Effect of Self-Management Support for Elderly People Post-Stroke: A Systematic Review
Abstract
1. Introduction
Self-Management
2. Method
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Study Selection and Data Extraction
2.4. Assessment of Risk of Bias
2.5. Data Synthesis
3. Results
3.1. Study Characteristics
3.2. Intervention Characteristics
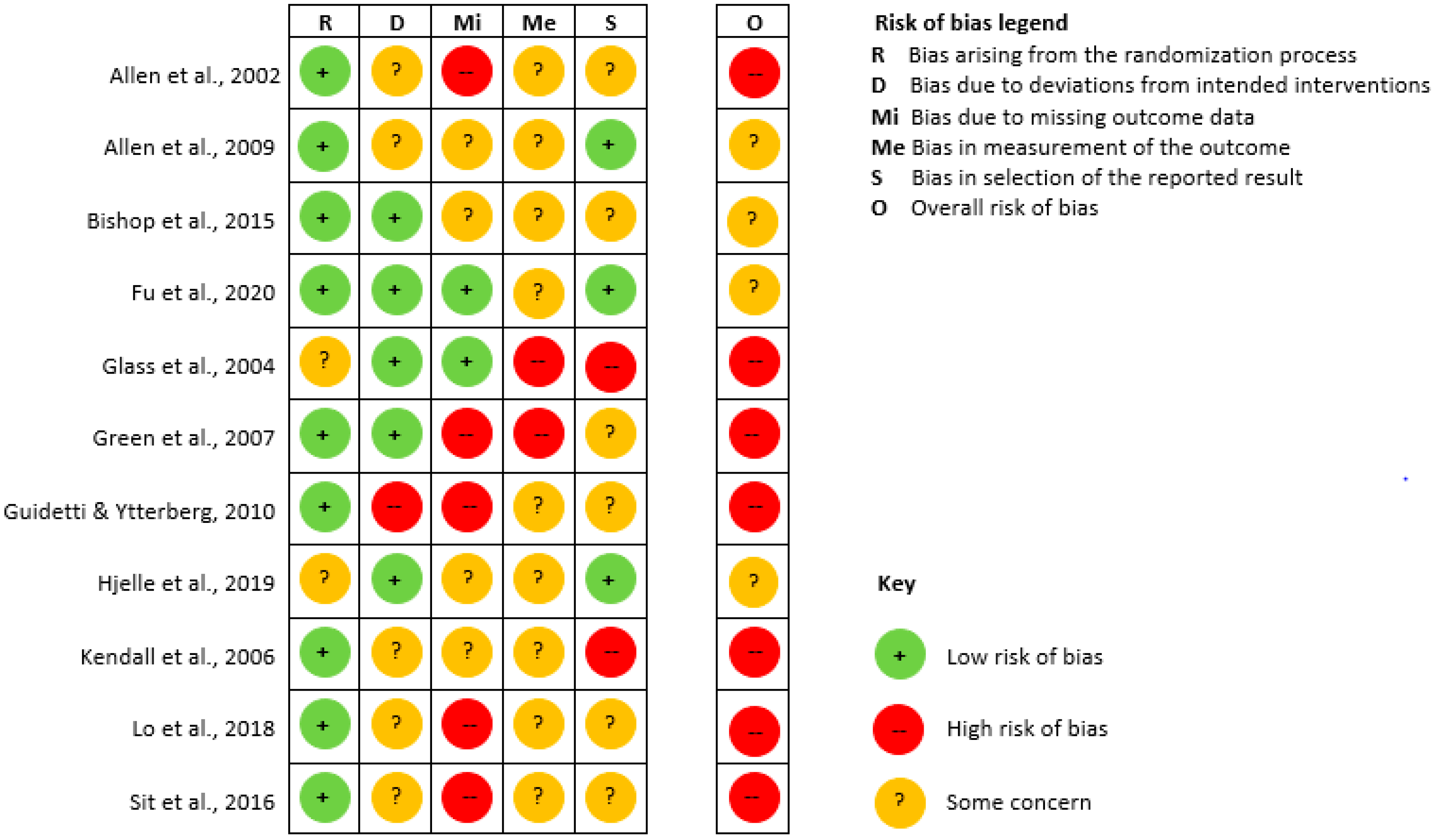
3.3. Risk of Bias
3.4. Evidence Synthesis
3.5. Efficacy of Self-Management Interventions
3.6. Self-Management
3.7. Self-Efficacy
3.8. Quality of Life
3.9. Depression
3.10. Activities of Daily Living
3.11. Active Lifestyle
3.12. Other Measures
4. Discussion
4.1. Methodological Quality of Included Studies
4.2. Limitations of the Review
4.3. Interpretation and Implications for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- The Danish Agency for Labour Market and Recruitment. Folkepensionsalderen nu og fremover. Available online: https://star.dk/ydelser/pension-og-efterloen/folkepension-og-foertidspension/folkepension/folkepensionsalderen-nu-og-fremover (accessed on 2 March 2020).
