Abstract
Background/Objectives: Alzheimer’s disease (AD) often begins with episodic memory deficits, detectable in Mild Cognitive Impairment (MCI). The Loewenstein–Acevedo Scales of Semantic Interference and Learning (LASSI-L) shows promise for early detection, but lacks validation in Mexico. Methods: We assessed 355 adults ≥ 60 years, classified as cognitively healthy (CHG), MCI, or mild AD, using DSM-V criteria. Participants completed neuropsychological testing including the LASSI-L. Construct, concurrent, and predictive validity were analyzed via ANOVA, correlations with the Hopkins Verbal Learning Test (HVLT), and logistic regression models controlling for age, education, and comorbidity. Results: LASSI-L scores significantly differed between groups (p < 0.0001), with recovery from proactive interference best discriminating CHG from MCI and mild AD. Strong correlations with HVLT indices supported concurrent validity. Predictive models identified semantically cued recall and free recall (CRA2 and FRB1) as robust markers, independent of education. Conclusions: LASSI-L is a valid, accessible tool for identifying typical AD-related memory impairment in older Mexican adults, supporting earlier diagnosis in low-biomarker-access settings.
1. Introduction
With the aging of the global population, the prevalence of neurocognitive disorders such as Alzheimer’s disease (AD) is steadily increasing [1]. This condition poses a significant challenge to healthcare systems, as it ultimately results in complete dependence of the affected individual. Consequently, early [2] and timely diagnosis is of paramount importance. A four-phase diagnostic process is currently recommended. In Phase 0, a basic assessment is performed to establish a probable diagnosis of Mild Cognitive Impairment (MCI) or dementia. In Phase 1, a specific diagnostic hypothesis is formed based on the classification of deficits into a clinical phenotype. At this point, a comprehensive neuropsychological evaluation and cognitive profile analysis are advised. In phases 2 and 3, biomarker analysis is recommended [3]. Throughout this process, a combined clinical–biological approach should be maintained, in which the presence of a specific clinical phenotype is linked to specific biomarkers [4].
MCI refers to cognitive impairment that has a detectable functional impact on complex daily activities but does not result in loss of independence [5,6]. In Mexico, its prevalence is estimated at 18% [7]. MCI is a known factorial risk to dementia in general [8] and to AD in up to 23.8% of cases [9]. The clinical phenotype of MCI most often associated with AD is the hippocampal-type amnestic syndrome [4], characterized by episodic memory deficits. Evidence suggests prevalence can go from 45.9% to 64% depending on measuring techniques [10,11]. Individuals with this syndrome perform worse than healthy controls on free and delayed recall tasks [12,13], with no improvement when semantic cues are provided. Other cognitive domains may also be affected.
Several instruments assess episodic memory by modifying the traditional serial word-learning paradigm to identify this phenotype [14]. One such tool is the Free and Cued Selective Reminding Test (FCSRT) [15,16], which has proven sensitive in detecting prodromal AD-related memory impairments [17]. It produces indices of immediate and delayed recall and evaluates the effect of semantic cues [18]. Some instruments have demonstrated even greater sensitivity [19], notably the Loewenstein–Acevedo Scales of Semantic Interference and Learning (LASSI-L) [20].
The LASSI-L involves learning two lists of 15 words each, clustered into three semantic categories, using active encoding by deliberately engaging participants with the material through reading and listening, maximizing the depth of processing of words to be remembered. It assesses delayed recall with and without cues, retroactive interference (RI), proactive interference (PI), and PI recovery [14]. Its biological validity has been demonstrated through evidence indicating an association between specific scores (PI and PI recovery) and biomarker positivity, such as elevated Tau levels in cerebrospinal fluid and atrophy of brain regions associated with Alzheimer’s disease [21,22]. Also, it has demonstrated the ability to identify individuals at high risk of converting from MCI to AD [23] and to detect alterations in cognitively normal individuals [24,25], those with subjective memory complaints [26], MCI [27], and mild AD [28]. Originally developed in a verbal format, the LASSI-L requires no more than 30 min to administer, does not need specialist interpretation, and is accessible to individuals with varying educational levels. A brief version and a computerized version are also available [29,30].
In summary, evidence indicates that the clinical phenotype associated with early and mild AD is characterized by episodic memory deficits detectable with tests such as the LASSI-L. These instruments are particularly useful for identifying deficits during the pre-dementia stage (MCI). However, most have not been validated in Latin America or Mexico. The present study seeks to validate the LASSI-L in Mexican participants by assessing construct, concurrent, and predictive validity in individuals with MCI and mild AD. This is particularly relevant in Latin America, where biomarker-based diagnosis is not widely available, and such tools could enable earlier and more accurate detection.
2. Materials and Methods
2.1. Subjects
A total of 355 individuals aged over 60 years were included in the present analysis. Participants were recruited through the National Institute of Geriatrics (INGER) at various day centers. Based on DSM-V diagnostic criteria for Minor Neurocognitive Disorder (mND) and Major Neurocognitive Disorder (MND), along with expert clinical judgment, participants were classified into three groups: cognitively healthy group (CHG), group with MCI or Minor Neurocognitive Disorder (mND), and group with mild AD or Major Neurocognitive Disorder (MND).
