A Systematic Review Examining Associations between Cardiovascular Conditions and Driving Outcomes among Older Drivers
Abstract
1. Introduction
2. Methods
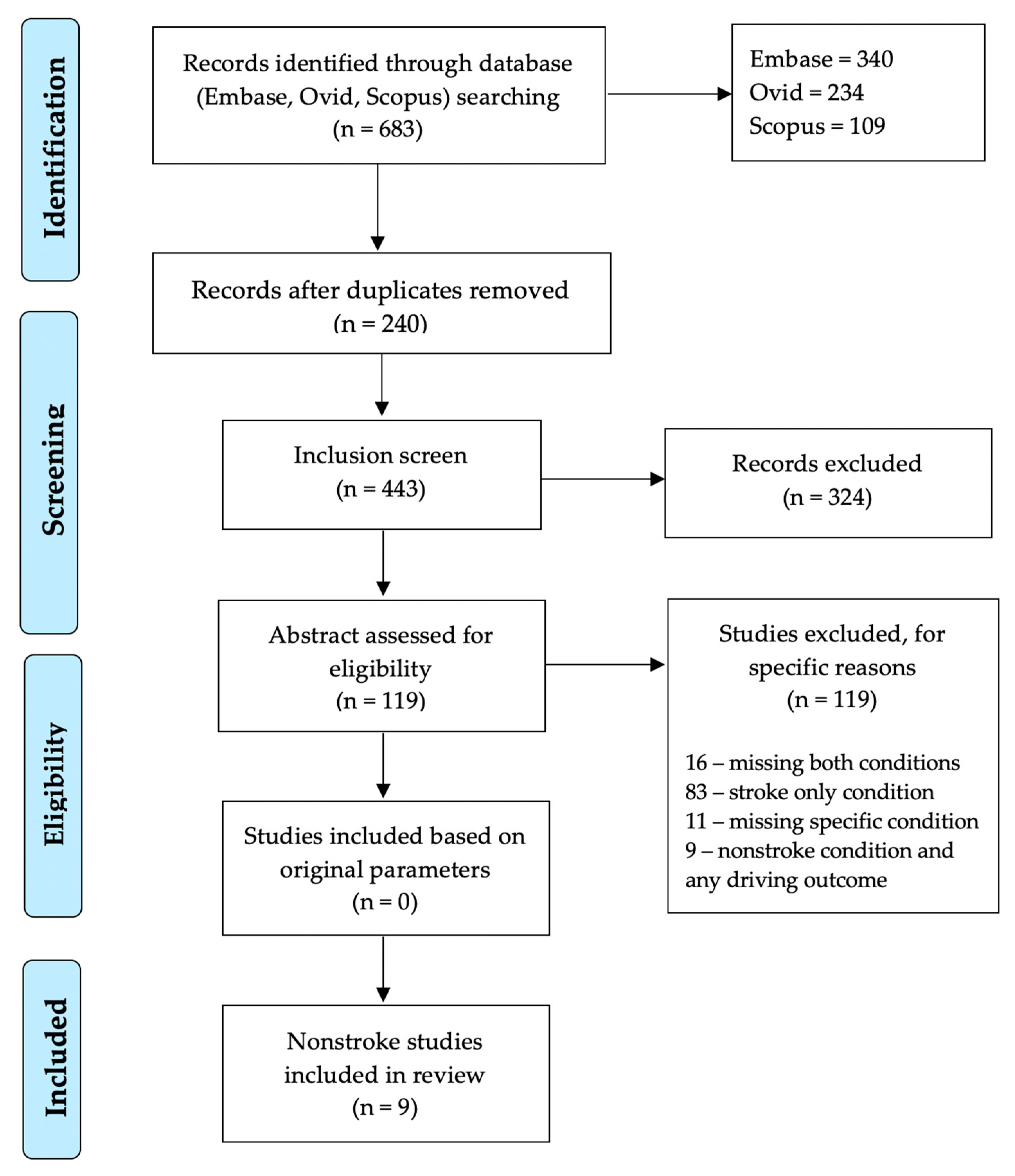
2.1. Literature Search Strategy
2.2. Inclusion/Exclusion Criteria
2.3. Data Extraction and Synthesis
3. Results
3.1. Heart Failure
3.2. Vascular Dementia
3.3. WMH/Leukoaraiosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control Prevention Web-based Injury Statistics Query and Reporting System (WISQARS). Available online: https://www.cdc.gov/motorvehiclesafety/older_adult_drivers/index.html (accessed on 31 March 2020).
