–2.30) | 0.785 | 1.47 (1.03–2.09) | 0.034 | (Base) |
| New faint | 0.68 (0.29–1.61) | 0.380 | 1.58 (0.37–6.80) | 0.538 | 0.94 (0.37–2.38) | 0.902 | 0.85 (0.47–1.52) | 0.578 | 0.83 (0.29–2.35) | 0.722 | 0.89 (0.12–6.74) | 0.910 | 0.28 (0.07–1.14) | 0.076 | (Base) |
| New ADL | 1.04 (0.74–1.47) | 0.822 | 1.58 (0.76–3.31) | 0.223 | 0.94 (0.62–1.43) | 0.775 | 0.92 (0.72–1.19) | 0.531 | 1.44 (0.95–2.21) | 0.089 | 1.08 (0.45–2.56) | 0.866 | 0.87 (0.59–1.28) | 0.480 | (Base) |
| New IADL | 1.01 (0.71–1.42) | 0.975 | 1.54 (0.73–3.24) | 0.252 | 0.97 (0.64–1.47) | 0.890 | 0.84 (0.65–1.09) | 0.181 | 1.39 (0.91–2.12) | 0.129 | 0.87 (0.35–2.18) | 0.767 | 0.83 (0.56–1.22) | 0.336 | (Base) |
| New cognitive decline | 1.25 (0.93–1.67) | 0.134 | 1.25 (0.63–2.50) | 0.524 | 0.79 (0.54–1.14) | 0.210 | 0.98 (0.79–1.22) | 0.876 | 1.63 (1.12–2.36) | 0.011 | 0.99 (0.46–2.16) | 0.988 | 0.82 (0.58–1.15) | 0.240 | (Base) |
| Mortality | 1.17 (0.60–2.29) | 0.653 | 0.49 (0.06–3.80) | 0.495 | 0.98 (0.48–2.76) | 0.970 | 0.95 (0.53–1.69) | 0.856 | 1.64 (0.77–3.49) | 0.203 | 0.47 (0.06–3.81) | 0.477 | 0.63 (0.22–1.76) | 0.377 | (Base) |
| Baseline Characteristic | Wave 3 Data Present | Wave 3 Data Missing | p | |
|---|---|---|---|---|
| (n = 2938) | (n = 1962) | |||
| Mean age in years (SD) | 59.8 (7.9) | 62.88 (9.7) | <0.001 | MWU |
| Female (n (%)) | 1609 (54.8) | 1094 (55.8) | 0.481 | chi |
| Non-frail (n (%)) | 2213 (76.6) | 1258 (66.4) | <0.001 | chi |
| Pre-frail/Frail (n (%)) | 676 (23.4) | 637 (33.6) | <0.001 | chi |
| Mean time to stand (SD) | 7.34 (2.6) | 8.07 (3.4) | <0.001 | MWU |
| Median MMSE (IQR) | 29 (2) | 29 (2) | <0.001 | MWU |
| Multimorbidity (n (%)) | 1239 (42.2) | 997 (50.8) | <0.001 | chi |
| Atrial Fibrillation (n (%)) | 52 (1.8) | 57 (3.0) | 0.008 | chi |
| Parkinson’s disease (n (%)) | 6 (0.2) | 9 (0.5) | 0.114 | chi |
| Diabetes Mellitus (n (%)) | 147 (5.0) | 153 (7.8) | <0.001 | chi |
| Hypertension (n (%)) | 1090 (37.2) | 844 (43.4) | <0.001 | chi |
| Polypharmacy (n (%)) | 396 (13.5) | 434 (22.3) | <0.001 | chi |
| Antihypertensive | chi | |||
| Overall (n (%)) | 803 (27.3) | 750 (38.3) | <0.001 | chi |
| Beta blockers (n (%)) | 274 (9.3) | 289 (14.8) | <0.001 | chi |
| Diuretics (n (%)) | 127 (4.3) | 162 (8.3) | <0.001 | chi |
| ACE inhibitors/Angiotensin receptor blockers (n (%)) | 536 (18.3) | 511 (26.2) | <0.001 | chi |
| Calcium channel blockers (n (%)) | 200 (6.8) | 202 (10.4) | <0.001 | chi |
| Alpha blockers (n (%)) | 28 (1.0) | 43 (2.2) | <0.001 | chi |
| Psychoactive medications | ||||
| Overall (n (%)) | 219 (7.5) | 225 (11.5) | <0.001 | chi |
| Z-drugs (n (%)) | 51 (1.7) | 58 (3.0) | 0.004 | chi |
| Benzodiazepines (n (%)) | 63 (2.2) | 77 (4.0) | <0.001 | chi |
| Antidepressants (n (%)) | 146 (5.0) | 135 (6.9) | 0.005 | chi |
| Orthostatic Intolerance during active stand (n (%)) | 1150 (39.2) | 730 (37.3) | 0.187 | chi |
| At least 1 fall in the past 12 months (n (%)) | 564 (19.2) | 396 (20.2) | 0.395 | chi |
| At least 1 blackout in the past 12 months (n (%)) | 129 (4.4) | 97 (5.0) | 0.369 | chi |
| Lifetime history of syncope (n (%)) | 589 (20.1) | 374 (19.1) | 0.398 | chi |
| Mean baseline SBP (SD) [mmHg] | 134.94 (21.9) | 137.04 (22.8) | <0.001 | MWU |
| Mean baseline HR (SD) [bpm] | 64.52 (9.6) | 65.61 (10.3) | <0.001 | MWU |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moloney, D.; Knight, S.P.; Newman, L.; Kenny, R.A.; Romero-Ortuno, R. Eight Orthostatic Haemodynamic Patterns in The Irish Longitudinal Study on Ageing (TILDA): Stability and Clinical Associations after 4 Years. Geriatrics 2021, 6, 50. https://doi.org/10.3390/geriatrics6020050
Moloney D, Knight SP, Newman L, Kenny RA, Romero-Ortuno R. Eight Orthostatic Haemodynamic Patterns in The Irish Longitudinal Study on Ageing (TILDA): Stability and Clinical Associations after 4 Years. Geriatrics. 2021; 6(2):50. https://doi.org/10.3390/geriatrics6020050
Chicago/Turabian StyleMoloney, David, Silvin P. Knight, Louise Newman, Rose Anne Kenny, and Roman Romero-Ortuno. 2021. "Eight Orthostatic Haemodynamic Patterns in The Irish Longitudinal Study on Ageing (TILDA): Stability and Clinical Associations after 4 Years" Geriatrics 6, no. 2: 50. https://doi.org/10.3390/geriatrics6020050
APA StyleMoloney, D., Knight, S. P., Newman, L., Kenny, R. A., & Romero-Ortuno, R. (2021). Eight Orthostatic Haemodynamic Patterns in The Irish Longitudinal Study on Ageing (TILDA): Stability and Clinical Associations after 4 Years. Geriatrics, 6(2), 50. https://doi.org/10.3390/geriatrics6020050