Inclusion criteria for the CHG were: no self-reported or informant-reported concerns regarding cognitive decline, ability to independently perform complex activities of daily living (Lawton score = 8, Barthel score = 100) [31,32], a MoCA score of 26 [33,34] or higher and other cognitive measures with less than 1.0 SD below normal limits for age and education (Category fluency and Trails A and B) [35,36]. Inclusion criteria for the MCI group were: concern from the participant, an informed third party, or a caregiver regarding a mild decline in cognitive abilities; a MoCA score below 26 and evidence of mild impairment in one or more cognitive domains reflected by neuropsychological test performance one to two standard deviations below age and education normative data (Category fluency and Trails A and B); and preserved ability to independently perform complex activities of daily living (Lawton score = 7–8, Barthel score = 100). Inclusion criteria for the mild AD group were: a MoCA score below 24, evidence of moderate impairment in one or more cognitive domains, indicated by neuropsychological test performance at least two standard deviations below age normative data (Category fluency and Trails A and B); and evidence of cognitive deficits interfering with the ability to independently perform activities of daily living (Lawton score < 7, Barthel score ≤ 95). Neuropsychological and other tests are described in the procedure.
2.2. Procedure
An invitation to participate was sent via INGER to various day centers. All participants who verbally agreed to participate completed the informed consent process and signed the corresponding form. Each participant underwent a battery of neuropsychological tests, health questionnaires, and functional assessment scales. Evaluations were conducted in two private sessions, each lasting approximately one hour, scheduled one week apart. Assessments were performed individually, although caregivers could be present upon request by the participant or themselves. All instruments were administered by a trained mental health professional.
During the first session, all tests were administered as follows: health history and sociodemographic questionnaire, detailed clinical history, MoCA [33,34], depression scales (CESD-7 and Geriatric Depression Scale) [37,38], and the Hopkins Verbal Learning Test [39]. The second session included the Charlson Comorbidity Index, Lawton & Brody functioning scale and Barthel’s dependency scale [31,32], the LASSI-L, verbal fluency tasks [40], and the Trail Making Test [41]. In the AD group, a comprehensive review of clinical history and medical records was undertaken to exclude alternative causes of cognitive impairment. In all cases, neuroimaging studies (magnetic resonance imaging or computed tomography) were available, confirming the absence of recent vascular lesions (within six months prior to the study). Also, to ensure mild severity, the Clinical Dementia Rating (CDR) scale was also applied (Global CDR scale of 1.0) [42] Upon completion, group classifications (CHG, MCI, or mild AD) were reviewed and confirmed by an expert.
2.3. Loewenstein–Acevedo Scales of Semantic Interference and Learning
For this study, the original Spanish version of the LASSI-L was used. For this purpose, some words were changed as follows: From list A “Armónica”, “banana”, “chaqueta”, and “media” were replaced with “tambor”, “plátano”, “chamarra”, and “calcetines”. From list B “melocotón”, “clarinete”, and “acordeón” were replaced with “durazno”, “pandero”, and “maraca”. No changes were made to semantic categories and order of presentation. LASSI-L procedure is as follows: the examiner presents a list of 15 common words (List A) with intermixed semantic categories (fruits, musical instruments, and clothing). The participant reads the list of words aloud at four-second intervals between each word. After reading, the participant recalls the words (free recall, FRA1), followed immediately by semantically cued recall (CRA1). The same procedure is repeated for a second cued recall (CRA2). A semantically related list of 15 words (List B) is then presented and learned in the same manner, followed by free recall (FRB1) and semantically cued recall (CRB1), then repeated for a second cued recall (CRB2). This is followed by delayed free recall of List A (SdFRA) and delayed cued recall of List A (SdCRA). Finally, delayed total free recall of all words from both lists is performed 20 min later (DR). For a graphic description see Figure 1.
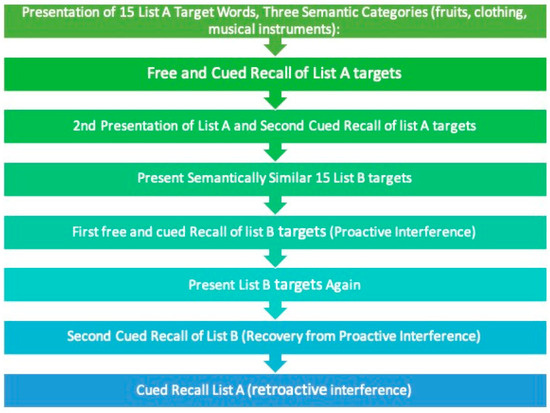
Figure 1.
Graphic description of LASSI-L. Based on Loewenstein et al. [14].
