The Impact of Age on the Effectiveness of Immune Checkpoint Inhibitors Therapy in Patients with Metastatic Non-Small-Cell Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. The Ethics Committee
2.2. Patient Population
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Treatment Duration
3.3. Treatment Response
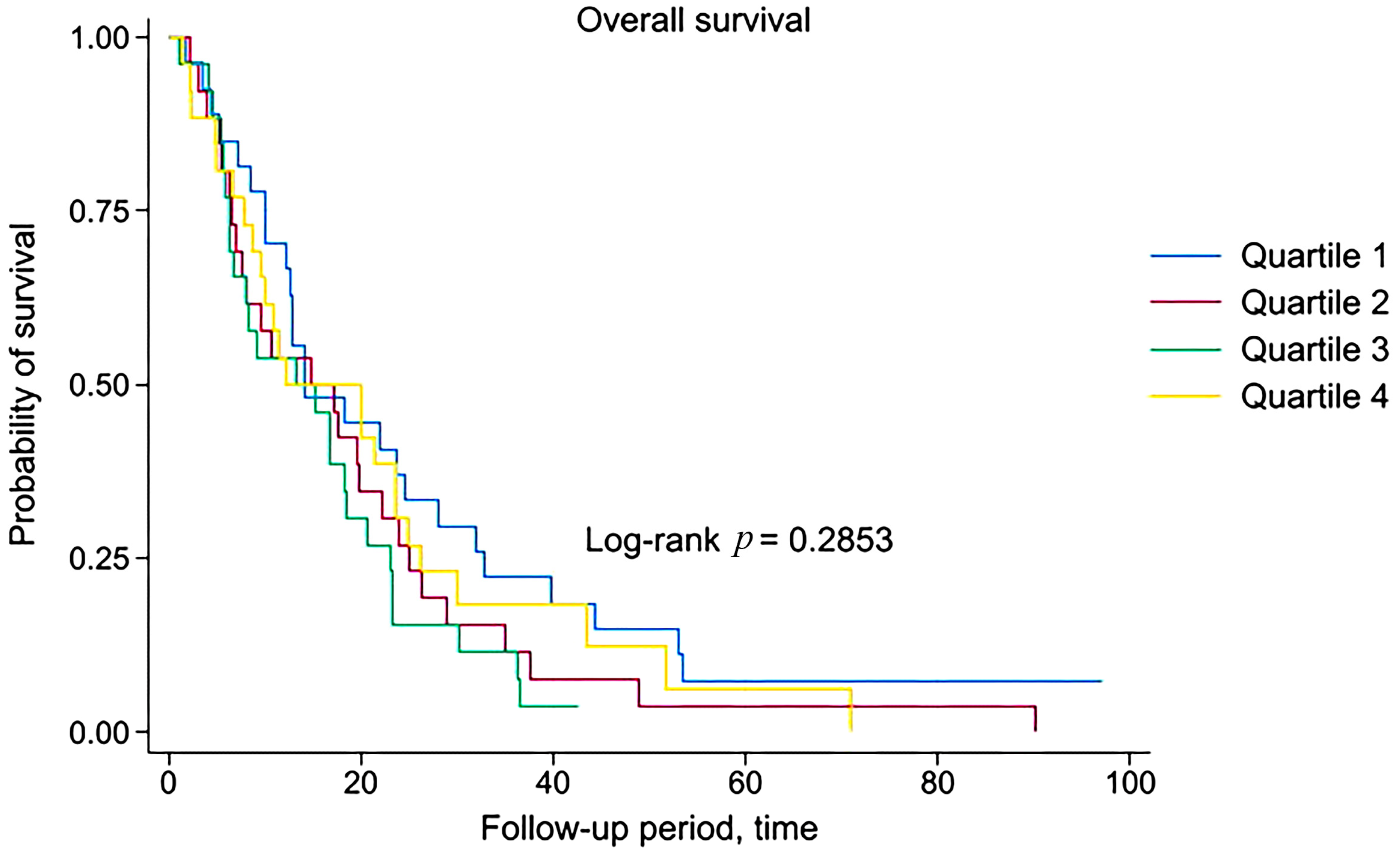
3.4. Survival Analysis
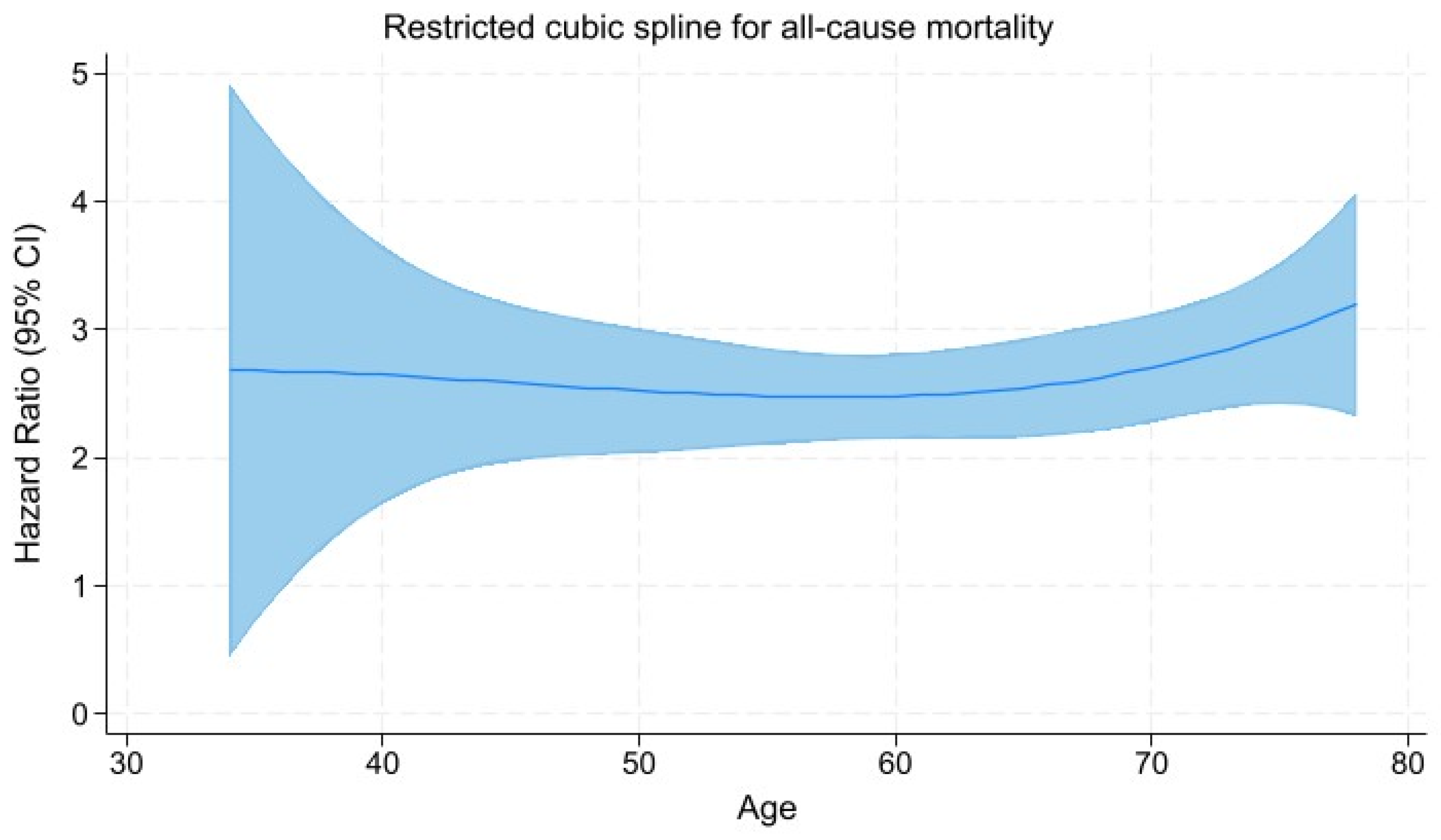
3.5. Restricted Cubic Spline Regression Analysis
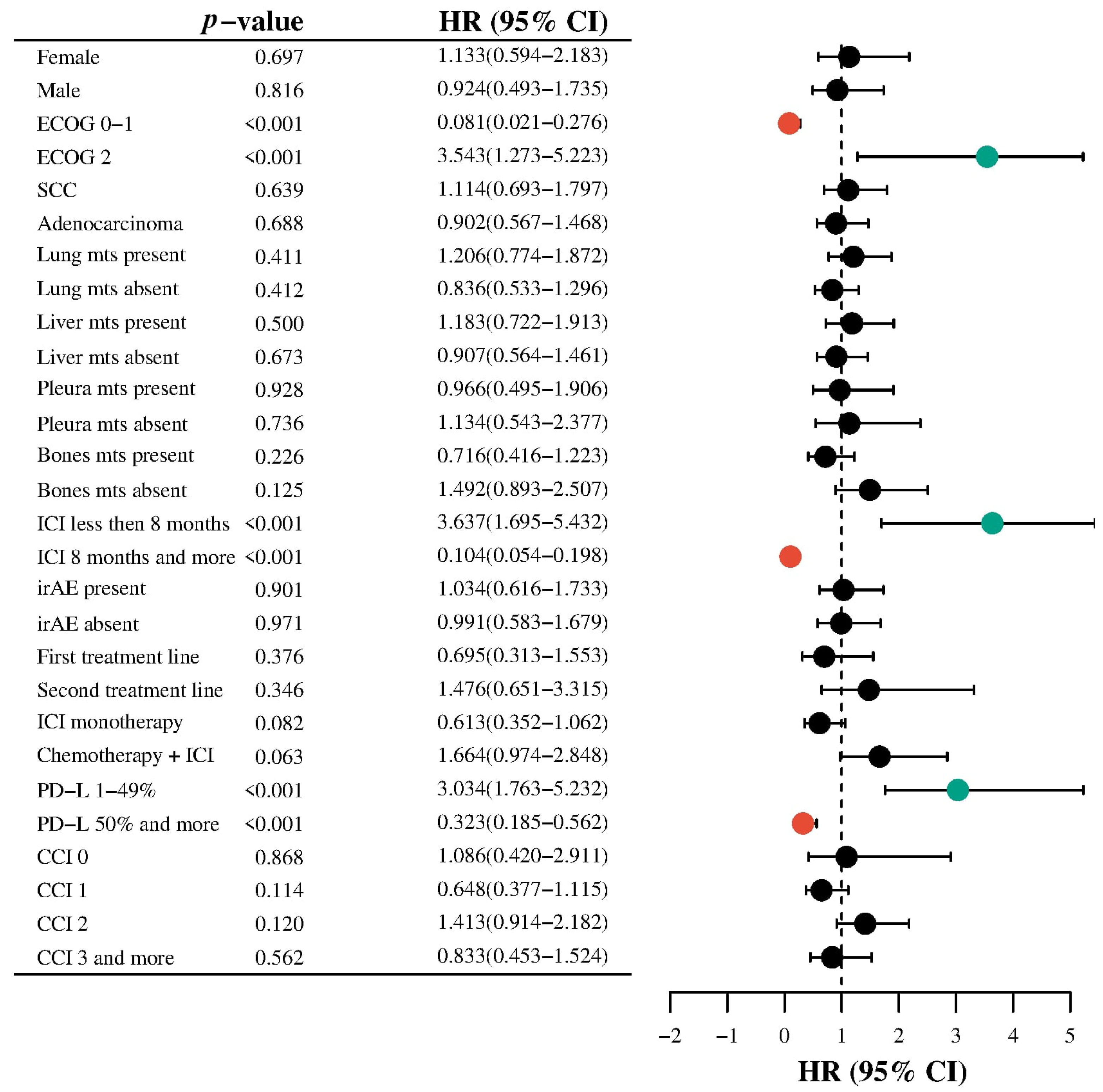
3.6. Subgroup Analysis
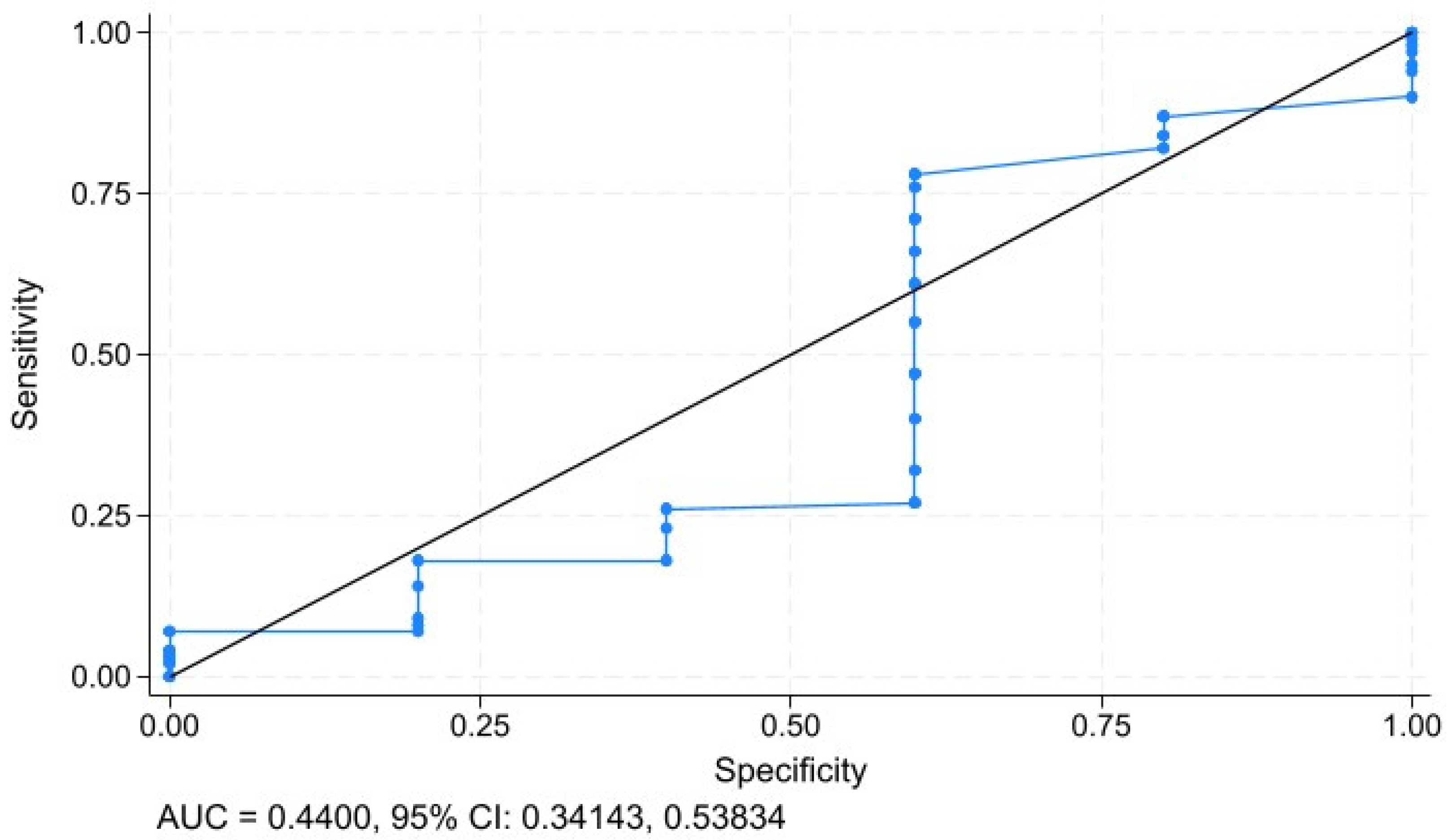
3.7. Sensitivity and Specifity Analysis
3.8. Immune-Related Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCI | Charlson Comorbidity Index |
| ICI | Immune checkpoint inhibitor |
| mNSCLC | Metastatic non-small-cell lung cancer |
References
- Pilleron, S.; Soto-Perez-de-Celis, E.; Vignat, J.; Ferlay, J.; Soerjomataram, I.; Bray, F.; Sarfati, D. Estimated global cancer incidence in the oldest adults in 2018 and projections to 2050. Int. J. Cancer 2021, 148, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Kudlova, N.; De Sanctis, J.B.; Hajduch, M. Cellular Senescence: Molecular Targets, Biomarkers, and Senolytic Drugs. Int. J. Mol. Sci. 2022, 23, 4168. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liang, Q.; Ren, Y.; Guo, C.; Ge, X.; Wang, L.; Cheng, Q.; Luo, P.; Zhang, Y.; Han, X. Immunosenescence: Molecular mechanisms and diseases. Signal Transduct. Target. Ther. 2023, 8, 200. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging: An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Santoro, A.; Bientinesi, E.; Monti, D. Immunosenescence and inflammaging in the aging process: Age-related diseases or longevity? Ageing Res. Rev. 2021, 71, 101422. [Google Scholar] [CrossRef] [PubMed]
