Quantifying Airway Invasion and Pharyngeal Residue in Patients with Dementia
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Incidence of Penetration, Aspiration, and Residue
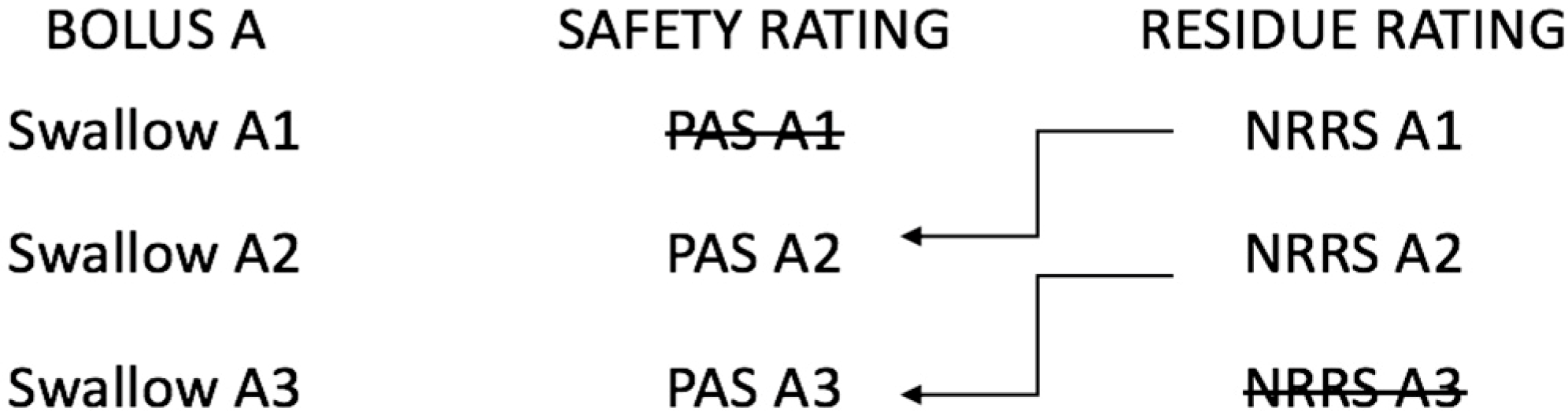
3.2. The Relationship between Residue and Aspiration on the Subsequent Swallow
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chambers, L.W.; Bancej, C.; McDowell, I. Prevalence and Monetary Costs of Dementia in Canada. Alzheimer Soc. Can. 2016, 36. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2017 Alzheimer’s disease facts and figures. Alzheimers Dement. 2017, 13, 325–373. [Google Scholar] [CrossRef]
- Affoo, R.H.; Foley, N.; Rosenbek, J.; Shoemaker, K.J.; Martin, R.E. Swallowing dysfunction and autonomic nervous system dysfunction in Alzheimer’s disease: A scoping review of the evidence. J. Am. Geriatr. Soc. 2013, 6, 2203–2213. [Google Scholar] [CrossRef] [PubMed]
- Clavé, P.; Rofes, L.; Carrión, S.; Ortega, O.; Cabré, M.; Serra-Prat, M.; Arreola, V. Pathophysiology, relevance and natural history of oropharyngeal dysphagia among older people. In Stepping Stones to Living Well with Dysphagia; Karger Publishers: Basel, Switzerland, 2012; pp. 57–66. [Google Scholar]
- Berlinger, W.G.; Potter, J.F. Low body mass index in demented outpatients. J. Am. Geriatr. Soc. 1991, 39, 973–978. [Google Scholar] [CrossRef]
- Watson, R. Undernutrition, weight loss and feeding difficulty in elderly patients with dementia: A nursing perspective. Rev. Clin. Gerontol. 1997, 7, 317–326. [Google Scholar] [CrossRef]
- Langmore, S.E.; Olney, R.K.; Lomen-Hoerth, C.; Miller, B.L. Dysphagia in patients with frontotemporal lobar dementia. Arch. Neurol. 2007, 64, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Priefer, B.A.; Robbins, J. Eating changes in mild-stage Alzheimer’s disease: A pilot study. Dysphagia 1997, 12, 212–221. [Google Scholar] [CrossRef]
- Londos, E.; Hanxsson, O.; Hirsch, I.A.; Janneskog, A.; Bülow, M.; Palmqvist, S. Dysphagia in Lewy body dementia—A clinical observational study of swallowing function by videofluoroscopic examination. BMC Neurol. 2013, 13, 140. [Google Scholar] [CrossRef]
- Feinberg, M.J.; Ekberg, O.; Segall, L.; Tully, J. Deglutition in elderly patients with dementia: Findings of videofluorographic evaluation and impact on staging and management. Radiology 1992, 183, 811–814. [Google Scholar] [CrossRef]
- Suh, M.K.; Kim, H.; Na, D.L. Dysphagia in patients with dementia: Alzheimer versus vascular. Alzheimer Dis. Assoc. Dis. 2009, 23, 178–184. [Google Scholar] [CrossRef]
- Humbert, I.A.; McLaren, D.G.; Kosmatka, K.; Fitzgerald, M.; Johnson, S.; Porcaro, E.; Kays, S.; Umoh, E.-O.; Robbins, J. Early deficits in cortical control of swallowing in Alzheimer’s disease. J. Alzheimers Dis. 2010, 19, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Horner, J.; Alberts, M.J.; Dawson, D.V.; Cook, G.M. Swallowing in Alzheimer’s disease. Alzheimer Dis. Assoc. Dis. 1994, 8, 177–189. [Google Scholar]