- Loughran, D.S.; Seabury, S.A.; Zakaras, L. What Risks Do Older Drivers Pose to Traffic Safety? RAND Corporation: Santa Monica, CA, USA, 2007. [Google Scholar]
- Cunningham, M.L.; Regan, M.A. The impact of emotion, life stress and mental health issues on driving performance and safety. Road Transp. Res. A J. Aust. N. Z. Res. Pract. 2016, 25, 40. [Google Scholar]
- Hunt, L.A.; Murphy, C.F.; Carr, D.; Duchek, J.M.; Buckles, V.; Morris, J.C. Reliability of the Washington University Road Test: A performance-based assessment for drivers with dementia of the Alzheimer type. Arch. Neurol. 1997, 54, 707–712. [Google Scholar] [CrossRef]
- Sifrit, K.J.; Stutts, J.; Martell, C.; Staplin, L. Intersection crashes among drivers in their 60s, 70s and 80s. In Proceedings of the Human Factors and Ergonomics Society; SAGE Publications: Los Angeles, CA, USA, 2010; pp. 2057–2061. [Google Scholar]
- Anstey, K.J.; Horswill, M.S.; Wood, J.M.; Hatherly, C. The role of cognitive and visual abilities as predictors in the Multifactorial Model of Driving Safety. Accid. Anal. Prev. 2012, 45, 766–774. [Google Scholar] [CrossRef]
- Eby, D.W.; Silverstein, N.M.; Molnar, L.J.; LeBlanc, D.; Adler, G. Driving behaviors in early stage dementia: A study using in-vehicle technology. Accid. Anal. Prev. 2012, 49, 330–337. [Google Scholar] [CrossRef]
- Molnar, L.J.; Charlton, J.L.; Eby, D.W.; Bogard, S.E.; Langford, J.; Koppel, S.; Kolenic, G.; Marshall, S.; Man-Son-Hing, M. Self-regulation of driving by older adults: Comparison of self-report and objective driving data. Transp. Res. Part F Traffic Psychol. Behav. 2013, 20, 29–38. [Google Scholar] [CrossRef]
- Molnar, L.J.; Charlton, J.L.; Eby, D.W.; Langford, J.; Koppel, S.; Kolenic, G.E.; Marshall, S. Factors affecting self-regulatory driving practices among older adults. Traffic Inj. Prev. 2014, 15, 262–272. [Google Scholar] [CrossRef]
- Ball, K.; Owsley, C.; Stalvey, B.; Roenker, D.L.; Sloane, M.E.; Graves, M. Driving avoidance and functional impairment in older drivers. Accid. Anal. Prev. 1998, 30, 313–322. [Google Scholar] [CrossRef]
- Ross, L.A.; Clay, O.J.; Edwards, J.D.; Ball, K.K.; Wadley, V.G.; Vance, D.E.; Cissell, G.M.; Roenker, D.L.; Joyce, J.J. Do older drivers at-risk for crashes modify their driving over time? J. Gerontol. Ser. B 2009, 64, 163–170. [Google Scholar] [CrossRef]
- Ross, L.A.; Dodson, J.E.; Edwards, J.D.; Ackerman, M.L.; Ball, K. Self-rated driving and driving safety in older adults. Accid. Anal. Prev. 2012, 48, 523–527. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; Floyd, J.; Fornage, M.; Gillespie, C.; Isasi, C. Heart disease and stroke statistics-2017 update: A report from the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Tong, X.; Yang, Q.; Ritchey, M.D.; George, M.G.; Jackson, S.L.; Gillespie, C.; Merritt, R.K. The Burden of Cerebrovascular Disease in the United States. Prev. Chronic Dis. 2019, 16. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, P.B. Risk factors for vascular dementia and Alzheimer disease. Stroke 2004, 35 (Suppl. 1), 2620–2622. [Google Scholar] [CrossRef]
- Meyer, J.S.; Rauch, G.M.; Rauch, R.A.; Haque, A.; Crawford, K. Cardiovascular and other risk factors for Alzheimer’s disease and vascular dementia. Ann. N. Y. Acad. Sci. 2000, 903, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Román, G.C. Vascular dementia prevention: A risk factor analysis. Cerebrovasc. Dis. 2005, 20 (Suppl. 2), 91–100. [Google Scholar] [CrossRef]
- Li, Q.; Yang, Y.; Reis, C.; Tao, T.; Li, W.; Li, X.; Zhang, J.H. Cerebral Small Vessel Disease. Cell Transplant. 2018, 27, 1711–1722. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef]
- Marek, M.; Horyniecki, M.; Frączek, M.; Kluczewska, E. Leukoaraiosis–new concepts and modern imaging. Pol. J. Radiol. 2018, 83, e76. [Google Scholar] [CrossRef]
