Impact of Abdominal Obesity on Frailty Development: A Web-Based Survey Using a Smartphone Health App
Abstract
1. Introduction
2. Materials and Methods
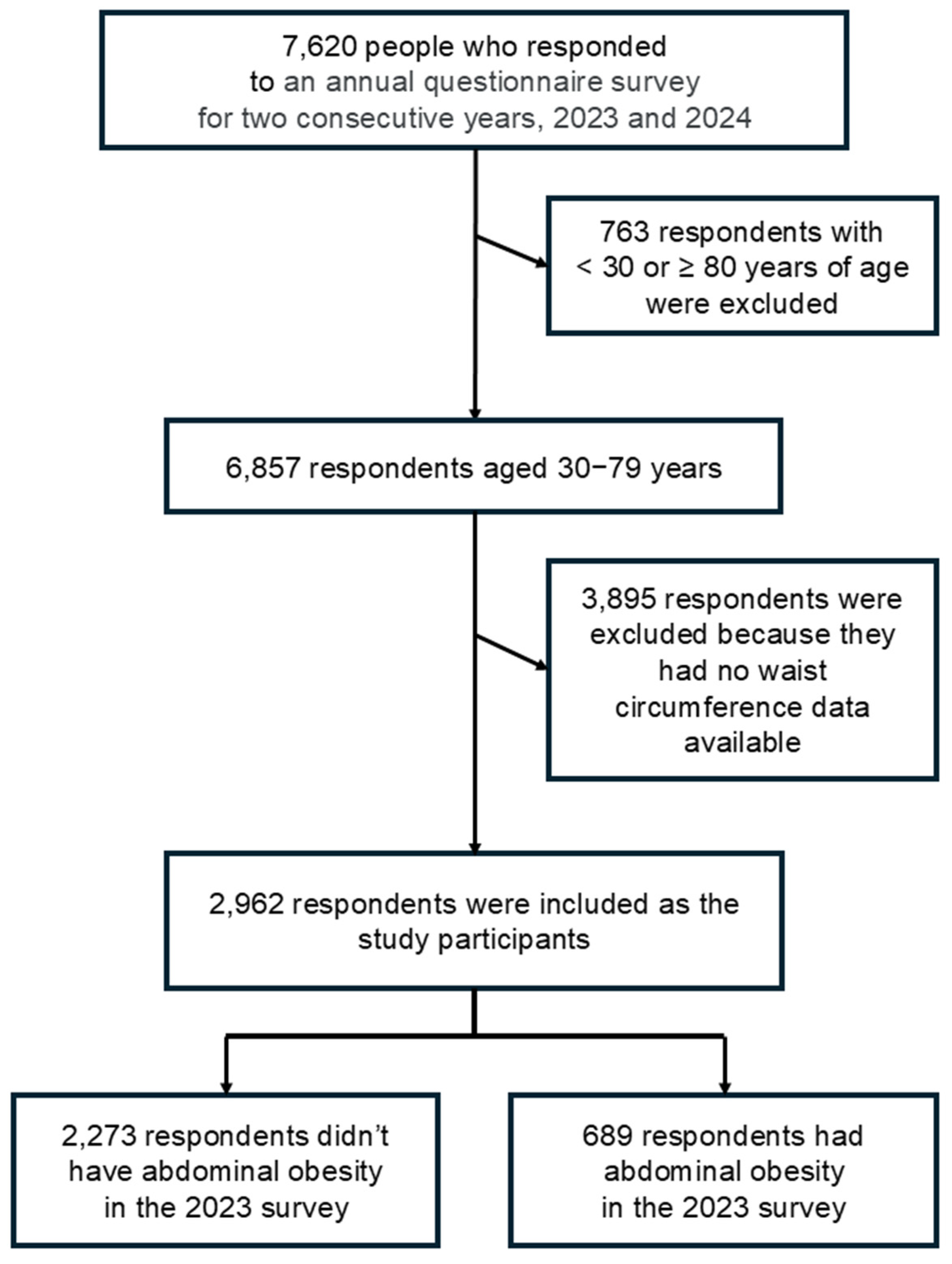
2.1. Study Design and Participants
2.2. Measures
2.3. Waist Circumference
2.4. Statistical Analysis
- Model 1: Age, sex
- Model 2: Model 1 + abdominal obesity
- Model 3: Model 2 + baseline KCL score
- Model 4: Model 3 + exercise habits and frailty awareness
- Model 5: Model 4 + mean daily steps
3. Results
3.1. Participant Characteristics and Abdominal Obesity Prevalence
3.2. Baseline Differences Between Participants with and Without Abdominal Obesity
3.3. Incidence of Frailty by Obesity Status and Related Factors
3.4. Multivariable Analysis of Predictors for Frailty Onset
3.5. Interaction Between Abdominal Obesity and Exercise Habit
3.6. Sensitivity Analysis and Model Robustness
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Japanese Ministry of Health, L., and Welfare. Average Life Expectancy and Healthy Life Expectancy. 2025. Available online: https://kennet.mhlw.go.jp/information/information/hale/h-01-002 (accessed on 8 September 2025).