- Statistics Denmark. Middellevetiden stiger fortsat. Available online: https://www.dst.dk/da/Statistik/nyt/NytHtml?cid=30217 (accessed on 1 April 2020).
- Jonsson, H.; Borell, L.; Sadlo, G. Retirement: An occupational transition with consequences for temporality, balance and meaning of occupations. J. Occup. Sci. 2000, 7, 29–37. [Google Scholar] [CrossRef]
- Grøn, L.; Ravn Andersen, C. Sårbarhed og handlekraft i alderdommen: Et etnografisk feltarbejde blandt fagpersoner og ældre i Horsens og omegn; Kora: Copenhagen, Danmark, 2014. [Google Scholar]
- Flachs, E.M.; Statens Institut for, F.; Sundhedsstyrelsen, S. Sygdomsbyrden i Danmark: Sygdomme; Sundhedsstyrelsen: København, Danmark, 2015; p. 382. [Google Scholar]
- McKevitt, C.; Fudge, N.; Redfern, J.; Sheldenkar, A.; Crichton, S.; Rudd, A.R.; Forster, A.; Young, J.; Nazareth, I.; Silver, L.E.; et al. Self-Reported Long-Term Needs After Stroke. Stroke 2011, 42, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Lamb, M.; Buchanan, D.; Godfrey, C.M.; Harrison, M.B.; Oakley, P. The psychosocial spiritual experience of elderly individuals recovering from stroke: A systematic review. Int. J. Evid. Based Health 2008, 6, 173–205. [Google Scholar] [CrossRef]
- Salter, K.; Hellings, C.; Foley, N.; Teasell, R. The experience of living with stroke: A qualitative meta-synthesis. J. Rehabil. Med. 2008, 40, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Secrest, J.A.; Thomas, S.P. Continuity and discontinuity: The quality of life following stroke. Rehabil. Nurs. 2001, 24, 240–246. [Google Scholar] [CrossRef]
- Becker, G. Continuity After a Stroke: Implications of Life-course Disruption in Old Age. Gerontol. 1993, 33, 148–158. [Google Scholar] [CrossRef]
- Pallesen, H. Fem år efter apopleksi: Fra sygdom til handicap. Ph.D. Thesis, Syddansk Universitet, Odense, Denmark, 2011. [Google Scholar]
- Crichton, S.L.; Bray, B.; McKevitt, C.; Rudd, A.; A Wolfe, C.D. Patient outcomes up to 15 years after stroke: Survival, disability, quality of life, cognition and mental health. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1091–1098. [Google Scholar] [CrossRef]
- Fryer, C.E.; A Luker, J.; McDonnell, M.N.; Hillier, S. Self management programmes for quality of life in people with stroke. Cochrane Database Syst. Rev. 2016, 2016. [Google Scholar] [CrossRef]
- Wray, F.; Clarke, D.; Forster, A. Post-stroke self-management interventions: A systematic review of effectiveness and investigation of the inclusion of stroke survivors with aphasia. Disabil. Rehabil. 2017, 40, 1237–1251. [Google Scholar] [CrossRef]
- Barlow, J.; Wright, C.; Sheasby, J.; Turner, A.; Hainsworth, J.; Turner, A. Self-management approaches for people with chronic conditions: A review. Patient Educ. Couns. 2002, 48, 177–187. [Google Scholar] [CrossRef]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; DeRuyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef] [PubMed]
- Jones, F.; McKevitt, C.; Riazi, A.; Liston, M. How is rehabilitation with and without an integrated self-management approach perceived by UK community-dwelling stroke survivors? A qualitative process evaluation to explore implementation and contextual variations. BMJ Open 2017, 7, e014109. [Google Scholar] [CrossRef] [PubMed]
- Pallesen, H.; Næss-Schmidt, E.; Kjeldsen, S.S.; Pedersen, S.K.S.; Sørensen, S.L.; Brunner, I.; Nielsen, J.F. Stroke - 65 Plus. Continued Active Life”: A study protocol for a randomized controlled cross-sectoral trial of the effect of a novel self-management intervention to support elderly people after stroke. Trials 2018, 19, 639. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, S.L.; Pedersen, S.K.S.; Pallesen, H. Social psychological mechanisms and processes in a novel, health professional-led, self-management intervention for older stroke individuals: A synthesis and phenomenological study. BMC Health Serv. Res. 2019, 19, 320. [Google Scholar] [CrossRef]
