Prognostic Factors for the Survival of Elderly Patients Who Were Hospitalized in the Medical Ward of Our Hospital in Japan
Abstract
1. Introduction
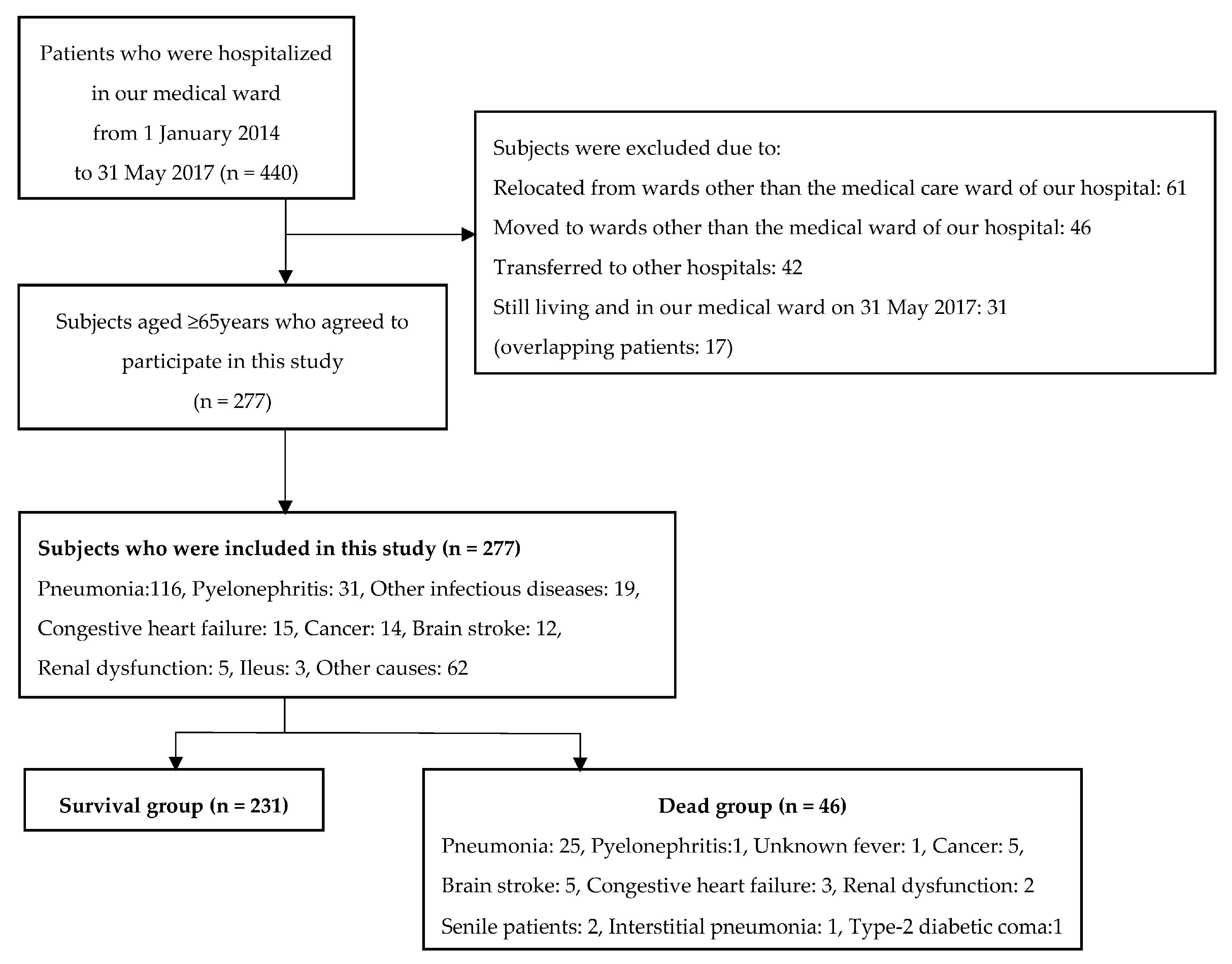
2. Materials and Methods
3. Results
4. Discussion
Acknowledgments
Conflicts of Interest
References
- Teno, J.M.; Harrell, F.E., Jr.; Knaus, W.; Phillips, R.S.; Wu, A.W.; Connors, A., Jr.; Wenger, N.S.; Wagner, D.; Galanos, A.; Desbiens, N.A.; et al. Prediction of survival for older hospitalized patients: The HELP survival model. Hospitalized Elderly Longitudinal Project. J. Am. Geriatr. Soc. 2000, 48 (Suppl. S5), S16–S24. [Google Scholar] [CrossRef] [PubMed]
- Stuck, A.E.; Siu, A.L.; Wieland, G.D.; Adams, J.; Rubenstein, L.Z. Comprehensive geriatric assessment: A meta-analysis of controlled trials. Lancet 1993, 342, 1032–1036. [Google Scholar] [CrossRef]
- Abbatecola, A.M.; Spazzafumo, L.; Corsonello, A.; Sirolla, C.; Bustacchini, S.; Guffanti, E. Development and validation of the HOPE prognostic index on 24-month posthospital mortality and rehospitalization: Italian National Research Center on Aging (INRCA). Rejuv. Res. 2011, 14, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Espaulella, J.; Arnau, A.; Cubí, D.; Amblàs, J.; Yánez, A. Time-dependent prognostic factors of 6-month mortality in frail elderly patients admitted to post-acute care. Age Ageing 2007, 36, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.; Ferrucci, L.; Franceschi, M.; D’Ambrosio, L.P.; Scarcelli, C.; Cascavilla, L.; Paris, F.; Placentino, G.; Seripa, D.; Dallapiccola, B.; et al. Development and validation of a multidimensional prognostic index for one-year mortality from comprehensive geriatric assessment in hospitalized older patients. Rejuv. Res. 2008, 11, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, A.; Murakami, T.; Kurumadani, H.; Shimizu, J.; Fujiwara, N. Relationship between criteria for evaluating the degree of independence of disabled elderly persons in performing activities of daily living. Sagyou Ryouhou 2002, 21, 455–462. (In Japanese) [Google Scholar]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [PubMed]
- Kokura, Y.; Maeda, K.; Wakabayashi, H.; Nishioka, S.; Higashi, S. High nutritional-related risk on admission predicts less improvement of functional independence measure in geriatric Stroke Patients: A retrospective cohort study. J. Stroke Cerebrovasc. Dis. 2016, 25, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Ignacio de Ulíbarri, J.; González-Madroño, A.; de Villar, N.G.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar] [PubMed]