- Helenius, J.; Soinne, L.; Salonen, O.; Kaste, M.; Tatlisumak, T. Leukoaraiosis, ischemic stroke and normal white matter on diffusion-weighted MRI. Stroke 2002, 33, 45–50. [Google Scholar] [CrossRef]
- Smith, E.E. Leukoaraiosis and stroke. Stroke 2010, 41 (10 Suppl. 1), S139–S143. [Google Scholar] [CrossRef]
- Schmidt, R.; Petrovic, K.; Ropele, S.; Enzinger, C.; Fazekas, F. Progression of leukoaraiosis and cognition. Stroke 2007, 38, 2619–2625. [Google Scholar] [CrossRef]
- Grool, A.M.; Van der Graaf, Y.; Mali, W.P.T.M.; Witkamp, T.D.; Vincken, K.L.; Geerlings, M.I. Location and progression of cerebral small-vessel disease and atrophy and depressive symptom profiles: The Second Manifestations of ARTerial disease (SMART)-Medea study. Psychol. Med. 2012, 42, 359–370. [Google Scholar] [CrossRef]
- Banerjee, G.; Wilson, D.; Jäger, H.R.; Werring, D.J. Novel imaging techniques in cerebral small vessel diseases and vascular cognitive impairment. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 926–938. [Google Scholar] [CrossRef]
- De Laat, K.F.; Tuladhar, A.M.; van Norden, A.G.W.; Norris, D.G.; Zwiers, M.P.; de Leeuw, F.-E. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain 2011, 134, 73–83. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Makin, S.J.; Hernández, M.C.V.; Armitage, P.A.; Heye, A.K.; Chappell, F.M.; Munoz-Maniega, S.; Sakka, E.; Shuler, K.; Dennis, M.S. Blood-brain barrier failure as a core mechanism in cerebral small vessel disease and dementia: Evidence from a cohort study. Alzheimer Dement. 2017, 13, 634–643. [Google Scholar] [CrossRef]
- Devos, H.; Akinwuntan, A.E.; Nieuwboer, A.; Truijen, S.; Tant, M.; De Weerdt, W. Screening for fitness to drive after stroke: A systematic review and meta-analysis. Neurology 2011, 76, 747–756. [Google Scholar] [CrossRef]
- Frith, J.; Hubbard, I.J.; James, C.L.; Warren-Forward, H. Returning to driving after stroke: A systematic review of adherence to guidelines and legislation. Br. J. Occup. Ther. 2015, 78, 349–355. [Google Scholar] [CrossRef]
- Marshall, S.C.; Molnar, F.; Man-Son-Hing, M.; Blair, R.; Brosseau, L.; Finestone, H.M.; Lamothe, C.; Korner-Bitensky, N.; Wilson, K.G. Predictors of driving ability following stroke: A systematic review. Top. Stroke Rehabil. 2007, 14, 98–114. [Google Scholar] [CrossRef]
- Perrier, M.-J.; Korner-Bitensky, N.; Petzold, A.; Mayo, N. The risk of motor vehicle crashes and traffic citations post stroke: A structured review. Top. Stroke Rehabil. 2010, 17, 191–196. [Google Scholar] [CrossRef]
- Golisz, K. Occupational therapy interventions to improve driving performance in older adults: A systematic review. Am. J. Occup. Ther. 2014, 68, 662–669. [Google Scholar] [CrossRef]
- Davis, R.L.; Ohman, J.M. Driving in early-stage Alzheimer’s disease: An integrative review of the literature. Res. Gerontol. Nurs. 2016, 10, 86–100. [Google Scholar] [CrossRef]
- De Winter, J.; Van Leeuwen, P.M.; Happee, R. Advantages and Disadvantages of Driving Simulators: A Discussion. In Proceedings of the Measuring Behavior 2012, Utrecht, The Netherlands, 28–31 August 2012. Citeseer: p 8th. [Google Scholar]
- Alosco, M.L.; Spitznagel, M.B.; Cleveland, M.J.; Gunstad, J. Cognitive deficits are associated with poorer simulated driving in older adults with heart failure. BMC Geriatr. 2013, 13, 58. [Google Scholar] [CrossRef]
- Alosco, M.L.; Penn, M.S.; Brickman, A.M.; Spitznagel, M.B.; Cleveland, M.J.; Griffith, E.Y.; Narkhede, A.; Gunstad, J. Preliminary observations on MRI correlates of driving independence and performance in persons with heart failure. Int. J. Neurosci. 2015, 125, 424–432. [Google Scholar] [CrossRef]