- Gulla, S.; Reddy, M.C.; Reddy, V.C.; Chitta, S.; Bhanoori, M.; Lomada, D. Role of thymus in health and disease. Int. Rev. Immunol. 2023, 42, 347–363. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Weng, N.P. The immunology and cell biology of T cell aging. Semin. Immunol. 2023, 70, 101843. [Google Scholar] [CrossRef]
- Dale, W.; Klepin, H.D.; Williams, G.R.; Alibhai, S.M.H.; Bergerot, C.; Brintzenhofeszoc, K.; Hopkins, J.O.; Jhawer, M.P.; Katheria, V.; Loh, K.P.; et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Systemic Cancer Therapy: ASCO Guideline Update. J. Clin. Oncol. 2023, 41, 4293–4312. [Google Scholar] [CrossRef]
- Kim, S.Y.; Halmos, B. Choosing the best first-line therapy: NSCLC with no actionable oncogenic driver. Lung Cancer Manag. 2020, 9, LMT36. [Google Scholar] [CrossRef]
- Choucair, K.; Naqash, A.R.; Nebhan, C.A.; Nipp, R.; Johnson, D.B.; Saeed, A. Immune Checkpoint Inhibitors: The Unexplored Landscape of Geriatric Oncology. Oncologist 2022, 27, 778–789. [Google Scholar] [CrossRef]
- Yamaguchi, O.; Imai, H.; Minemura, H.; Suzuki, K.; Wasamoto, S.; Umeda, Y.; Osaki, T.; Kasahara, N.; Uchino, J.; Sugiyama, T.; et al. Efficacy and safety of immune checkpoint inhibitor monotherapy in pretreated elderly patients with non-small cell lung cancer. Cancer Chemother. Pharmacol. 2020, 85, 761–771. [Google Scholar] [CrossRef]
- Al-Danakh, A.; Safi, M.; Jian, Y.; Yang, L.; Zhu, X.; Chen, Q.; Yang, K.; Wang, S.; Zhang, J.; Yang, D. Aging-related biomarker discovery in the era of immune checkpoint inhibitors for cancer patients. Front. Immunol. 2024, 15, 1348189. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.M.; Wang, Y.; Sun, X.X.; Chen, J.; Gong, Z.P.; Meng, H.Y. Clinical Efficacy of Immune Checkpoint Inhibitors in Older Non-small-Cell Lung Cancer Patients: A Meta-Analysis. Front. Oncol 2020, 10, 558454. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, M.R.L.; Nipp, R.D.; Muzikansky, A.; Goodwin, K.; Anderson, D.; Newcomb, R.A.; Gainor, J.F. Impact of Age on Outcomes with Immunotherapy in Patients with Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2019, 14, 547–552. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Q.; Xie, J.; Chen, M.; Liu, H.; Zhan, P.; Lv, T.; Song, Y. The Predictive Value of Clinical and Molecular Characteristics or Immunotherapy in Non-Small Cell Lung Cancer: A Meta-Analysis of Randomized Controlled Trials. Front. Oncol. 2021, 11, 732214. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, G.; Das, S.; Paul, S.; Rakshit, S.; Sarkar, K. Clinical relevance and therapeutic aspects of professional antigen-presenting cells in lung cancer. Med. Oncol. 2022, 39, 237. [Google Scholar] [CrossRef]
- Wang, C.; Chen, L.; Fu, D.; Liu, W.; Puri, A.; Kellis, M.; Yang, J. Antigen presenting cells in cancer immunity and mediation of immune checkpoint blockade. Clin. Exp. Metastasis 2024, 41, 333–349. [Google Scholar] [CrossRef]
- Granier, C.; Gey, A.; Roncelin, S.; Weiss, L.; Paillaud, E.; Tartour, E. Immunotherapy in older patients with cancer. Biomed. J. 2021, 44, 260–271. [Google Scholar] [CrossRef]
- Tang, S.; Qin, C.; Hu, H.; Liu, T.; He, Y.; Guo, H.; Yan, H.; Zhang, J.; Tang, S.; Zhou, H. Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer: Progress, Challenges, and Prospects. Cells 2022, 11, 320. [Google Scholar] [CrossRef]
- Fasano, M.; Corte, C.M.D.; Liello, R.D.; Viscardi, G.; Sparano, F.; Iacovino, M.L.; Paragliola, F.; Piccolo, A.; Napolitano, S.; Martini, G.; et al. Immunotherapy for head and neck cancer: Present and future. Crit. Rev. Oncol. Hematol. 2022, 174, 103679. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.N.; Wang, L.S.; Dang, Y.Q.; Ji, G. Evaluating the efficacy of immunotherapy in gastric cancer: Insights from immune checkpoint inhibitors. World J. Gastroenterol. 2024, 30, 3726–3729. [Google Scholar] [CrossRef]