- Narumi, T.; Arimoto, T.; Funayama, A.; Kadowaki, S.; Otaki, Y.; Nishiyama, S.; Takahashi, H.; Shishido, T.; Miyashita, T.; Miyamoto, T.; et al. Prognostic importance of objective nutritional indexes in patients with chronic heart failure. J. Cardiol. 2013, 62, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Hak, E.; Bont, J.; Hoes, A.W.; Verheij, T.J. Prognostic factors for serious morbidity and mortality from community-acquired lower respiratory tract infections among the elderly in primary care. Fam. Pract. 2005, 22, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, F.; Ghaderpanahi, M.; Fakhrzadeh, H.; Mirarefin, M.; Badamchizadeh, Z.; Tajalizadekhoob, Y.; Fadayivatan, R.; Philp, I.; Larijani, B. Older people’s mortality index: Development of a practical model for prediction of mortality in nursing homes (Kahrizak Elderly Study). Geriatr. Gerontol. Int. 2012, 12, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Hu, X.; Chen, J.; Wang, H.; Zhang, L.; Dong, B.; Yang, M. Predicting long-term mortality in hospitalized elderly patients using the new ESPEN definition. Sci. Rep. 2017, 7, 4067. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Hertan, H.; Pitchumoni, C.S. Hypoalbuminemia is a poor predictor of survival after percutaneous endoscopic gastrostomy in elderly patients with dementia. Am. J. Gastroenterol. 2000, 95, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Gom, I.; Fukushima, H.; Shiraki, M.; Miwa, Y.; Ando, T.; Takai, K.; Moriwaki, H. Relationship between serum albumin level and aging in community-dwelling self-supported elderly population. J. Nutr. Sci. Vitaminol. Tokyo 2007, 53, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Hayakawa, T.; Hozawa, A.; Kadowaki, T.; Murakami, Y.; Kita, Y.; Abbott, R.D.; Okayama, A.; Ueshima, H.; NIPPON DATA80 Research Group. Lower levels of serum albumin and total cholesterol associated with decline in activities of daily living and excess mortality in a 12-year cohort study of elderly Japanese. J. Am. Geriatr. Soc. 2008, 56, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Avelino-Silva, T.J.; Farfel, J.M.; Curiati, J.A.; Amaral, J.R.; Campora, F.; Jacob-Filho, W. Comprehensive geriatric assessment predicts mortality and adverse outcomes in hospitalized older adults. BMC Geriatr. 2014, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.M.; Tang, W.H.; Woo, J. Predictors of in-hospital mortality of older patients admitted for community-acquired pneumonia. Age Ageing 2011, 40, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Rosolem, M.M.; Rabello, L.S.; Lisboa, T.; Caruso, P.; Costa, R.T.; Leal, J.V.; Salluh, J.I.; Soares, M. Critically ill patients with cancer and sepsis: Clinical course and prognostic factors. J. Crit. Care 2012, 27, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Ohura, T.; Nakajo, T.; Okada, S.; Omura, K.; Adachi, K. Evaluation of effects of nutrition intervention on healing of pressure ulcers and nutritional states (randomized controlled trial). Wound Repair Regen. 