- Alosco, M.L.; Penn, M.S.; Spitznagel, M.B.; Cleveland, M.J.; Ott, B.R.; Gunstad, J. Reduced physical fitness in patients with heart failure as a possible risk factor for impaired driving performance. Am. J. Occup. Ther. 2015, 69, 6902260010p1–6902260010p8. [Google Scholar] [CrossRef]
- Sims, R.V.; Mujib, M.; McGwin, G., Jr.; Zhang, Y.; Ahmed, M.I.; Desai, R.V.; Aban, I.B.; Sawyer, P.; Anker, S.D.; Ahmed, A. Heart failure is a risk factor for incident driving cessation among community-dwelling older adults: Findings from a prospective population study. J. Card. Fail. 2011, 17, 1035–1040. [Google Scholar] [CrossRef]
- Fitten, L.J.; Perryman, K.M.; Wilkinson, C.J.; Little, R.J.; Burns, M.M.; Pachana, N.; Mervis, J.R.; Malmgren, R.; Siembieda, D.W.; Ganzell, S. Alzheimer and vascular dementias and driving: A prospective road and laboratory study. JAMA 1995, 273, 1360–1365. [Google Scholar] [CrossRef]
- Piersma, D.; Fuermaier, A.B.; De Waard, D.; Davidse, R.J.; De Groot, J.; Doumen, M.J.; Bredewoud, R.A.; Claesen, R.; Lemstra, A.W.; Scheltens, P. Assessing fitness to drive in patients with different types of dementia. Alzheimer Dis. Assoc. Disord. 2018, 32, 70. [Google Scholar] [CrossRef]
- Nakano, K.; Park, K.; Zheng, R.; Fang, F.; Ohori, M.; Nakamura, H.; Kumagai, Y.; Okada, H.; Teramura, K.; Nakayama, S. Leukoaraiosis significantly worsens driving performance of ordinary older drivers. PLoS ONE 2014, 9, e108333. [Google Scholar] [CrossRef]
- Jang, M.; Hong, C.H.; Kim, H.-C.; Choi, S.H.; Seo, S.W.; Kim, S.Y.; Na, D.L.; Lee, Y.; Chang, K.J.; Roh, H.W. Subcortical ischemic change as a predictor of driving cessation in the elderly. Psychiatr. Investig. 2018, 15, 1162. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Morris, J.C. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 1993, 43, 2412. [Google Scholar] [CrossRef]
- Vincent, G.K.; Velkoff, V.A. The Next Four Decades: The Older Population in the United States: 2010 to 2050; US Department of Commerce, Economics and Statistics Administration: Washington, DC, USA, 2010.
- Khavjou, O.; Phelps, D.; Leib, A. Projections of cardiovascular disease prevalence and costs: 2015–2035. Am. Heart Assoc. 2016, 214680, 1–54. [Google Scholar]
- Babulal, G.M.; Vivoda, J.; Harmon, A.; Carr, D.B.; Roe, C.M.; Zikmund-Fisher, B. Older Adults’ Expectations about Mortality, Driving Life and Years Left without Driving. J. Gerontol. Soc. Work 2019, 62, 794–811. [Google Scholar] [CrossRef]
- Babulal, G.M.; Stout, S.H.; Benzinger, T.L.S.; Ott, B.R.; Carr, D.B.; Webb, M.; Traub, C.M.; Addison, A.; Morris, J.C.; Warren, D.K.; et al. A Naturalistic Study of Driving Behavior in Older Adults and Preclinical Alzheimer Disease. J. Appl. Gerontol. 2017, 38, 277–289. [Google Scholar] [CrossRef]
- Davis, J.D.; Papandonatos, G.D.; Miller, L.A.; Hewitt, S.D.; Festa, E.K.; Heindel, W.C.; Ott, B.R. Road test and naturalistic driving performance in healthy and cognitively impaired older adults: Does environment matter? J. Am. Geriatr. Soc. 2012, 60, 2056–2062. [Google Scholar] [CrossRef]
- Roe, C.M.; Stout, S.H.; Rajasekar, G.; Ances, B.M.; Jones, J.M.; Head, D.; Benzinger, T.L.S.; Williams, M.M.; Davis, J.D.; Ott, B.R. A 2.5-year longitudinal assessment of naturalistic driving in preclinical Alzheimer’s disease. J. Alzheimers Dis. 2019, 68, 1625–1633. [Google Scholar] [CrossRef]
| First Author (Year) | Study Design | Purpose/Aim | Vascular Condition | Driving Outcome | N/Age Mean (SD) | Notable Findings |
|---|---|---|---|---|---|---|
| M. L. Alosco (2013) | Cross-sectional Case-control | Association between cognitive functioning and driving performance | Heart Failure | Simulator (STISIM: Build 2.08.03) | Cases N = 18:/67.7 (8.6) Controls N = 97:/19.9(3.0) | HF cases drove worse compared to controls; poor cognitive function was directly associated with poorer driving |