- Li, H.; Lin, S.; Wang, Y.; Shi, Y.; Fang, X.; Wang, J.; Cui, H.; Bian, Y.; Qi, X. Immunosenescence: A new direction in anti-aging research. Int. Immunopharmacol. 2024, 141, 112900. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Boby, J.M.; Benny, S.J.; Ghazali, N.; Vermeulen, E.; George, M. Immunotherapy in Older Patients with Cancer: A Narrative Review. Int. J. Gen. Med. 2024, 17, 305–313. [Google Scholar] [CrossRef]
- Arias Ron, D.; Areses Manrique, M.C.; Mosquera Martínez, J.; García González, J.; Afonso Afonso, F.J.; Lázaro Quintela, M.; Fernández Núñez, N.; Azpitarte Raposeiras, C.; Amenedo Gancedo, M.; Santomé Couto, L.; et al. Efficacy and safety of Nivolumab in older patients with pretreated lung cancer: A subgroup analysis of the Galician lung cancer group. J. Geriatr. Oncol. 2021, 12, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Luciani, A.; Marra, A.; Toschi, L.; Cortinovis, D.; Fava, S.; Filipazzi, V.; Tuzi, A.; Cerea, G.; Rossi, S.; Perfetti, V.; et al. Efficacy and Safety of Anti-PD-1 Immunotherapy in Patients Aged ≥ 75 Years With Non-small-cell Lung Cancer (NSCLC): An Italian, Multicenter, Retrospective Study. Clin. Lung Cancer 2020, 21, e567–e571. [Google Scholar] [CrossRef]
- Tagliamento, M.; Frelaut, M.; Baldini, C.; Naigeon, M.; Nencioni, A.; Chaput, N.; Besse, B. The use of immunotherapy in older patients with advanced non-small cell lung cancer. Cancer Treat. Rev. 2022, 106, 102394. [Google Scholar] [CrossRef]
- Huang, X.; Wu, S.; Chen, S.; Qiu, M.; Zhao, Y.; Wei, J.; He, J.; Zhao, W.; Tan, L.; Su, C.; et al. Prognostic impact of age in advanced non-small cell lung cancer patients undergoing first-line checkpoint inhibitor immunotherapy and chemotherapy treatment. Int. Immunopharmacol. 2024, 132, 111901. [Google Scholar] [CrossRef]