2011, 19, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Pearte, C.A.; Furberg, C.D.; O’Meara, E.S.; Psaty, B.M.; Kuller, L.; Powe, N.R.; Manolio, T. Characteristics and baseline clinical predictors of future fatal versus nonfatal coronary heart disease events in older adults: The cardiovascular health study. Circulation 2006, 113, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.H.; Kim, J.S.; Kwon, S.U.; Yun, S.C.; Koh, J.Y.; Kang, D.W. Undernutrition as a predictor of poor clinical outcomes in acute ischemic stroke patients. Arch. Neurol. 2008, 65, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Nouvenne, A.; Ticinesi, A.; Lauretani, F.; Maggio, M.; Lippi, G.; Prati, B.; Borghi, L.; Meschi, T. The prognostic value of high-sensitivity C-reactive protein and prealbumin for short-term mortality in acutely hospitalized multimorbid elderly patients: A prospective cohort study. J. Nutr. Health Aging 2016, 20, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Norman, D.C. Fever in the elderly. Clin. Infect. Dis. 2000, 31, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.T. Epidemiology and unique aspects of aging and infectious disease. Clin. Infect. Dis. 2000, 30, 931–933. [Google Scholar] [CrossRef] [PubMed]
- Dewan, S.K.; Zheng, S.B.; Xia, S.J.; Bill, K. Senescent remodeling of the immune system and its contribution to the predisposition of the elderly to infections. Chin. Med. J. Engl. 2012, 125, 3325–3331. [Google Scholar] [PubMed]
- Zalacain, R.; Torres, A.; Celis, R.; Blanquer, J.; Aspa, J.; Esteban, L.; Menéndez, R.; Blanquer, R.; Borderías, L. Commmity-acquired pneumonia in the elderly: Spanish multicentre study. Eur. Respir. J. 2003, 21, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.L.; Miller, S.C.; Teno, J.M.; Kiely, D.K.; Davis, R.B.; Shaffer, M.L. Prediction of 6-month survival of nursing home residents with advanced dementia using ADEPT vs hospice eligibility guidelines. JAMA 2010, 304, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
| Self-supported | Rank J | Some disabilities, but daily living is mostly independent; capable of going outdoors unassisted. |
| 1 | Goes outdoors with means of transportation, etc. | |
| 2 | Goes out near home. | |
| Quasi-bedridden | Rank A | Indoor living predominantly independent, but unable to go out without assistance. |
| 1 | Goes out with assistance, spending most of the time during the daytime out of bed. | |
| 2 | Does not go out frequently, repeating cycles of lying down on and getting up from the bed during the daytime. | |
| Bedridden | Rank B | Some assistance needed for indoor living, also lies in bed for much of the daytime, although sitting position is possible. |