For scoring, the examiner records the number of correct words from both target lists and the number of intrusions (words not included on the lists) on each attempt. Each attempt yields a score between 0 and 15 for both correct words and intrusions. Nine measures are obtained: Free recall List A (FRA1), Free recall List B (FRB1), Semantically cued recall List A1 (CRA1), Semantically cued recall List A2 (CRA2), Semantically cued recall List B1 (CRB1), Semantically cued recall List B2 (CRB2), Free deferred recall (SdFR), semantically cued deferred recall (SdCRA) and delayed total recall (DR).
2.4. Statistical Analysis
A descriptive analysis of the total sample was conducted, stratified by group, to define distribution similar to normal we use the asymmetry (±0.5) and kurtosis stats (±2.0). Discriminant validity was obtained examining whether the LASSI-L could differentiate between groups. A One-way ANOVA was conducted to compare test performance between groups (CHG, MCI, AD). Tukey’s post hoc tests were used to identify group differences, the effect size of cognitive status on each evaluated variable was calculated using partial eta squared (η2p). ANOVA assumptions were assessed through residual analysis (normality [by QQ-plots, asymmetry and kurtosis stats] and independence of residuals [Durbin–Watson test]) and Levene’s test for homogeneity of variances.
Criterion validity was assessed via concurrent validity by comparing LASSI-L performance with that of the Hopkins Verbal Learning Test (HVLT). Spearman correlations were calculated between LASSI-L indices (FRA1, CRA1, CRA2, FRB1, CRB1, CRB2, SdFRA, SdCRA, DR) and HVLT indices (immediate recall free one (IFR1), immediate recall interference one (IRI1), delayed recall free one (DLR1), delayed recall with cues one (DLRC1), delayed recall free two (DLR2), and delayed recall with cues two (DLRC2)).
Predictive validity was examined through binary logistic regression followed by ordinal logistic regression. The binary model assessed associations between LASSI-L scores and the presence or absence of MCI/AD, with variables entered using a stepwise backward method. Three models were constructed: a full model including all nine variables, and a reduced model including four variables selected based on the stepwise approach using the likelihood-ratio test and changes in information criteria (AIC and BIC). The reduced model was adjusted for age (in years), years of education, and comorbidity (Charlson Comorbidity Index score), which were considered potential confounding variables for the outcome.
The ordinal model estimated the likelihood of more severe cognitive impairment. Initially, a proportional odds model was fitted using the Backward method with all nine variables, which identified the same four predictors as the binary model. These were then tested in proportional odds and generalized ordinal models. Since the proportional odds assumption was not met, the generalized model provided a better fit according to log-likelihood. Results for both models are reported as odds ratios (OR) with 95% confidence intervals, and regression assumptions were verified through residual analysis.
A p-value < 0.05 was considered statistically significant. All analyses were performed using SPSS (version 21), and the binary and ordinal logistic regression models were conducted in R (version 4.4.3).
3. Results
3.1. Descriptive Analysis
The results of the descriptive analysis are presented in Table 1. Statistically significant differences (p < 0.0001) were observed in almost all evaluated variables between groups. The CHG group had a mean age of 64.68 years, whereas the mild AD group averaged 78.44 years. A marked decrease in years of schooling was noted as the severity of cognitive impairment increased, with the CHG group averaging 16.34 years of education compared to 8.24 years in the AD group. The presence of comorbidities was similar across all three groups. Regarding global cognitive functioning, the CHG group scored an average of 26, the MCI group 18, and the mild AD group 15.

Table 1.
Descriptive analysis.
3.2. Discriminant Validity
To evaluate the discriminant validity of the instrument, an analysis of variance (ANOVA) was conducted, with results shown in Table 2. Mean scores and standard deviations for the different LASSI-L measures are presented across the three groups. This analysis revealed statistically significant differences (p < 0.0001) in all LASSI-L variables. Performance demonstrated a progressive decline across groups, with the mild AD group showing the lowest scores and greatest difficulty in free recall tasks (e.g., FRA1 means: 9.45 for CHG, 6.70 for MCI, 5.39 for mild AD) and cued recall (CRA2 means: 13.46 for CHG, 10.72 for MCI, 9.47 for mild AD). Cues improved recall less effectively in impaired individuals compared to cognitively healthy participants. Cued recall scores were higher than free recall scores for List B, suggesting a greater effect of proactive interference (PI) across groups.

Table 2.
Discriminant validity, analysis of variance (ANOVA).
3.3. Concurrent Validity
Regarding concurrent validity, correlation results are presented in Table 3. Statistically significant associations (p < 0.01) were found among the indices. Notably, the HVLT-H variables EIL1, EDL1, EDC1, EDL2, and EDC2 showed moderate to strong correlations with LASSI-L indices FRA1, CRA1, CRA2, FRB1, CRB1, and CRB2. EDL1 exhibited correlation coefficients above 0.45 across all associations, particularly with CRA2 (0.550) and CRB2 (0.552), indicating a robust relationship between the two instruments at delayed recall. EII1 showed lower but still significant correlations (ranging from 0.229 to 0.353), suggesting a moderate association with other dimensions.

Table 3.
Concurrent validity, correlation analysis.