- E Walsh, M.; Galvin, R.; Loughnane, C.; Macey, C.; Horgan, N.F. Factors associated with community reintegration in the first year after stroke: A qualitative meta-synthesis. Disabil. Rehabil. 2014, 37, 1–10. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, U.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Reichardt, C. Refworks. Public Serv. Q. 2010, 6, 366–367. [Google Scholar] [CrossRef]
- Cochrane Methods 2016. Cochrane Methods 2016 2016. [CrossRef]
- Radomski, M.V. Impact of Post-Discharge Habit Training of Self Care Skills on Independence, Caregiver Burden, and Development of Automaticity for Survivors of Recent Stroke; ProQuest Information & Learning: Ann Arbor, MI, USA, 2007. [Google Scholar]
- Sahebalzamani, M.; Aliloo, L.; Shakibi, A. The efficacy of self-care education on rehabilitation of stroke patients. Saudi Med. J. 2009, 30, 550–554. [Google Scholar]
- Sit, J.W.; Chair, S.Y.; Chan Yip, C.W.; Choi, K.C.; Lee, D.T.; Leung, K.P.; Tang, S.W.; Chan, P.S. Effect of health empowerment intervention for stroke self-management on behaviour and health in stroke rehabilitation patients. Hong Kong Med J. = Xianggang Yi Xue Za Zhi 2018, 24, 12–15. [Google Scholar]
- Ada, L.; Dean, C.; I Lindley, R.; Lloyd, G. Improving community ambulation after stroke: The AMBULATE trial. BMC Neurol. 2009, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.; Myint, P.K.; Elender, F.; Barton, G.; Pfeil, M.; Price, G.M.; Wyatt, N.; Ravenhill, G.; Thomas, E.; Jagger, J.; et al. A Depression Recognition and Treatment package for families living with Stroke (DepReT-Stroke): Study protocol for a randomised controlled trial. Trials 2011, 12, 105. [Google Scholar] [CrossRef] [PubMed]
- AlAbdulwahab, S.S.; Ahmad, F.; Singh, H. Effects of Functional Limb Overloading on Symmetrical Weight Bearing, Walking Speed, Perceived Mobility, and Community Participation among Patients with Chronic Stroke. Rehabil. Res. Pr. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Aben, L.; Heijenbrok-Kal, M.; Van Loon, E.M.P.; Groet, E.; Ponds, R.W.H.M.; Busschbach, J.J.; Ribbers, G. Training Memory Self-efficacy in the Chronic Stage After Stroke. Neurorehabilit. Neural Repair 2012, 27, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Ada, L.; Dean, C.; I Lindley, R. Randomized Trial of Treadmill Training to Improve Walking in Community-Dwelling People after Stroke: The AMBULATE Trial. Int. J. Stroke 2013, 8, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, Y.; Chen, X.; Shen, X.; Wang, Q.; Sun, C. Longitudinal Study of Effectiveness of a Patient-Centered Self-Management Empowerment Intervention During Predischarge Planning on Stroke Survivors. Worldviews Evid. -Based Nurs. 2018, 15, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Damush, T.; Ofner, S.; Yu, Z.; Plue, L.; Nicholas, G.; Williams, L.S. Implementation of a stroke self-management program. Transl. Behav. Med. 2011, 1, 561–572. [Google Scholar] [CrossRef]
- Eames, S.; Hoffmann, T.; Worrall, L.; Read, S.; Wong, A. Randomised controlled trial of an education and support package for stroke patients and their carers. BMJ Open 2013, 3, e002538. [Google Scholar] [CrossRef]
- Harwood, M.; Weatherall, M.; Talemaitoga, A.; Barber, P.A.; Gommans, J.; Taylor, W.; McPherson, K.; McNaughton, H. Taking charge after stroke: Promoting self-directed rehabilitation to improve quality of life—A randomized controlled trial. Clin. Rehabil. 2011, 26, 493–501. [Google Scholar] [CrossRef]
- Sabariego, C.; Barrera, A.E.; Neubert, S.; Bostan, C.; Cieza, A.; Stier-Jarmer, M. Evaluation of an ICF-based patient education programme for stroke patients: A randomized, single-blinded, controlled, multicentre trial of the effects on self-efficacy, life satisfaction and functioning. Br. J. Health Psychol. 2012, 18, 707–728. [Google Scholar] [CrossRef]
- Wolf, T.; Baum, C.M.; Lee, D.; Hammel, J. The Development of the Improving Participation after Stroke Self-Management Program (IPASS): An Exploratory Randomized Clinical Study. Top. Stroke Rehabil. 2016, 23, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Minshall, C.; Castle, D.J.; Thompson, D.R.; Pascoe, M.; Cameron, J.; McCabe, M.P.; Apputhurai, P.; Knowles, S.R.; Jenkins, Z.; Ski, C.F. A psychosocial intervention for stroke survivors and carers: 12–month outcomes of a randomized controlled trial. Top. Stroke Rehabil. 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chumbler, N.R.; Quigley, P.; Li, X.; Morey, M.; Rose, D.; Sanford, J.; Griffiths, P.; Hoenig, H. Effects of Telerehabilitation on Physical Function and Disability for Stroke Patients. Stroke 2012, 43, 2168–2174. [Google Scholar] [CrossRef] [PubMed]