| 1 | Uses a wheelchair without assistance, takes meals, and excretes/urinates off the bed. | |
| 2 | Uses a wheelchair with assistance. | |
| Rank C | Bedridden all day, requires assistance with excretion/urination, meals, and dressing/undressing. | |
| 1 | Capable of changing posture in bed. | |
| 2 | Unable to change posture in bed without assistance. |
| Rank I | Has some type of dementia, but almost independent in terms of daily living at home and in society. |
| Rank II | Some daily life-disturbing symptoms, behaviors and problems in communication seen but can lead daily life independently if kept watched by someone. |
| IIa | Condition II, mentioned above, seen outside home. |
| IIb | Condition II, mentioned above, seen at home. |
| Rank III | Daily life-disturbing symptoms, behaviors, and problems in communication that require assistance. |
| IIIa | Condition III, mentioned above, seen primarily during the daytime. |
| IIIb | Condition III, mentioned above, seen primarily at night. |
| Rank IV | Daily life-disturbing symptoms, behaviors, and problems in communication frequently require assistance. |
| Rank M | Marked psychiatric symptoms/related symptoms or serious physical disorders require expert management. |
| Variable | Survived (n = 231) | Died (n = 46) | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|---|---|
| Sex | 0.878 | 0.443–1.753 | 0.745 | ||
| Male | 98 | 21 | |||
| Female | 133 | 25 | |||
| Hospitalization source | |||||
| Home | 28 | 4 | |||
| nursing home | 150 | 26 | |||
| other hospital | 53 | 16 | |||
| Independence degree of daily living for the disabled elderly | 0.200 | ||||
| JA | 12 | 0 | |||
| B | 73 | 4 | |||
| C | 146 | 42 | |||
| Independence degree of daily living for the demented elderly | 0.029 * | ||||
| I | 39 | 0 | |||
| II | 38 | 3 | |||
| III | 73 | 9 | |||
| IV/M | 81 | 34 | |||
| History of bone fracture | 97 | 17 | 0.810 | 0.394–1.624 | 0.623 |
| History of brain stroke | 117 | 15 | 0.473 | 0.224–0.959 | 0.0349 * |
| History of heart failure | 80 | 20 | 1.450 | 0.719–2.891 | 0.313 |
| History of malignant disease | 46 | 14 | 1.755 | 0.798–3.721 | 0.120 |
| Intake of steroid | 15 | 3 | 1.005 | 0.179–3.775 | 1.000 |
| Intake of sleeping pill | 57 | 6 | 0.459 | 0.151–1.168 | 0.122 |
| Intake of psychotropic drug | 40 | 11 | 1.449 | 0.632–3.342 | 0.301 |
| Intake of dementia treatment drug | 42 | 5 | 0.550 | 0.160–1.551 | 0.285 |
| Variable | Survived (n = 231) | Died (n = 46) | p-Value |
|---|---|---|---|
| Age (y.o.) | 84.1 ± 8.6 | 88.8 ± 6.3 | 0.0001 * |
| Height (cm) | 150.5 ± 10.2 | 148.1 ± 9.4 | 0.180 |
| Weight (kg) | 46.1 ± 9.6 | 42.0 ± 9.7 | 0.021 * |