3.4. Predictive Validity
For predictive validity, first, a binary logistic regression model was performed to estimate the probability of MCI or mild AD based on LASSI-L scores and to identify which scores were most strongly associated with diagnosis. A stepwise backward procedure was applied, starting with all scores and sequentially removing non-significant variables. The results are summarized in Table 4. Three models were generated: the initial full model, a reduced model including only significant predictors from the full model, and a final reduced and adjusted model controlled for age, education, and comorbidity. The full model showed an odds ratio (OR) of 0.736 (95% CI: 0.630–0.860, p = 0.000), while the reduced and adjusted model yielded an OR of 0.720 (95% CI: 0.616–0.843, p < 0.0001). In the adjusted model, each additional point in CRA2 significantly reduced the likelihood of cognitive impairment, even after controlling for age and education. FRB1 was the other score consistently associated with outcome across all models (OR = 0.745, 95% CI: 0.622–0.893, p = 0.001). Other scores (FRA1, CRA1, CRB1, SdCRA, DR) lost significance in the reduced and adjusted model, indicating their effects were confounded by sociodemographic factors. Age (OR = 1.114, 95% CI: 1.060–1.170, p < 0.0001) and education (OR = 0.835, 95% CI: 0.778–0.896, p < 0.0001) were inversely associated with cognitive impairment, whereas comorbidity (OR = 1.001; p = 0.382) showed no significant effect.

Table 4.
Predictive validity of LASSI-L scores and the presence of MCI/AD throughout binary logistic regression model.
Second, a generalized ordinal logistic regression model, which did not assume proportional odds, was conducted to assess whether LASSI-L performance predicted transitions between cognitive states. Results (Table 5) indicated that CRA2 (OR = 0.73, p < 0.001) and FRB1 (OR = 0.75, p < 0.001) were significantly associated with lower odds of progression from normal cognition to MCI, suggesting a protective effect. However, these scores did not significantly predict progression from MCI to mild AD. Increasing age significantly influenced both transitions (OR ≥ 1.11, p < 0.001), while education primarily protected against the initial transition from CHG to MCI (OR = 0.87, p < 0.001). The effect of education on dementia progression approached but did not reach significance (p = 0.058). The Charlson comorbidity index was not associated with cognitive diagnosis.

Table 5.
Predictive validity, generalized ordinal logistic regression model.
4. Discussion
Current evidence suggests that certain memory tests—particularly those assessing free and cued recall—can detect the specific clinical phenotype associated with typical Alzheimer’s disease (AD) during its preclinical and mild stages, such as mild cognitive impairment (MCI). The value of these tests lies in enabling earlier and more timely diagnoses, especially in contexts like Latin America, where biomarker-based diagnosis is not widely accessible. However, most of these instruments have not yet been validated in Latin American populations, including Mexico. The LASSI-L exemplifies this type of tool, and thus the present study sought to validate the LASSI-L among cognitively healthy Mexican subjects, as well as individuals with MCI and mild AD.
To this end, analyses of discriminant, criterion, and predictive validity were conducted. Discriminant validity was demonstrated through analysis of variance and post hoc tests, which revealed significant differences across the three groups (CHG, MCI, and AD) in all LASSI-L indices, confirming the instrument’s discriminative capacity. As expected, scores declined progressively with increasing severity of cognitive impairment, consistent with literature showing that AD-related cognitive deficits become more severe and widespread as the disease progresses [43]. Importantly, individuals in the preclinical and early stages show deficits in using semantic cues to retrieve information, reflecting neurodegeneration in medial temporal regions such as the hippocampus [44]. Notably, recovery from proactive interference (CRB2) emerged as the measure with the largest group differences. This result aligns with prior evidence indicating that performance on this particular subtest has high discriminative properties, even among cognitively healthy individuals [29].
Criterion validity was supported by correlation analyses demonstrating significant associations between analogous indices from the LASSI-L and the Hopkins Verbal Learning Test (HVLT). High correlations were particularly evident between delayed recall with cues (EDL1, EDC2 on HVLT and CRA2, CRB2 on LASSI-L). Lower, though still significant, correlations likely reflect methodological differences: while HVLT follows a traditional serial word learning paradigm, LASSI-L incorporates controlled, in-depth learning paradigms. These findings underline the conceptual coherence between the instruments despite procedural differences [27].
Regarding predictive validity, logistic regression models revealed that LASSI-L performance is significantly associated with MCI and mild AD diagnoses, aligning with previous reports [26,27,28]. Notably, LASSI-L scores related to semantic cuing and proactive interference (CRA2 and FRB1) remained significant predictors even after adjusting for age, education, and comorbidity. This suggests that these LASSI-L indices may serve as markers relatively independent of education, a particularly valuable attribute in Mexico where educational attainment varies widely [45]. This is crucial given the predominance of low educational levels in the older Mexican population [46] and evidence that education interacts with age to influence cognitive performance [47]. Age itself is a well-known factor affecting cognitive test performance [48], especially memory, which is particularly vulnerable to aging [49]. The retention of the association after adjusting for age highlights the LASSI-L’s robustness against such biases, an important consideration given the heterogeneity of aging processes across Latin America [50].