- Chumbler, N.R.; Li, X.; Quigley, P.; Morey, M.C.; Rose, D.; Griffiths, P.; Sanford, J.; Hoenig, H. A randomized controlled trial on Stroke telerehabilitation: The effects on falls self-efficacy and satisfaction with care. J. Telemed. Telecare 2015, 21, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Chaiyawat, P.; Kulkantrakorn, K. Effectiveness of home rehabilitation program for ischemic stroke upon disability and quality of life: A randomized controlled trial. Clin. Neurol. Neurosurg. 2012, 114, 866–870. [Google Scholar] [CrossRef]
- Sackley, C.; Atkinson, J.C.; Walker, M. Occupational Therapy in Nursing and Residential Care Settings: A Description of a Randomised Controlled Trial Intervention. Br. J. Occup. 2004, 67, 104–110. [Google Scholar] [CrossRef]
- Sackley, C.; Wade, D.T.; Mant, D.; Atkinson, J.C.; Yudkin, P.; Cardoso, K.; Levin, S.; Lee, V.B.; Reel, K. Cluster Randomized Pilot Controlled Trial of an Occupational Therapy Intervention for Residents With Stroke in UK Care Homes. Stroke 2006, 37, 2336–2341. [Google Scholar] [CrossRef]
- Marsden, D.; Quinn, R.; Pond, N.; Golledge, R.; Neilson, C.; White, J.; McElduff, P.; Pollack, M. A multidisciplinary group programme in rural settings for community-dwelling chronic stroke survivors and their carers: A pilot randomized controlled trial. Clin. Rehabil. 2010, 24, 328–341. [Google Scholar] [CrossRef]
- SUPPORT-HF 2 Investigators and Committees; Rahimi, K. Home monitoring with IT-supported specialist management versus home monitoring alone in patients with heart failure: Design and baseline results of the SUPPORT-HF 2 randomized trial. Am. Heart J. 2019, 208, 55–64. [Google Scholar] [CrossRef]
- Lindquist, L.A.; Ramirez-Zohfeld, V.; Sunkara, P.D.; Forcucci, C.; Campbell, D.S.; Mitzen, P.; Ciolino, J.D.; Gregory, D.; Kricke, G.; Cameron, K. PlanYourLifeSpan.org—An intervention to help seniors make choices for their fourth quarter of life: Results from the randomized clinical trial. Patient Educ. Couns. 2017, 100, 1996–2004. [Google Scholar] [CrossRef]
- Hoffmann, T.; McKenna, K.; Worrall, L.; Read, S. Randomised trial of a computer-generated tailored written education package for patients following stroke. Age Ageing 2007, 36, 280–286. [Google Scholar] [CrossRef]
- Allison, R.; Dennett, R. Pilot randomized controlled trial to assess the impact of additional supported standing practice on functional ability post stroke. Clin. Rehabil. 2007, 21, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Harel-Katz, H.; Adar, T.; Milman, U.; Carmeli, E. Examining the feasibility and effectiveness of a culturally adapted participation-focused stroke self-management program in a day-rehabilitation setting: A randomized pilot study. Top. Stroke Rehabil. 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Aben, L.; Heijenbrok-Kal, M.; Ponds, R.W.H.M.; Busschbach, J.J.; Ribbers, G. Long-Lasting Effects of a New Memory Self-efficacy Training for Stroke Patients. Neurorehabilit. Neural Repair 2013, 28, 199–206. [Google Scholar] [CrossRef] [PubMed]
- A Cadilhac, D.; Kilkenny, M.F.; Srikanth, V.; I Lindley, R.; Lalor, E.; Osborne, R.H.; Batterbsy, M. Do cognitive, language, or physical impairments affect participation in a trial of self-management programs for stroke? Int. J. Stroke 2015, 11, 77–84. [Google Scholar] [CrossRef] [PubMed]
- A Joice, S.; Johnston, M.; Bonetti, D.; Morrison, V.; MacWalter, R. Stroke survivors’ evaluations of a stroke workbook-based intervention designed to increase perceived control over recovery. Health Educ. J. 2010, 71, 17–29. [Google Scholar] [CrossRef]
- Fido, R. Facilitating Diary Keeping and Participation in Valued Activities with Individuals Who Have Had a Stroke: A Randomised Controlled Trial. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 15 November 2010. [Google Scholar]