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L.P. Frailty: Implications for clinical practice and public health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Honda, T.; Narazaki, K.; Chen, T.; Kishimoto, H.; Kumagai, S. Physical Frailty and Risk of Needing Long-Term Care in Community-Dwelling Older Adults: A 6-Year Prospective Study in Japan. J. Nutr. Health Aging 2019, 23, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Beals, J.W.; Burd, N.A.; Moore, D.R.; van Vliet, S. Obesity Alters the Muscle Protein Synthetic Response to Nutrition and Exercise. Front. Nutr. 2019, 6, 87. [Google Scholar] [CrossRef] [PubMed]
- Osaka Prefecture. Osaka Prefecture’s Health App “ASMILE. Available online: https://www.asmile.pref.osaka.jp (accessed on 18 January 2024).
- Japanese Ministry of Health, L., and Welfare. Manual on Daily-Living Function Assessment for Long-Term Care Prevention (Revised Edition). 2009. Available online: https://www.mhlw.go.jp/topics/2009/05/dl/tp0501-1 c_0001.pdf (accessed on 23 January 2024).
- Kyoto-Kameoka study group; Yamaguchi, M.; Yoshida, T.; Yamada, Y.; Watanabe, Y.; Nanri, H.; Yokoyama, K.; Date, H.; Miyake, M.; Itoi, A.; et al. Sociodemographic and physical predictors of non-participation in community based physical checkup among older neighbors: A case-control study from the Kyoto-Kameoka longitudinal study, Japan. BMC Public Health 2018, 18, 568. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y. Examination Committee of Criteria for ‘Metabolic Syndrome’ in Japan. Definition and criteria of meta-bolic syndrome. J. Jpn. Soc. Study Obes. 2005, 94, 794–809. [Google Scholar]
- International Diabetes Federation. The IDF Consensus Worldwide Definition of the Metabolic Syndrome; International Diabetes Federation: Brussels, Belgium, 2005. [Google Scholar]
- Pérez-Tasigchana, R.F.; León-Muñoz, L.M.; Lopez-Garcia, E.; Gutierrez-Fisac, J.L.; Laclaustra, M.; Rodríguez-Artalejo, F.; Guallar-Castillón, P. Metabolic syndrome and insulin resistance are associated with frailty in older adults: A prospective cohort study. Age Ageing 2017, 46, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Lynch, G.M.; Murphy, C.H.; Castro, E.d.M.; Roche, H.M. Inflammation and metabolism: The role of adiposity in sarcopenic obesity. Proc. Nutr. Soc. 2020, 79, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Xia, J.; Yu, J.; Wang, J.; Yin, S.; Yang, J.; Wu, T.; Zhang, Z.; Yan, W.; Wang, S.; et al. Pathophysiological Mechanisms Underlying Sarcopenia and Sarcopenic Obesity: A Systematic Review and Meta-Analysis of Biomarker Evidence. Int. J. Mol. Sci. 2025, 26, 5113. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tan, Y.; Shi, Y.; Wang, X.; Liao, Z.; Wei, P. Diabetes and Sarcopenic Obesity: Pathogenesis, Diagnosis, and Treatments. Front. Endocrinol. 2020, 11, 568. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-C.; Ki, S.-W.; Kim, H.; Kang, S.; Kim, H.; Go, G.-W. Recent Advances in Nutraceuticals for the Treatment of Sarcopenic Obesity. Nutrients 2023, 15, 3854. [Google Scholar] [CrossRef] [PubMed]
- Fraenkel, M.; Kim, M.; Faggiano, A.; De Herder, W.W.; Valk, G.D. Incidence of gastroenteropancreatic neuroendocrine tumours: A systematic review of the literature. Endocr. Relat. Cancer 2014, 21, R153–R163. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-H.; Yang, H.-Y.; Shun, S.-C. Effect of exercise intervention dosage on reducing visceral adipose tissue: A systematic review and network meta-analysis of randomized controlled trials. Int. J. Obes. 2021, 45, 982–997. [Google Scholar] [CrossRef] [PubMed]
- Eidam, A.; Durga, J.; Bauer, J.M.; Zimmermann, S.; Vey, J.A.; Rapp, K.; Schwenk, M.; Cesari, M.; Benzinger, P. Interventions to prevent the onset of frailty in adults aged 60 and older (PRAE-Frail): A systematic review and network meta-analysis. Eur. Geriatr. Med. 2024, 15, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Shirvani, T.; Javadivala, Z.; Azimi, S.; Shaghaghi, A.; Fathifar, Z.; Bhalla, H.D.R.D.; Abdekhoda, M.; Nadrian, H. Community-based educational interventions for prevention of type II diabetes: A global systematic review and meta-analysis. Syst. Rev. 2021, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Costenoble, A.; Knoop, V.; Debain, A.; Bautmans, I.; Van Laere, S.; Lieten, S.; Rossi, G.; Verté, D.; Gorus, E.; De Vriendt, P.; et al. Transitions in robust and prefrail octogenarians after 1 year: The influence of activities of daily living, social participation, and psychological resilience on the frailty state. BMC Geriatr. 2023, 23, 485. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Huang, X.; Wang, X.; Dai, M.; Yue, J. Development and validation of PRE-FRA (PREdiction of FRAilty risk in community older adults) frailty prediction model. Front. Public Health 2025, 13, 1593668. [Google Scholar] [CrossRef] [PubMed]
- E-Stat, G.S.P. National Health and Nutrition Survey Waist Circumference Distribution—Waist Cir-Cumference Classification. 2019. Available online: https://www.e-stat.go.jp/dbview?sid=0003234716 (accessed on 2 December 2024).