- Rofes, L.; Arreola, V.; Almirall, J.; Cabré, M.; Campins, L.; García-Peris, P.; Speyer, R.; Clavé, P. Diagnosis and management of oropharyngeal dysphagia and its nutritional and respiratory complications in the elderly. Gastroenterol. Res. Pract. 2011, 2011, 818979. [Google Scholar] [CrossRef]
- Rosenbek, J.C.; Robbins, J.A.; Roecker, E.B.; Coyle, J.L.; Wood, J.L. A penetration-aspiration scale. Dysphagia 1996, 11, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Pearson, W.G.; Molfenter, S.M.; Smith, Z.M.; Steele, C.M. Image-based measurement of post-swallow residue: The normalized residue ratio scale. Dysphagia 2013, 28, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Fleiss, J.L. Design and Analysis of Clinical Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Steele, C.M.; Peladeau-Pigeon, M.; Barbon, C.E.A.; Guida, B.T.; Namasivayam-MacDonald, A.M.; Tapson, M.S.; Valenzano, T.; Waito, A.; Wolkin, T. Post-swallow pharyngeal residue: How much is normal? In Proceedings of the Dysphagia Research Society 2018 Annual Meeting, Baltimore, MD, USA, 16 March 2018. [Google Scholar]
- Allen, J.E.; White, C.J.; Leonard, R.J.; Belafsky, P.C. Prevalence of penetration and aspiration on videofluoroscopy in normal individuals without dysphagia. Otolaryngol. Head Neck Surg. 2010, 142, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Daggett, A.; Logemann, J.; Rademaker, A.; Pauloski, B. Laryngeal penetration during deglutition in normal subjects of various ages. Dysphagia 2006, 21, 270–274. [Google Scholar] [CrossRef]
- Butler, S.G.; Stuart, A.; Markley, L.; Rees, C. Penetration and Aspiration in Healthy Older Adults as Assessed during Endoscopic Evaluation of Swallowing. Ann. Otol. Rhinol. Laryngol. 2009, 118, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Shaker, R.; Dodds, W.J.; Dantas, R.O.; Hogan, W.J.; Arndorfer, R.C. Coordination of deglutitive glottic closure with oropharyngeal swallowing. Gastroenterology 1990, 98, 1478–1484. [Google Scholar] [CrossRef]
- Inamoto, Y.; Fujii, N.; Saitoh, E.; Baba, M.; Okada, S.; Katada, K.; Ozeki, Y.; Kanamori, D.; Palmer, J.B. Evaluation of Swallowing Using 320-detector-row Multislice CT. Part II: Kinematic Analysis of Laryngeal Closure during Normal Swallowing. Dysphagia 2011, 26, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Ekberg, O. Closure of the Laryngeal Vestibule During Deglutition. Acta Oto-Laryngol. 1982, 93, 123–129. [Google Scholar] [CrossRef]
- Ohmae, Y.; Logemann, J.A.; Kaiser, P.; Hanson, D.G.; Kahrilas, P.J. Timing of glottic closure during normal swallow. Head Neck 1995, 17, 394–402. [Google Scholar] [CrossRef]
- Molfenter, S.M.; Steele, C.M. The Relationship Between Residue and Aspiration on the Subsequent Swallow: An Application of the Normalized Residue Ratio Scale. Dysphagia 2013, 28, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Alagiakrishnan, K.; Bhanji, R.A.; Kurian, M. Evaluation and management of oropharyngeal dysphagia in different types of dementia: A systematic review. Arch. Gerontol. Geriat. 2013, 56, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dejaeger, E.; Pelemans, W.; Ponette, E.; Joosten, E. Mechanisms Involved in Postdeglutition Retention in the Elderly. Dysphagia 1997, 12, 63–67. [Google Scholar] [CrossRef]