- Ramos, M.J.; Mendes, A.S.; Romão, R.; Febra, J.; Araújo, A. Immunotherapy in Elderly Patients-Single-Center Experience. Cancers 2023, 16, 145. [Google Scholar] [CrossRef]
- Mebarki, S.; Pamoukdjian, F.; Pierro, M.; Poisson, J.; Baldini, C.; Lahlou, W.; Taieb, J.; Fabre, E.; Canoui-Poitrine, F.; Oudard, S.; et al. Safety and efficacy of immunotherapy according to the age threshold of 80 years. Bull. Cancer 2023, 110, 570–580. [Google Scholar] [CrossRef]
- Bogani, G.; Cinquini, M.; Signorelli, D.; Pizzutilo, E.G.; Romanò, R.; Bersanelli, M.; Raggi, D.; Alfieri, S.; Buti, S.; Bertolini, F.; et al. A systematic review and meta-analysis on the optimal treatment duration of checkpoint inhibitors in solid tumors: The OTHERS study. Crit. Rev. Oncol. Hematol. 2023, 187, 104016. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, D.W.; Kim, M.; Lee, Y.; Ahn, H.K.; Cho, J.H.; Kim, I.H.; Lee, Y.G.; Shin, S.H.; Park, S.E.; et al. Long-term outcomes in patients with advanced and/or metastatic non-small cell lung cancer who completed 2 years of immune checkpoint inhibitors or achieved a durable response after discontinuation without disease progression: Multicenter, real-world data (KCSG LU20-11). Cancer 2022, 128, 778–787. [Google Scholar] [CrossRef] [PubMed]
| Variables | Age | p-Value (Test) | |||
|---|---|---|---|---|---|
| Quartile 1, n = 27 | Quartile 2, n = 26 | Quartile 3, n = 26 | Quartile 4, n = 26 | ||
| Sex, n (%) | |||||
| Male | 21 (77.8) | 25 (96.2) | 23 (88.5) | 20 (76.9) | 0.162 (χ2) |
| Female | 6 (22.2) | 1 (3.8) | 3 (11.5) | 6 (23.1) | |
| Histology, n (%) | |||||
| Adenocarcinoma | 17 (63.0) | 18 (69.2) | 9 (34.6) | 14 (53.8) | 0.066 (χ2) |
| Squamous cell carcinoma | 10 (37.0) | 8 (30.8) | 17 (65.4) | 12 (46.2) | |
| Metastasis in the lung, n (%) | |||||