Finally, findings from the ordinal regression model emphasize the role of cued recall (CRA2) and proactive interference (FRB1) as risk factors for transitioning from normal cognition to MCI. This highlights LASSI-L potential for differential diagnosis when MCI etiology is not clear, also this aligns with evidence that individuals with MCI are more susceptible to proactive interference due to deficits in information encoding [51], highlighting the importance of assessing these effects in populations at increased risk for AD progression. It also supports prior work indicating that while memory tests emphasizing cued recall are superior in detecting early AD-related changes, they may not be as effective in predicting conversion to dementia [9]. Additionally, years of schooling emerged as a protective factor against the transition from normal cognition to MCI but did not significantly influence progression from MCI to AD, consistent with the cognitive reserve hypothesis that education delays symptom onset primarily in earlier disease stages [52].
Several limitations should be noted. Despite comprehensive clinical evaluation and specialist diagnosis, the absence of biomarker data limits the ability to confirm prodromal AD etiology in most MCI cases, reflecting a common challenge in Mexico and Latin America where biomarker access is limited. The cross-sectional design also restricts conclusions regarding the LASSI-L’s capacity to predict conversion from MCI to AD, highlighting the need for longitudinal studies.
5. Conclusions
To our knowledge, the present study is the first one in Latin America to demonstrate that the Loewenstein–Acevedo Scales of Semantic Interference and Learning (LASSI-L) is a valid tool, capable of distinguishing cognitively healthy older Mexican adults from those with varying degrees of cognitive impairment. The test’s strength lies particularly in measures related to cued recall and proactive interference effects. Its utility in detecting memory disturbances in individuals with MCI represents an important advancement for the Latin American context, where biomarker-based diagnostics are scarce and validated neuropsychological tests for subtle cognitive changes are lacking. Validating the LASSI-L in the Mexican population represents a critical step toward more contextualized, accessible, and cost-effective neuropsychological assessment. This instrument can enhance early detection and diagnosis, inform timely intervention planning, and promote equitable access to quality cognitive evaluation, even in resource-limited settings.
Author Contributions
Conceptualization, A.K.-G. and P.R.-R.; methodology, A.K.-G. and P.R.-R.; formal analysis A.K.-G. and P.R.-R.; investigation, J.S.-A., P.P.-G., T.Á.-C. and P.R.-R.; data curation J.S.-A., P.P.-G., T.Á.-C., and P.R.-R.; writing—original draft preparation, P.R.-R.; writing—review and editing, J.S.-A. and P.R.-R.; project administration, T.Á.-C., P.P.-G., and P.R.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Instituto Nacional de Geriatría (protocol code DI-PI-002/2018, 02/01/2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is unavailable due to privacy and ethical restrictions.
Acknowledgments
During the preparation of this manuscript, the authors used AI for the purposes of grammar and style correcting. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| AD | Alzheimer’s disease |
| MCI | Mild Cognitive Impairment |
| FCSRT | Free and Cued Selective Reminding Test |
| LASSI-L | Loewenstein–Acevedo Scales of Semantic Interference and Learning |
| INGER | National Institute of Geriatrics |
| mND | Minor Neurocognitive Disorder |
| MND | Mayor Neurocognitive Disorder |
| CHG | cognitively healthy group |
| MoCA | Montreal Cognitive Assessment |
| HVLT | Hopkins Verbal Learning Test |
| CDR | Clinical Dementia Rating Scale |
| FRA1 | Free recall List A |
| FRB1 | Free recall List B |
| CRA1 | Semantically cued recall List A1 |
| CRA2 | Semantically cued recall List A2 |
| CRB1 | Semantically cued recall List B1 |
| CRB2 | Semantically cued recall List B2 |
| SdFR | Free deferred recall |
| SdCRA | Semantically cued deferred recall |
| DR | Total Delayed Recall |
| IFR1 | Immediate recall free one |
| IRI1 | Immediate recall interference one |
| DLR1 | Delayed recall free one |
| DLRC1 | Delayed recall with cues one |
| DLR2 | Delayed recall free two |
| DLRC2 | Delayed recall with cues two |
References
- GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rasmussen, J.; Langerman, H. Alzheimer’s Disease—Why We Need Early Diagnosis. Degener. Neurol. Neuromuscul. Dis. 2019, 9, 123–130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frisoni, G.B.; Festari, C.; Massa, F.; Cotta Ramusino, M.; Orini, S.; Aarsland, D.; Agosta, F.; Babiloni, C.; Borroni, B.; Cappa, S.F.; et al. European intersocietal recommendations for the biomarker-based diagnosis of neurocognitive disorders. Lancet Neurol. 2024, 23, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Villain, N.; Frisoni, G.B.; Rabinovici, G.D.; Sabbagh, M.; Cappa, S.; Bejanin, A.; Bombois, S.; Epelbaum, S.; Teichmann, M.; et al. Clinical diagnosis of Alzheimer’s disease: Recommendations of the International Working Group. Lancet Neurol. 2021, 20, 484–496. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Cummings, J.L.; Dekosky, S.T.; Barberger-Gateau, P.; Delacourte, A.; Frisoni, G.; Fox, N.C.; Galasko, D.; et al. Revising the definition of Alzheimer’s disease: A new lexicon. Lancet Neurol. 2010, 9, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Jr Andrews, J.S.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E.; et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimer’s Dement. 2024, 20, 5143–5169. [Google Scholar] [CrossRef] [PubMed]
- Magallón-Zertuche, V.; Garrido-Dzib, A.G.; Salazar-Gonzalez, E.; González-Castro, D.G.; Chávez-Loría, G.; Avila-Nava, A.; Gutiérrez-Solis, A.L. A Systematic Review and Meta-Analysis on the Prevalence of Mild Cognitive Impairment and Dementia in Mexico. Dement. Geriatr. Cogn. Disord. 2024, 53, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Breton, A.; Casey, D.; Arnaoutoglou, N.A. Cognitive tests for the detection of mild cognitive impairment (MCI), the prodromal stage of dementia: Meta-analysis of diagnostic accuracy studies. Int. J. Geriatr. Psychiatry 2019, 34, 233–242. [Google Scholar] [CrossRef] [PubMed]
- McGrattan, A.M.; Pakpahan, E.; Siervo, M.; Mohan, D.; Reidpath, D.D.; Prina, M.; Allotey, P.; Zhu, Y.; Shulin, C.; Yates, J.; et al. Risk of conversion from mild cognitive impairment to dementia in low- and middle-income countries: A systematic review and meta-analysis. Alzheimer’s Dement. 2022, 8, e12267. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bouteloup, V.; Villain, N.; Vidal, J.S.; Gonzalez-Ortiz, F.; Yuksekel, I.; Santos, C.; Schraen-Maschken, S.; Pellegrin, I.; Lehmann, S.; Blennow, K.; et al. Cognitive Phenotyping and Interpretation of Alzheimer Blood Biomarkers. JAMA Neurol. 2025, 82, 506–515. [Google Scholar] [CrossRef]
- Salvadori, N.; Torrigiani, E.G.; Paoletti, F.P.; Chipi, E.; Montanucci, C.; Verderosa, C.; Siena, E.; Fruttini, D.; Parnetti, L. Predictive value for cerebrospinal fluid Alzheimer’s disease profile of different measures of verbal episodic memory in patients with MCI. Sci. Rep. 2024, 14, 12235. [Google Scholar] [CrossRef]
- Weissberger, G.H.; Strong, J.V.; Stefanidis, K.B.; Summers, M.J.; Bondi, M.W.; Stricker, N.H. Diagnostic Accuracy of Memory Measures in Alzheimer’s Dementia and Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. Neuropsychol. Rev. 2017, 27, 354–388. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gainotti, G.; Quaranta, D.; Vita, M.G.; Marra, C. Neuropsychological predictors of conversion from mild cognitive impairment to Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 38, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Loewenstein, D.A.; Curiel, R.E.; Duara, R.; Buschke, H. Novel Cognitive Paradigms for the Detection of Memory Impairment in Preclinical Alzheimer’s Disease. Assessment 2018, 25, 348–359. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grober, E.; Ocepek-Welikson, K.; Teresi, J.A. The free and cued selective reminding test: Evidence of psychometric adequacy Psychol. Sci. Q. 2009, 51, 266–282. [Google Scholar]
- Sarazin, M.; Chauviré, V.; Gerardin, E.; Colliot, O.; Kinkingnéhun, S.; de Souza, L.C.; Hugonot-Diener, L.; Garnero, L.; Lehéricy, S.; Chupin, M.; et al. The amnestic syndrome of hippocampal type in Alzheimer’s disease: An MRI study. J. Alzheimer’s Dis. 2010, 22, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Sarazin, M.; Berr, C.; De Rotrou, J.; Fabrigoule, C.; Pasquier, F.; Legrain, S.; Michel, B.; Puel, M.; Volteau, M.; Touchon, J.; et al. Amnestic syndrome of the medial temporal type identifies prodromal AD: A longitudinal study. Neurology 2007, 69, 1859–1867, Erratum in Neurology 2008, 70, 2016. [Google Scholar] [CrossRef] [PubMed]