- Johnston, M.; Bonetti, D.; Joice, S.; Pollard, B.; Morrison, V.; Francis, J.J.; MacWalter, R. Recovery from disability after stroke as a target for a behavioural intervention: Results of a randomized controlled trial. Disabil. Rehabil. 2007, 29, 1117–1127. [Google Scholar] [CrossRef]
- Ellis-Hill, C.; Thomas, S.; Gracey, F.; Lamont-Robinson, C.; Cant, R.; Marques, E.; Thomas, P.W.; Grant, M.; Nunn, S.; Paling, T.; et al. HeART of Stroke: Randomised controlled, parallel-arm, feasibility study of a community-based arts and health intervention plus usual care compared with usual care to increase psychological well-being in people following a stroke. BMJ Open 2019, 9, e021098. [Google Scholar] [CrossRef]
- Allen, K.R.; Hazelett, S.; Jarjoura, D.; Wickstrom, G.C.; Hua, K.; Weinhardt, J.; Wright, K. Effectiveness of a postdischarge care management model for stroke and transient ischemic attack: A randomized trial. J. Stroke Cereb. Dis. 2002, 11, 88–98. [Google Scholar] [CrossRef]
- Allen, K.; Hazelett, S.; Jarjoura, D.; Hua, K.; Wright, K.; Weinhardt, J.; Kropp, D. A Randomized Trial Testing the Superiority of a Postdischarge Care Management Model for Stroke Survivors. J. Stroke Cereb. Dis. 2009, 18, 443–452. [Google Scholar] [CrossRef]
- Bishop, D.; Miller, I.; Weiner, D.; Guilmette, T.; Mukand, J.; Feldmann, E.; Keitner, G.; Springate, B. Family Intervention: Telephone Tracking (FITT): A Pilot Stroke Outcome Study. Top. Stroke Rehabil. 2014, 21, S63–S74. [Google Scholar] [CrossRef]
- Fu, V.; Weatherall, M.; McPherson, K.; Taylor, W.; McRae, A.; Thomson, T.; Gommans, J.; Green, G.; Harwood, M.; Ranta, A.; et al. Taking Charge after Stroke: A randomized controlled trial of a person-centered, self-directed rehabilitation intervention. Int. J. Stroke 2020. [Google Scholar] [CrossRef] [PubMed]
- Glass, T.; Berkman, L.F.; Hiltunen, E.F.; Furie, K.; Glymour, M.M.; Fay, M.; Ware, J. The Families In Recovery From Stroke Trial (FIRST): Primary Study Results. Psychosom. Med. 2004, 66, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Green, T.; Haley, E.; Eliasziw, M.; Hoyte, K. Education in stroke prevention: Efficacy of an educational counselling intervention to increase knowledge in stroke survivors. Can. J. Neurosci. Nurs. 2007, 29, 13–20. [Google Scholar] [PubMed]
- Guidetti, S.; Ytterberg, C. A randomised controlled trial of a client-centred self-care intervention after stroke: A longitudinal pilot study. Disabil. Rehabil. 2010, 33, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Hjelle, E.G.; Kildal, L.; Kirkevold, M.; Zucknick, M.; Bronken, B.; Martinsen, R.; Kvigne, K.J.; Kitzmüller, G.; Mangset, M.; Thommessen, B.; et al. Effect of a dialogue-based intervention on psychosocial well-being 6 months after stroke in Norway: A randomized controlled trial. J. Rehabil. Med. 2019, 51, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Kendall, E.; Catalano, T.; Kuipers, P.; Posner, N.; Buys, N.J.; Charker, J. Recovery following stroke: The role of self-management education. Soc. Sci. Med. 2007, 64, 735–746. [Google Scholar] [CrossRef]
- Lo, S.H.S.; Chang, A.M.; Chau, J.P. Stroke Self-Management Support Improves Survivors’ Self-Efficacy and Outcome Expectation of Self-Management Behaviors. Stroke 2018, 49, 758–760. [Google Scholar] [CrossRef]
- Sit, J.W.; Chair, S.Y.; Choi, K.C.; Chan, C.W.H.; Lee, T.F.D.; Chan, A.W.; Cheung, J.L.; Tang, S.W.; Chan, P.S.; Taylor-Piliae, R.E. Do empowered stroke patients perform better at self-management and functional recovery after a stroke? A randomized controlled trial. Clin. Interv. Aging 2016, 11, 1441–1450. [Google Scholar] [CrossRef]
- Higgins, J.P.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A. Assessing risk of bias in a randomized trial. Cochrane Handb. Syst. Rev. Interv. 2019, 205–228. [Google Scholar] [CrossRef]
- Centre for Reviews and Dissemination. Systematic Reviews. CRD’s guidance for undertaking reviews in health care. Available online: https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf (accessed on 2 April 2020).