| Absent | 12 (44.4) | 14 (53.8) | 17 (65.4) | 13 (50.0) | 0.477 (χ2) |
| Present | 15 (55.6) | 12 (46.2) | 9 (34.6) | 13 (50.0) | |
| Metastasis in the pleura, n (%) | |||||
| Absent | 25 (92.6) | 26 (100.0) | 25 (96.2) | 17 (65.4) | 0.0001 † |
| Present | 2 (7.4) | 0 (0.0) | 1 (3.8) | 9 (34.6) | |
| Metastasis in the liver, n (%) | |||||
| Absent | 22 (81.5) | 20 (76.9) | 17 (65.4) | 17 (65.4) | 0.447 (χ2) |
| Present | 5 (18.5) | 6 (23.1) | 9 (34.6) | 9 (34.6) | |
| Metastasis in the bones, n (%) | |||||
| Absent | 21 (77.8) | 15 (57.7) | 16 (61.5) | 20 (76.9) | 0.269 (χ2) |
| Present | 6 (22.2) | 11 (42.3) | 10 (38.5) | 6 (23.1) | |
| Treatment line, n (%) | |||||
| First | 25 (92.6) | 22 (84.6) | 23 (88.5) | 23 (88.5) | 0.801 † |
| Second | 2 (7.4) | 4 (15.4) | 3 (11.5) | 3 (11.5) | |
| Immunotherapy regimen, n (%) | |||||
| ICIs monotherapy | 10 (37.0) | 13 (50.0) | 6 (23.1) | 9 (34.6) | 0.249 (χ2) |
| Chemoimmunotherapy | 17 (63.0) | 13 (50.0) | 20 (76.9) | 17 (65.4) | |
| irAE, n (%) | |||||
| Absent | 22 (81.5) | 23 (88.5) | 21 (80.8) | 13 (50.0) | 0.010 † |
| Present | 5 (18.5) | 3 (11.5) | 5 (19.2) | 13 (50.0) | |
| PD-L1 expression, n (%) | |||||
| 1–49% | 22 (81.5) | 23 (88.5) | 19 (73.1) | 18 (69.2) | 0.327 † |
| ≥50% | 5 (18.5) | 3 (11.5) | 7 (26.9) | 8 (30.8) | |
| ECOG performance status, n (%) | |||||
| 0–1 | 26 (96.3) | 24 (84.6) | 24 (84.6) | 26 (100.0) | 0.568 † |
| ≥2 | 1 (3.7) | 2 (15.4) | 2 (15.4) | 0 (0.0) | |
| CCI, n (%) | |||||
| 0 | 7 (26.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.0001 † |
| 1 | 12 (44.4) | 11 (42.3) | 0 (0.0) | 0 (0.0) | |
| 2 | 8 (29.6) | 10 (38.5) | 21 (80.8) | 10 (38.5) | |
| ≥3 | 0 (0.0) | 5 (19.2) | 5 (19.2) | 16 (61.5) | |
| Age Groups | The Median Duration of ICI Treatment (Range), Months | p |
|---|---|---|
| Quartile 1, n = 27 | 8.1 (1.3–20.0) | 0.5976 |