- Brugnolo, A.; Girtler, N.; Doglione, E.; Orso, B.; Massa, F.; Donegani, M.I.; Bauckneht, M.; Morbelli, S.; Arnaldi, D.; Nobili, F.; et al. Brain Resources: How Semantic Cueing Works in Mild Cognitive Impairment due to Alzheimer’s Disease (MCI-AD). Diagnostics 2021, 11, 108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matias-Guiu, J.A.; Cabrera-Martín, M.N.; Curiel, R.E.; Valles-Salgado, M.; Rognoni, T.; Moreno-Ramos, T.; Carreras, J.L.; Loewenstein, D.A.; Matías-Guiu, J. Comparison between FCSRT and LASSI-L to Detect Early Stage Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 61, 103–111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Curiel, R.E.; Crocco, E.; Acevedo, A.; Duara, R.; Agron, J.; Loewenstein, D.A. A new scale for the evaluation of proactive and retroactive interference in mild cognitive impairment and early Alzheimer’s disease. J. Aging Sci. 2013, 1, 102. [Google Scholar] [CrossRef]
- Valles-Salgado, M.; Gil-Moreno, M.J.; Cid, R.E.C.; Delgado-Álvarez, A.; Ortega-Madueño, I.; Delgado-Alonso, C.; Palacios-Sarmiento, M.; López-Carbonero, J.I.; Cárdenas, M.C.; Díez-Cirarda, M.; et al. Detection of cerebrospinal fluid biomarkers changes of Alzheimer’s disease using a cognitive stress test in persons with subjective cognitive decline and mild cognitive impairment. Front. Psychol. 2024, 15, 1373541. [Google Scholar] [CrossRef]
- Zheng, D.D.; Cid, R.E.C.; Duara, R.; Kitaigorodsky, M.; Crocco, E.; Loewenstein, D.A. Semantic intrusion errors as a function of age, amyloid, and volumetric loss: A confirmatory path analysis. Int. Psychogeriatr. 2022, 34, 991–1001. [Google Scholar] [CrossRef]
- Crocco, E.A.; Curiel Cid, R.; Kitaigorodsky, M.; Grau, G.A.; Garcia, J.M.; Duara, R.; Barker, W.; Chirinos, C.L.; Rodriguez, R.; Loewenstein, D.A. Intrusion Errors and Progression of Cognitive Deficits in Older Adults with Mild Cognitive Impairment and PreMCI States. Dement. Geriatr. Cogn. Disord. 2021, 50, 135–142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Loewenstein, D.A.; Greig, M.T.; Schinka, J.A.; Barker, W.; Shen, Q.; Potter, E.; Raj, A.; Brooks, L.; Varon, D.; Schoenberg, M.; et al. An investigation of PreMCI: Subtypes and longitudinal outcomes. Alzheimer’s Dement. 2012, 8, 172–179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Loewenstein, D.A.; Greig, M.T.; Curiel, R.; Rodriguez, R.; Wicklund, M.; Barker, W.W.; Hidalgo, J.; Rosado, M.; Duara, R. Proactive Semantic Interference is Associated with Total and Regional Abnormal Amyloid Load in Non-Demented Community-Dwelling Elders: A Preliminary Study. Am. J. Geriatr. Psychiatry 2015, 23, 1276–1279. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Loewenstein, D.A.; Curiel, R.E.; Greig, M.T.; Bauer, R.M.; Rosado, M.; Bowers, D.; Wicklund, M.; Crocco, E.; Pontecorvo, M.; Joshi, A.D.; et al. A Novel Cognitive Stress Test for the Detection of Preclinical Alzheimer Disease: Discriminative Properties and Relation to Amyloid Load. Am. J. Geriatr. Psychiatry 2016, 24, 804–813. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Loewenstein, D.A.; Curiel, R.E.; Wright, C.; Sun, X.; Alperin, N.; Crocco, E.; Czaja, S.J.; Raffo, A.; Penate, A.; Melo, J.; et al. Recovery from Proactive Semantic Interference in Mild Cognitive Impairment and Normal Aging: Relationship to Atrophy in Brain Regions Vulnerable to Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 56, 1119–1126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crocco, E.; Curiel, R.E.; Acevedo, A.; Czaja, S.J.; Loewenstein, D.A. An evaluation of deficits in semantic cueing and proactive and retroactive interference as early features of Alzheimer’s disease. Am. J. Geriatr. Psychiatry. 2014, 22, 889–897. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crocco, E.; Curiel-Cid, R.E.; Kitaigorodsky, M.; González-Jiménez, C.J.; Zheng, D.; Duara, R.; Loewenstein, D.A. A brief version of the LASSI-L detects prodromal Alzheimer’s disease states. J. Alzheimer’s Dis. 2020, 78, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.; Curiel-Cid, R.; Kallestrup, P.; Mueller, A.; Rivera-Molina, A.; Czaja, S.; Crocco, E.; Loewenstein, D. Digital Migration of the Loewenstein Acevedo Scales for Semantic Interference and Learning (LASSI-L): Development and Validation Study in Older Participants. JMIR Ment. Health 2025, 12, e64716. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duarte-Ayala, R.E.; Velasco-Rojano, A.E. Psychometric validation of the Barthel index in older Mexican adults. Horiz. Sanit. 2022, 21, 113–120. [Google Scholar] [CrossRef]