| M. L. Alosco (2015a) | Cross-sectional | Association between white matter hyperintensities and brain volume on driving | Heart Failure | Simulator (STISIM: Build 2.08.03) | N = 49:/69.1 (8.27) | MRI indices correlate with driving performance and cognitive functioning in patients with HF |
| M. L. Alosco (2015b) | Cross-sectional | Association between driving, physical fitness and cognitive functioning | Heart Failure | Simulator (STISIM: Build 2.08.03) | N = 18:/67.7 (8.6) | Poor physical fitness was associated with worse driving performance among patients with HF |
| B. A. Fausto (2017) | Retrospective Longitudinal | Whether heart failure predicts driving cessation | Heart Failure | Driving Habits Questionnaire Self-report | Cases N = 29:/74.7 (5.5) Controls N = 821/72.8 (5.4) | HF cases at a higher risk for driving cessation and may be mediated by poor cognitive functioning |
| L. J. Fitten (1995) | Cross-sectional | Examine driving performance between vascular and Alzheimer’s dementia | Vascular Dementia | Sepulveda Road Test | AD N = 13:/70.0 (7.4) VD N = 12:/71.8 (5.1) | The vascular dementia group showed greater variation in scores on the road test but higher cognitive scores compared to the AD group |
| M. Jang (2018) | Retrospective Longitudinal | Determine if WMH predicts driving cessation over time | WMH | Self-report Cessation | Mild N = 389:/66.0 (8.7) Mod. N = 116:/70.7 (7.4) Severe N = 35:/73.4 (6.4) | WMH and severity are associated with driving cessation and faster change in status from ‘currently driving’ to ‘no longer driving.’ |
| K. Nakano (2014) | Cross-sectional | Association between WMH and driving performance | WMH | Standard licensing road test | Young: N = 9/24.7 (3.6) No WMH N = 13/70.0 (6.6) WMH N = 13/69.5 (6.1) | Both older groups performed worse that the young group. Older adults with WMH were distracted and made more errors on the road test |
| D Piersma (2018) | Cross-sectional | To examine prediction of fitness-to-drive among older adults with different dementia | Vascular Dementia | Road test and simulator (Jentig 50) | VD N = 12:/75.0 (5.3) FTD N = 14:/67.3 (10.3) DLB N = 8:/71.7 (10.3) | Compared to the other groups, the vascular dementia group made more errors on the road test and driving simulator and had poor cognitive functioning |
| R. V. Sims (2011) | Prospective Longitudinal | Whether heart failure predicts driving cessation | Heart Failure | Self-report Cessation | No HF N = 4544/73 (5) HF N = 839/76 (6) | HR is an independent risk factor for driving cessation over time while accounting for multiple covariates. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babulal, G.M.; Kolady, R.; Stout, S.H.; Roe, C.M. A Systematic Review Examining Associations between Cardiovascular Conditions and Driving Outcomes among Older Drivers. Geriatrics 2020, 5, 27. https://doi.org/10.3390/geriatrics5020027
Babulal GM, Kolady R, Stout SH, Roe CM. A Systematic Review Examining Associations between Cardiovascular Conditions and Driving Outcomes among Older Drivers. Geriatrics. 2020; 5(2):27. https://doi.org/10.3390/geriatrics5020027
Chicago/Turabian StyleBabulal, Ganesh M., Ramana Kolady, Sarah H. Stout, and Catherine M. Roe. 2020. "A Systematic Review Examining Associations between Cardiovascular Conditions and Driving Outcomes among Older Drivers" Geriatrics 5, no. 2: 27. https://doi.org/10.3390/geriatrics5020027
APA StyleBabulal, G. M., Kolady, R., Stout, S. H., & Roe, C. M. (2020). A Systematic Review Examining Associations between Cardiovascular Conditions and Driving Outcomes among Older Drivers. Geriatrics, 5(2), 27. https://doi.org/10.3390/geriatrics5020027