| Quartile 2, n = 26 | 7.6 (1.3–18.7) | |
| Quartile 3, n = 26 | 7.5 (1.0–18.5) | |
| Quartile 4, n = 26 | 8.7 (1.0–24.0) |
| Treatment Response | Quartile 1, n (%) | Quartile 2, n (%) | Quartile 3, n (%) | Quartile 4, n (%) | p-Value (Test) |
|---|---|---|---|---|---|
| ORR: | |||||
| Yes (n = 54) | 13 (48.1) | 11 (42.3) | 14 (53.8) | 16 (61.5) | 0.551 (χ2) |
| No (n = 51) | 14 (51.9) | 15 (57.7) | 12 (46.2) | 10 (38.5) | |
| DCR: | |||||
| Yes (n = 91) | 21 (77.8) | 23 (88.5) | 25 (96.2) | 22 (84.6) | 0.257 † |
| No (n = 14) | 6 (22.2) | 3 (11.5) | 1 (3.8) | 4 (15.4) |
| Age for All-Cause Mortality | HR | 95% CI | p-Value | P for Trend | |
|---|---|---|---|---|---|
| Model 1 | Q1 | Reference | – | – | 0.370 |
| Q2 | 1.19 | 0.90–1.57 | 0.208 | ||
| Q3 | 1.19 | 0.99–1.44 | 0.063 | ||
| Q4 | 1.05 | 0.91–1.21 | 0.468 | ||
| Model 2 | Q1 | Reference | – | – | 0.577 |
| Q2 | 1.24 | 0.92–1.67 | 0.143 | ||
| Q3 | 1.17 | 0.95–1.43 | 0.122 | ||
| Q4 | 1.02 | 0.87–1.19 | 0.795 | ||
| Model 3 | Q1 | Reference | – | – | 0.716 |
| Q2 | 1.19 | 0.84–1.68 | 0.317 | ||
| Q3 | 1.24 | 0.95–1.62 | 0.110 | ||
| Q4 | 1.03 | 0.83–1.27 | 0.762 |
| irAE | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
|---|---|---|---|---|
| Pruritus | 0 (0.0%) | 1 (3.8%) | 0 (0.0%) | 1 (3.8%) |
| Hypothyroidism | 0 (0.0%) | 1 (3.8%) | 0 (0.0%) | 0 (0.0%) |
| Hyperthyroidism | 0 (0.0%) | 1 (3.8%) | 1 (3.8%) | 4 (15.4%) |
| Hepatitis | 1 (3.7) | 0 (0.0%) | 0 (0.0%) | 1 (2.0%) |
| Pneumonitis | 0 (0.0%) | 0 (0.0%) | 1 (3.8%) | 1 (6.7%) |
| Nephritis | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (7.7%) |
| Colitis | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (7.7%) |
| Arthralgia | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (3.8%) |
| Myalgia | 0 (0.0%) | 0 (0.0%) | 1 (3.8%) | 0 (0.0%) |