- Vergara, I.; Bilbao, A.; Orive, M.; Garcia-Gutierrez, S.; Navarro, G.; Quintana, J.M. Validation of the Spanish version of the Lawton IADL Scale for its application in elderly people. Health Qual. Life Outcomes 2012, 10, 130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699, Erratum in J. Am. Geriatr. Soc. 2019, 67, 1991. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Navarro, S.G.; Mimenza-Alvarado, A.J.; Palacios-García, A.A.; Samudio-Cruz, A.; Gutiérrez-Gutiérrez, L.A.; Ávila-Funes, J.A. Validity and Reliability of the Spanish Version of the Montreal Cognitive Assessment (MoCA) for the Detection of Cognitive Impairment in Mexico. Rev. Colomb. Psiquiatr. (Engl. Ed.) 2018, 47, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Arango-Lasprilla, J.C.; Rivera, D.; Aguayo, A.; Rodríguez, W.; Garza, M.T.; Saracho, C.P.; Rodríguez-Agudelo, Y.; Aliaga, A.; Weiler, G.; Luna, M.; et al. Trail making test: Normative data for the Latin American Spanish speaking adult population. NeuroRehabilitation 2015, 37, 639–661. [Google Scholar] [CrossRef]
- Olabarrieta-Landa, L.; Rivera, D.; Galarza-Del-Angel, J.; Garza, M.T.; Saracho, C.P.; Rodríguez, W.; Chávez-Oliveros, M.; Rábago, B.; Leibach, G.; Schebela, S.; et al. Verbal fluency tests: Normative data for the Latin American Spanish speaking adult population. NeuroRehabilitation 2015, 37, 515–561. [Google Scholar] [CrossRef]
- García-Peña, C.; Wagner, F.A.; Sánchez-Garcia, S.; Juárez-Cedillo, T.; Espinel-Bermúdez, C.; García-Gonzalez, J.J.; Gallegos-Carrillo, K.; Franco-Marina, F.; Gallo, J.J. Depressive symptoms among older adults in Mexico City. J. Gen. Intern. Med. 2008, 23, 1973–1980. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1982, 17, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Arango-Lasprilla, J.C.; Rivera, D.; Garza, M.T.; Saracho, C.P.; Rodríguez, W.; Rodríguez-Agudelo, Y.; Aguayo, A.; Schebela, S.; Luna, M.; Longoni, M.; et al. Hopkins Verbal Learning Test- Revised: Normative data for the Latin American Spanish speaking adult population. NeuroRehabilitation 2015, 37, 699–718. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Oliveros, M.; Rodríguez-Agudelo, Y.; Acosta-Castillo, I.; García-Ramírez, N.; Rojas de la Torre, G.; Sosa-Ortiz, A.L. Semantic verbal fluency in elderly Mexican adults: Reference values. Neurologia 2015, 30, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Suarez, P.A.; Díaz-Santos, M.; Marquine, M.J.; Kamalyan, L.; Mindt, M.R.; Umlauf, A.; Heaton, R.K.; Grant, I.; Cherner, M. Demographically adjusted norms for the Trail Making Test in native Spanish speakers: Results from the neuropsychological norms for the US-Mexico border region in Spanish (NP-NUMBRS) project. Clin. Neuropsychol. 2021, 35, 308–323. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morris, J.C. The Clinical Dementia Rating (CDR) current version and scoring rules. Neurology 1993, 43, 2412. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.A.S.; González, H.M.; Léger, G.C. Alzheimer’s disease. In Handbook of Clinical Neurology; Aminoff, M.J., Boller, F., Swaab, D.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 231–255. [Google Scholar]
- Bondi, M.W.; Edmonds, E.C.; Salmon, D.P. Alzheimer’s Disease: Past, Present, and Future. J. Int. Neuropsychol. Soc. 2017, 23, 818–831. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Díaz-Venegas, C.; Samper-Ternent, R.; Michaels-Obregón, A.; Wong, R. The effect of educational attainment on cognition of older adults: Results from the Mexican Health and Aging Study 2001 and 2012. Aging Ment Health 2019, 23, 1586–1594. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wong, R.; Michaels-Obregon, A.; Palloni, A. Cohort Profile: The Mexican Health and Aging Study (MHAS). Int. J. Epidemiol. 2017, 46, e2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ardila, A.; Ostrosky-Solis, F.; Rosselli, M.; Gómez, C. Age-related cognitive decline during normal aging: The complex effect of education. Arch. Clin. Neuropsychol. 2000, 15, 495–513. [Google Scholar] [PubMed]
- Murman, D.L. The Impact of Age on Cognition. Semin. Hear. 2015, 36, 111–121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Small, G.W. What we need to know about age related memory loss. BMJ 2002, 324, 1502–1505. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ibanez, A.; Maito, M.; Botero-Rodríguez, F.; Fittipaldi, S.; Coronel, C.; Migeot, J.; Lacroix, A.; Lawlor, B.; Duran-Aniotz, C.; Baez, S.; et al. Healthy aging meta-analyses and scoping review of risk factors across Latin America reveal large heterogeneity and weak predictive models. Nat. Aging. 2024, 4, 1153–1165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hanseeuw, B.J.; Seron, X.; Ivanoiu, A. Increased sensitivity to proactive and retroactive interference in amnestic mild cognitive impairment: New insights. Brain Cogn. 2012, 80, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Corbo, I.; Marselli, G.; Di Ciero, V.; Casagrande, M. The Protective Role of Cognitive Reserve in Mild Cognitive Impairment: A Systematic Review. J. Clin. Med. 2023, 12, 1759. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).