- Miller, W.; Lasiter, S.; Ellis, R.J.B.; Buelow, J.M. Chronic disease self-management: A hybrid concept analysis. Nurs. Outlook 2014, 63, 154–161. [Google Scholar] [CrossRef]
- Ryan, P.; Sawin, K.J. The Individual and Family Self-Management Theory: Background and perspectives on context, process, and outcomes. Nurs. Outlook 2009, 57, 217–225.e6. [Google Scholar] [CrossRef] [PubMed]
- Holman, H.R.; Lorig, K. Patient self-management: A key to effectiveness and efficiency in care of chronic disease. Public Health Rep. 2004, 119, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Osborne, R.H.; Wilson, T.; Lorig, K.R.; McColl, G.J. Does self-management lead to sustainable health benefits in people with arthritis? A 2-year transition study of 452 Australians. J. Rheumatol. 2007, 34, 1112–1117. [Google Scholar] [PubMed]
- Richards, D.A. The complex interventions framework. In Complex Interventions in Health—An Overview of Research Methods; Hallberg, I.R., Richards, D.A., Eds.; Routledge: New York, NY, USA, 2015; pp. 1–15. [Google Scholar]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo-Reed, A.; Chaddock, L.; Kim, J.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- Netz, Y.; Dwolatzky, T.; Zinker, Y.; Argov, E.; Agmon, R. Aerobic fitness and multidomain cognitive function in advanced age. Int. Psychogeriatr. 2010, 23, 114–124. [Google Scholar] [CrossRef]
- Yuenyongchaiwat, K.; Pongpanit, K.; Hanmanop, S. Physical activity and depression in older adults with and without cognitive impairment. Dement. Neuropsychol. 2018, 12, 12–18. [Google Scholar] [CrossRef]
- Van Achterberg, T.; Waal, G.G.J.H.-D.; Ketelaar, N.A.B.M.; Oostendorp, R.A.B.; Jacobs, J.E.; Wollersheim, H.C.H. How to promote healthy behaviours in patients? An overview of evidence for behaviour change techniques. Health Promot Int. 2010, 26, 148–162. [Google Scholar] [CrossRef]
- Pallesen, H. Body, coping and self-identity. A qualitative 5-year follow-up study of stroke. Disabil. Rehabil. 2013, 36, 232–241. [Google Scholar] [CrossRef]

| Database | Fields Searched | Articles Identified (Hits) |
|---|---|---|
| PubMed | Title, Abstract and Keywords | 488 |
| Embase | Title, Abstract and Keywords | 603 |
| PsycInfo | Title, Abstract and Keywords | 120 |
| Total (n) | 1211 |
| Authors, Title, Year, Journal, Country | Sample Size | Age (Mean and SD) | Study Design | Population | Aim | Theoretical Foundation | Intervention/ Comparator | Psychosocial Outcome Measures | Follow-up Time Point |
|---|---|---|---|---|---|---|---|---|---|
| Allen, K.R.; Hazelett, S.; Jarjoura, D.; Wickstrom, G.C.; Hua, K.; Weinhardt; J., and Wright K. Effectiveness of a Post discharge Care Management Model for Stroke and Transient Ischemic Attack: A Randomized Trial 2002 Journal of Stroke and Cerebrovascular Diseases USA [56] | 96 | Intervention group 69.0 (SD not reported) Control group 72.0 (SD not reported) | RCT- Two arms | Ischemic stroke and transient ischemic attack | To test the effectiveness of comprehensive, interdisciplinary post-discharge care management for improvement of a profile of indicators of health recovery and secondary prevention in stroke and transient ischemic attack (TIA) patients. | Health literacy, but no clear theoretical rationales were defined. | Intervention: An advanced practice nurse-care manager provided care management focused on health promotion and psychosocial well-being. Comparator: Usual care | 1) QOL1 2) Depression | Three months post-discharge. |
| Allen, K.R.; Hazelett, S.; Jarjoura, D.; Hua, K.; Wright, K.; Weinhardt, J., and Kropp D.A. Randomized Trial Testing the Superiority of a Post discharge Care Management Model for Stroke Survivors 2009 Journal of Stroke and Cerebrovascular Diseases USA [57] | 380 | Intervention group 68.0 (SD not reported) Control group 69.0 (SD not reported) | RCT- Two arms | Ischemic stroke | To test the superiority of comprehensive interdisciplinary post-discharge stroke care management for improving outcomes for stroke survivors as compared with organized acute stroke department care with enhanced discharge planning. | Health literacy, but no clear theoretical rationales were defined. | Intervention: An advanced practice nurse-care manager provided care management including self-management support. Comparator: Usual care | 1) QOL1 2) Depression 3) Active Lifestyle | Six months post-discharge. |