| Bullous pemphigus | 1 (3.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Onycholysis | 0 (0.0%) | 1 (3.8%) | 0 (0.0%) | 1 (3.8%) |
| Optic neuritis | 0 (0.0%) | 0 (0.0%) | 1 (3.8%) | 0 (0.0%) |
| Rash | 0 (0.0%) | 0 (0.0%) | 1 (3.8%) | 0 (0.0%) |
| Aseptic bone necrosis | 0 (0.0%) | 0 (0.0%) | 1 (3.8%) | 0 (0.0%) |
| Infusion reaction | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (7.7%) |
| Total number of irAE of any grade of toxicity | 2 (7.4%) | 4 (15.4%) | 6 (23.1) | 15 (57.7%) |
| Number of irAE ≥ grade 3 of toxicity | 2 (7.4%) | 3 (11.5%) | 3 (11.5) | 1 (3.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moskalenko, Y.; Yazykov, O.; Vasylieva, O.; Smiian, K.; Ivakhniuk, T.; Budko, H.; Moskalenko, R. The Impact of Age on the Effectiveness of Immune Checkpoint Inhibitors Therapy in Patients with Metastatic Non-Small-Cell Lung Cancer. Geriatrics 2025, 10, 85. https://doi.org/10.3390/geriatrics10040085
Moskalenko Y, Yazykov O, Vasylieva O, Smiian K, Ivakhniuk T, Budko H, Moskalenko R. The Impact of Age on the Effectiveness of Immune Checkpoint Inhibitors Therapy in Patients with Metastatic Non-Small-Cell Lung Cancer. Geriatrics. 2025; 10(4):85. https://doi.org/10.3390/geriatrics10040085
Chicago/Turabian StyleMoskalenko, Yuliia, Oleksandr Yazykov, Olena Vasylieva, Kateryna Smiian, Tetiana Ivakhniuk, Hanna Budko, and Roman Moskalenko. 2025. "The Impact of Age on the Effectiveness of Immune Checkpoint Inhibitors Therapy in Patients with Metastatic Non-Small-Cell Lung Cancer" Geriatrics 10, no. 4: 85. https://doi.org/10.3390/geriatrics10040085
APA StyleMoskalenko, Y., Yazykov, O., Vasylieva, O., Smiian, K., Ivakhniuk, T., Budko, H., & Moskalenko, R. (2025). The Impact of Age on the Effectiveness of Immune Checkpoint Inhibitors Therapy in Patients with Metastatic Non-Small-Cell Lung Cancer. Geriatrics, 10(4), 85. https://doi.org/10.3390/geriatrics10040085