| BMI (kg/m2) | 20.4 ± 3.8 | 19.1 ± 3.8 | 0.048 * |
| The highest body temperature (°C) | 37.4 ± 0.9 | 37.1 ± 0.8 | 0.038 * |
| Systolic blood pressure (mmHg) | 133.3 ± 22.5 | 135.0 ± 23.5 | 0.718 |
| Heart rate (beats/min) | 81.0 ± 14.9 | 83.5 ± 21.7 | 0.607 |
| Respiratory rate (times/min) | 20.1 ± 6.5 | 21.5 ± 7.3 | 0.082 |
| SpO2 (%) | 93.2 ± 7.2 | 91.4 ± 5.0 | 0.002 * |
| Oxygen flow (L/min) | 0.5 ± 1.1 | 1.5 ± 2.3 | 0.001 * |
| White blood Cell (×103/μL) | 8.6 ± 4.1 | 9.0 ± 4.2 | 0.445 |
| Hematocrit (%) | 33.8 ± 5.0 | 33.1 ± 5.0 | 0.282 |
| Hemoglobin (g/dL) | 11.5 ± 1.8 | 11.1 ± 1.7 | 0.235 |
| Platelet (×104/μL) | 24.8 ± 12.6 | 22.5 ± 11.0 | 0.317 |
| C-reactive protein (mg/dL) | 4.2 ± 4.9 | 4.6 ± 5.2 | 0.217 |
| Blood urea nitrogen (mg/dL) | 19.3 ± 11.1 | 33.7 ± 34.7 | 0.012 * |
| Creatinine (mg/dL) | 0.89 ± 0.54 | 1.24 ± 1.03 | 0.010 * |
| Serum sodium (mEq/L) | 136.9 ± 5.1 | 135.7 ± 7.2 | 0.019 * |
| Serum potassium (mEq/L) | 4.1 ± 0.7 | 4.3 ± 0.9 | 0.263 |
| Total protein (g/dL) | 6.5 ± 0.8 | 6.4 ± 0.8 | 0.555 |
| Serum albumin (g/dL) | 3.2 ± 0.6 | 2.9 ± 0.6 | 0.001 * |
| GNRI | 84.5 ± 9.7 | 77.9 ± 10.5 | 0.0003 * |
| CONUT | 4.8 ± 2.6 | 6.2 ± 2.6 | 0.002 * |
| Cholinesterase (IU/L) | 190.4 ± 63.9 | 152.8 ± 60.7 | 0.0002 * |
| Total cholesterol (mg/dL) | 161.4 ± 43.8 | 150.4 ± 51.3 | 0.085 |
| Blood glucose (mg/dL) | 128.5 ± 47.5 | 163.6 ± 203.4 | 0.370 |
| HbA1c (NGSP) (%) | 6.5 ± 10.3 | 5.9 ± 1.3 | 0.976 |
| Variable | Odds Ratio (Adjusted) | 95% Confidence Interval (Adjusted) | p-Value |
|---|---|---|---|
| Age (y.o.) | 1.09 (1.07) | 1.04–1.15 (1.00–1.14) | 0.027 |
| Blood urea nitrogen (mg/dL) | 1.04 (1.04) | 1.02–1.06 (1.02–1.07) | <0.001 |
| Serum albumin (g/dL) | 0.37 (0.39) | 0.21–0.66 (0.19–0.78) | 0.006 |
| Independence degree of daily living for the demented elderly | 3.06 (2.76) | 1.85–5.08 (1.63–4.68) | <0.001 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abe, S. Prognostic Factors for the Survival of Elderly Patients Who Were Hospitalized in the Medical Ward of Our Hospital in Japan. Geriatrics 2017, 2, 32. https://doi.org/10.3390/geriatrics2040032
Abe S. Prognostic Factors for the Survival of Elderly Patients Who Were Hospitalized in the Medical Ward of Our Hospital in Japan. Geriatrics. 2017; 2(4):32. https://doi.org/10.3390/geriatrics2040032
Chicago/Turabian StyleAbe, Shuichi. 2017. "Prognostic Factors for the Survival of Elderly Patients Who Were Hospitalized in the Medical Ward of Our Hospital in Japan" Geriatrics 2, no. 4: 32. https://doi.org/10.3390/geriatrics2040032
APA StyleAbe, S. (2017). Prognostic Factors for the Survival of Elderly Patients Who Were Hospitalized in the Medical Ward of Our Hospital in Japan. Geriatrics, 2(4), 32. https://doi.org/10.3390/geriatrics2040032