| Bishop, D.; Miller, I.; Weiner, D.; Guilmette, T.; Mukand, J.; Feldmann, E.; Keitner, G., and Springate, B. Family Intervention: Telephone Tracking (FITT): A Pilot Stroke Outcome Study 2015 Topics in Stroke Rehabilitation USA [58] | 49 | Both groups Stroke individuals: 70.1 (SD 11.6) Caregivers: 56.8 (SD 16.4) Only a total mean age for both groups is reported | RCT- Two arms | Stroke and their caregivers (Not sub-arachnoid hemorrhage) | To preliminarily test the efficacy of a telephone intervention. | Grounded in psychosocial theories (A family system approach). | Intervention: A family intervention by telephone tracking designed to assist people with stroke and their primary caregivers during the first 6 months after stroke. Comparator: Usual care | 1) Depression 2) Functional independence2 3) Family functioning2 | Three- and six-months post-stroke. |
| Fu, V.; Weatherall, M.; McPherson, K.; Taylor, W.; McRae, A.; Thomson, T.; Gommans, J.; Green, G.; Harwood, M.; Ranta, A.; Hanger, C.; Riley., and McNaughton, H. Taking Charge after Stroke: A randomized controlled trial of a person-centered, self-directed rehabilitation intervention 2020 International Journal of Stroke New Zealand [59] | 400 | Intervention groups Take Charge 1: 71.4 (SD 12.6) Take Charge 2: 71.7 (SD 12.6) Control group 73.0 (SD 12.2) | RCT- Three arms | Stroke | To confirm whether the Take Charge intervention improved quality of life at 12 months after stroke and whether two sessions were more effective than one. | Grounded in psychosocial theories (Self Determination Theory). | Intervention: Take Charge: A one-to-one, non-directive exploration of the stroke individuals views on what and who was important to them in their lives, and what they wanted to prioritize for the next 12 months Take Charge 1: A single Take Charge session. Take Charge 2: Two Take Charge sessions six weeks apart. Comparator: Were given written educational material about stroke covering common issues following stroke and risk factor management. | 1) QOL1 2) Actual activities2 3) Caregiver strain2 | Six- and 12-months post-stroke. |
| Glass, T.A.; Berkman, L.F.; Hiltunen, E.F.; Furie, K.; Glymour, M.; Fay, M.E., and Ware, J. The Families in Recovery from Stroke Trial (FIRST): Primary Study 2004 Psychosomatic Medicine USA [60] | 291 | Intervention group 69.3 (SD 11.0) Control group 70.4 (SD 11.0) | RCT- Two arms | Ischemic or non-traumatic hemorrhagic stroke | To examine whether a family-system intervention designed to influence social support and self-efficacy affects functional outcome in older stroke patients. | Grounded in psychosocial theories (A family system approach). | Intervention: An integrative psychosocial intervention for stroke individuals and their families tailored to each family’s needs Comparator: Usual care | 1) Self-efficacy 2) QOL1 3) Depression 4) Active lifestyle 5) Social support2 | Three and six months post-randomization. |
| Green, T.; Harley, E.; Eliasziw, M., and Hoyte, K. Education in stroke prevention: Efficacy of an educational counselling intervention to increase knowledge in stroke survivors 2007 Canadian Journal of Neuroscience Nursing Canada [61] | 200 | Intervention group 66.3 (SD 12.4) Control group 67.2 (SD 12.4) | RCT- Two arms | Stroke and transient ischemic attack | To examine the impact of one-to-one brief nurse/patient interview on acquisition of knowledge of stroke and influence on lifestyle behavior changes. | Trans-theoretical stages of change model. | Intervention: An education-counselling interview, where participants mapped their individual risk factors on a stages-of-change model and received an appointment to the next group lifestyle class. Comparator: Usual care | 1) Active lifestyle 2) Stress2 | Three months post-appointment. |
| Guidetti, S. and Ytterberg, C. A randomised controlled trial of a client-centred self-care intervention after stroke: a longitudinal pilot study 2010 Disability and Rehabilitation Sweden [62] | 40 | Intervention group Stroke individuals: 66.0 (SD 14.0) Caregivers: 64.0 (SD not reported) Control group Stroke individuals: 69.0 (SD 15.0) Caregiver: 63.0 (SD not reported) | RCT- Two arms | Stroke and their caregivers | To study (i) the feasibility of the study design, (ii) effects up to 12 months on activities of daily living, use of informal care and home help services, and caregiver burden. | Health literacy, but no clear theoretical rationales were defined. | Intervention: A new client-centered self-care intervention after stroke focusing on learning to use and implement a global problem-solving strategy, goal-plan-do-check when performing self-care activities. Caregivers were invited to collaborate. Comparator: Usual care | 1) ADL3 2) Social/ Lifestyle activities2 3) Participation2 4) Satisfaction with life2 | Three, six and 12 months post-intervention. |