- Stokely, S.L.; Peladeau-Pigeon, M.; Leigh, C.; Molfenter, S.M.; Steele, C.M. The Relationship between Pharyngeal Constriction and Post-swallow Residue. Dysphagia 2015, 30, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Molfenter, S.M.; Lenell, C.; Lazarus, C.L. Volumetric Changes to the Pharynx in Healthy Aging: Consequence for Pharyngeal Swallow Mechanics and Function. Dysphagia 2018. [Google Scholar] [CrossRef]
- Daniels, S.K.; Schroeder, M.F.; DeGeorge, P.C.; Corey, D.M.; Rosenbek, J.C. Effects of Verbal Cue on Bolus Flow During Swallowing. Am. J. Speech-Lang. Pathol. 2007, 16, 140–147. [Google Scholar] [CrossRef]

| PAS Score | PAS Description | n (%) of Thin Liquid Swallows | n (%) of Extremely Thick Liquid Swallows |
|---|---|---|---|
| 1 | Material does not enter the airway. | 216 (55%) | 152 (64%) |
| 2 | Material enters the airway, remains above the vocal folds, and is ejected from the airway. | 59 (15%) | 23 (10%) |
| 3 | Material enters the airway, remains above the vocal folds, and is not ejected from the airway. | 43 (11%) | 35 (15%) |
| 4 | Material enters the airway, contacts the vocal folds, and is ejected from the airway. | 8 (2%) | 1 (0.33%) |
| 5 | Material enters the airway, contacts the vocal folds, and is not ejected from the airway. | 16 (4%) | 1 (0.33%) |
| 6 | Material enters the airway, passes below the vocal folds, and is ejected into the larynx or out of the airway. | 4 (1%) | 1 (0.33%) |
| 7 | Material enters the airway, passes below the vocal folds, and is not ejected from the trachea despite effort. | 20 (5%) | 9 (4%) |
| 8 | Material enters the airway, passes below the vocal folds, and no effort is made to eject. | 27 (7%) | 15 (6%) |
| Stimuli | Swallow Status | n (%) | NRRSv | NRRSp | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | 95% CI | Mean | SD | 95% CI | |||
| Thin liquid | Nonterminal | 122 (31%) | 0.32 | 0.32 | 0.26–0.37 | 0.15 | 0.34 | 0.10–0.19 |
| Terminal | 271 (69%) | 0.16 | 0.25 | 0.13–0.19 | 0.04 | 0.09 | 0.03–0.05 | |
| Extremely thick liquid | Nonterminal | 67 (28%) | 0.41 | 0.57 | 0.28–0.55 | 0.08 | 0.19 | 0.04–0.13 |
| Terminal | 170 (72%) | 0.21 | 0.30 | 0.16–0.25 | 0.02 | 0.07 | 0.00–0.03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namasivayam-MacDonald, A.M.; Riquelme, L.F. Quantifying Airway Invasion and Pharyngeal Residue in Patients with Dementia. Geriatrics 2019, 4, 13. https://doi.org/10.3390/geriatrics4010013
Namasivayam-MacDonald AM, Riquelme LF. Quantifying Airway Invasion and Pharyngeal Residue in Patients with Dementia. Geriatrics. 2019; 4(1):13. https://doi.org/10.3390/geriatrics4010013
Chicago/Turabian StyleNamasivayam-MacDonald, Ashwini M., and Luis F. Riquelme. 2019. "Quantifying Airway Invasion and Pharyngeal Residue in Patients with Dementia" Geriatrics 4, no. 1: 13. https://doi.org/10.3390/geriatrics4010013
APA StyleNamasivayam-MacDonald, A. M., & Riquelme, L. F. (2019). Quantifying Airway Invasion and Pharyngeal Residue in Patients with Dementia. Geriatrics, 4(1), 13. https://doi.org/10.3390/geriatrics4010013