| Male | Female | |||||
|---|---|---|---|---|---|---|
| n = 1250 | n = 1712 | |||||
| abdominal obesity | abdominal obesity | |||||
| n | no (%) | yes (%) | n | no (%) | yes (%) | |
| 30–39 years | 10 | 80.0 | 20.0 | 17 | 100.0 | 0.0 |
| 40–49 years | 75 | 72.0 | 28.0 | 159 | 93.7 | 6.3 |
| 50–59 years | 252 | 62.3 | 37.7 | 450 | 90.0 | 10.0 |
| 60–69 years | 488 | 57.8 | 42.2 | 734 | 90.7 | 9.3 |
| 70–79 years | 425 | 55.1 | 44.9 | 352 | 85.5 | 14.5 |
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
|---|---|---|---|---|---|
| Variables | age + sex | Model 1 + Abdominal obesity | Model 2 + KCL score | Model 3 + Exercise habit + Frailty awareness | Model 4 + Mean daily steps |
| Age (per year) | 0.995 (0.979–1.011) p = 0.523 | 0.993 (0.977–1.010) p = 0.428 | 0.997 (0.980–1.015) p = 0.779 | 1.010 (0.992–1.030) p = 0.276 | 1.011 (0.992–1.030) p = 0.262 |
| Female (vs. male) | 0.729 (0.543–0.980) p = 0.036 | 0.819 (0.595–1.128) p = 0.221 | 0.970 (0.693–1.357) p = 0.859 | 1.147 (0.808–1.630) p = 0.443 | 1.169 (0.812–1.683) p = 0.402 |
| Abdominal obesity (yes) | 1.425 (1.003–2.023) p = 0.048 | 1.097 (0.757–1.589) p = 0.624 | 1.107 (0.762–1.608) p = 0.594 | 1.117 (0.767–1.627) p = 0.566 | |
| KCL score (per point) | 1.815 (1.648–1.998) p < 0.001 | 1.767 (1.602–1.950) p < 0.001 | 1.767 (1.602–1.950) p < 0.001 | ||
| Frailty awareness (vs. “do not know”) | |||||
| “have heard the word before” | 0.678 (0.436–1.054) p = 0.085 | 0.681 (0.438–1.060) p = 0.088 | |||
| “know a little” | 0.363 (0.233–0.567) p < 0.001 | 0.364 (0.233–0.569) p < 0.001 | |||
| “know well” | 0.341 (0.212–0.548) p < 0.001 | 0.341 (0.212–0.548) p < 0.001 | |||
| Exercise habit (yes) | 0.611 (0.399–0.935) p = 0.023 | 0.596 (0.382–0.930) p = 0.023 | |||
| Mean daily steps (per 1000 step) | 1.007 (0.972–1.043) p = 0.710 | ||||
| Model χ2 (df) | 4.465 (2), p < 0.107 | 8.278 (3), p < 0.041 | 186.90 (4), p < 0.001 | 221.84 (8), p < 0.001 | 221.98 (9), p < 0.001 |
| −2 Log Likelihood | 1366.55 | 1362.74 | 1161.24 | 1126.29 | 1126.16 |
| Nagelkerke R2 | 0.004 | 0.008 | 0.174 | 0.205 | 0.205 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokoyama, H. Impact of Abdominal Obesity on Frailty Development: A Web-Based Survey Using a Smartphone Health App. Geriatrics 2025, 10, 147. https://doi.org/10.3390/geriatrics10060147
Yokoyama H. Impact of Abdominal Obesity on Frailty Development: A Web-Based Survey Using a Smartphone Health App. Geriatrics. 2025; 10(6):147. https://doi.org/10.3390/geriatrics10060147
Chicago/Turabian StyleYokoyama, Hisayo. 2025. "Impact of Abdominal Obesity on Frailty Development: A Web-Based Survey Using a Smartphone Health App" Geriatrics 10, no. 6: 147. https://doi.org/10.3390/geriatrics10060147
APA StyleYokoyama, H. (2025). Impact of Abdominal Obesity on Frailty Development: A Web-Based Survey Using a Smartphone Health App. Geriatrics, 10(6), 147. https://doi.org/10.3390/geriatrics10060147