| Hjelle, E.; Bragsted, L.K.; Kirkevold, M.; Zucknivk, M.; Bronken, B.A.; Martinsen, R.; Kvigne, K.J.; Kitzmüller, G.; Mangset, M.; Thommessen, B., and Sveen, U. Effect of a dialogue-based intervention on psychosocial wellbeing 6 months after stroke in Norway: a randomized controlled trial 2019 Journal of Rehabilitation Medicine Norway [63] | 322 | Intervention group 66.0 (SD 12.1) Control group 65.0 (SD 13.3) | RCT- Two arms | Stroke | To evaluate the effect of a dialogue-based intervention in addition to usual care on psychosocial well-being 6 months after stroke. | Grounded in psychosocial theories (Salutogenesis, sense of coherence, narrative theory, and ideas from guided self-determination). | Intervention: A dialogue-based intervention that aimed to support the coping and life skills of stroke. Comparator: Usual care | 1) QOL1 2) Depression 3) Well-being2 4) Sense of coherence2 | Six months post-stroke. |
| Kendall, E.; Catalano, T.; Kuipers, P.; Posner N, Buys, N., and Charker, J. Recovery following stroke: the role of self-management education 2007 Social Science & Medicine Australia [64] | 100 | Intervention group 66.4 (SD 15.3) Control group 66.4 (SD 14.9) | RCT- Two arms | Stroke | To examine the utility of the Chronic Disease Self-Management course as a way of promoting progressive psychosocial recovery pathways among people with stroke. | Grounded in psychosocial theories (Lazarus and Folkman’s theory of stress and coping) | Intervention: An existing self-management intervention, the Chronic Disease Self-Management Course, was used to operationalize the concept of psychosocial skill expansion. Comparator: Usual care | 1) Self-efficacy 2) QOL1 | Three, six, nine- and 12-months post-stroke. |
| Lo, S.H.S.; Chang, A.M., and Chau, J.P.C. Stroke Self-Management Support Improves Survivors’ Self-Efficacy and Outcome Expectation of Self-Management Behaviours 2018 Stroke Australia [65] | 128 | Both groups 67.5 (SD 11.95) Only a total mean age for both groups is reported | RCT- Two arms | Stroke | To determine the effectiveness of a new nurse-led self-efficacy-based stroke self-management program. | Grounded in psychosocial theories (Bandura construct of self-efficacy) | Intervention: A nurse-led intervention facilitating stroke self-management. Comparator: Usual care | 1) Self-management 2) Self-efficacy | Eight weeks after randomization. |
| Sit, J.W.; Chair, S.Y.; Choi, K.C.; Chan, C.W.; Lee, D.T.; Chan, A.W.; Cheung, J.L.; Tang, S.W.; Chan, P.S., and Taylor-Piliae, R.E. Do empowered stroke patients perform better at self-management and functional recovery after a stroke? A randomized controlled trial 2016 Clinical Interventions in Aging Hong Kong [66] | 210 | Intervention group 67.8 (SD 14.2) Control group 70.7 (SD 13.9) | RCT- Two arms | Stroke | To examine the effects of the empowerment intervention on stroke patients’ self-efficacy, self-management behavior, and functional recovery. | Grounded in psychosocial theories (Shearer’s theory of health empowerment) | Intervention: An intervention to empower stroke individuals with “how to” knowledge and skills to enhance self-management in conjunction with their post-stroke rehabilitation journey. Comparator: Usual care | 1) Self-management 2) Self-efficacy 3) ADL3 | One week, three and six months post-intervention |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kristine Stage Pedersen, S.; Lillelund Sørensen, S.; Holm Stabel, H.; Brunner, I.; Pallesen, H. Effect of Self-Management Support for Elderly People Post-Stroke: A Systematic Review. Geriatrics 2020, 5, 38. https://doi.org/10.3390/geriatrics5020038
Kristine Stage Pedersen S, Lillelund Sørensen S, Holm Stabel H, Brunner I, Pallesen H. Effect of Self-Management Support for Elderly People Post-Stroke: A Systematic Review. Geriatrics. 2020; 5(2):38. https://doi.org/10.3390/geriatrics5020038
Chicago/Turabian StyleKristine Stage Pedersen, Sedsel, Susanne Lillelund Sørensen, Henriette Holm Stabel, Iris Brunner, and Hanne Pallesen. 2020. "Effect of Self-Management Support for Elderly People Post-Stroke: A Systematic Review" Geriatrics 5, no. 2: 38. https://doi.org/10.3390/geriatrics5020038
APA StyleKristine Stage Pedersen, S., Lillelund Sørensen, S., Holm Stabel, H., Brunner, I., & Pallesen, H. (2020). Effect of Self-Management Support for Elderly People Post-Stroke: A Systematic Review. Geriatrics, 5(2), 38. https://doi.org/10.3390/geriatrics5020038





