The Influence of Frailty on Pharmacotherapy Adherence and Adverse Drug Reactions in Older Psychiatric Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Frailty Measurements
2.3. Variable Definitions
2.4. Statistical Analyses
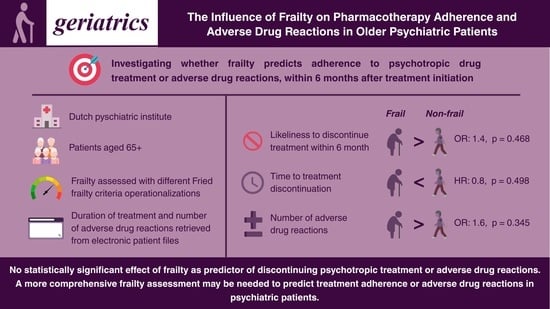
3. Results
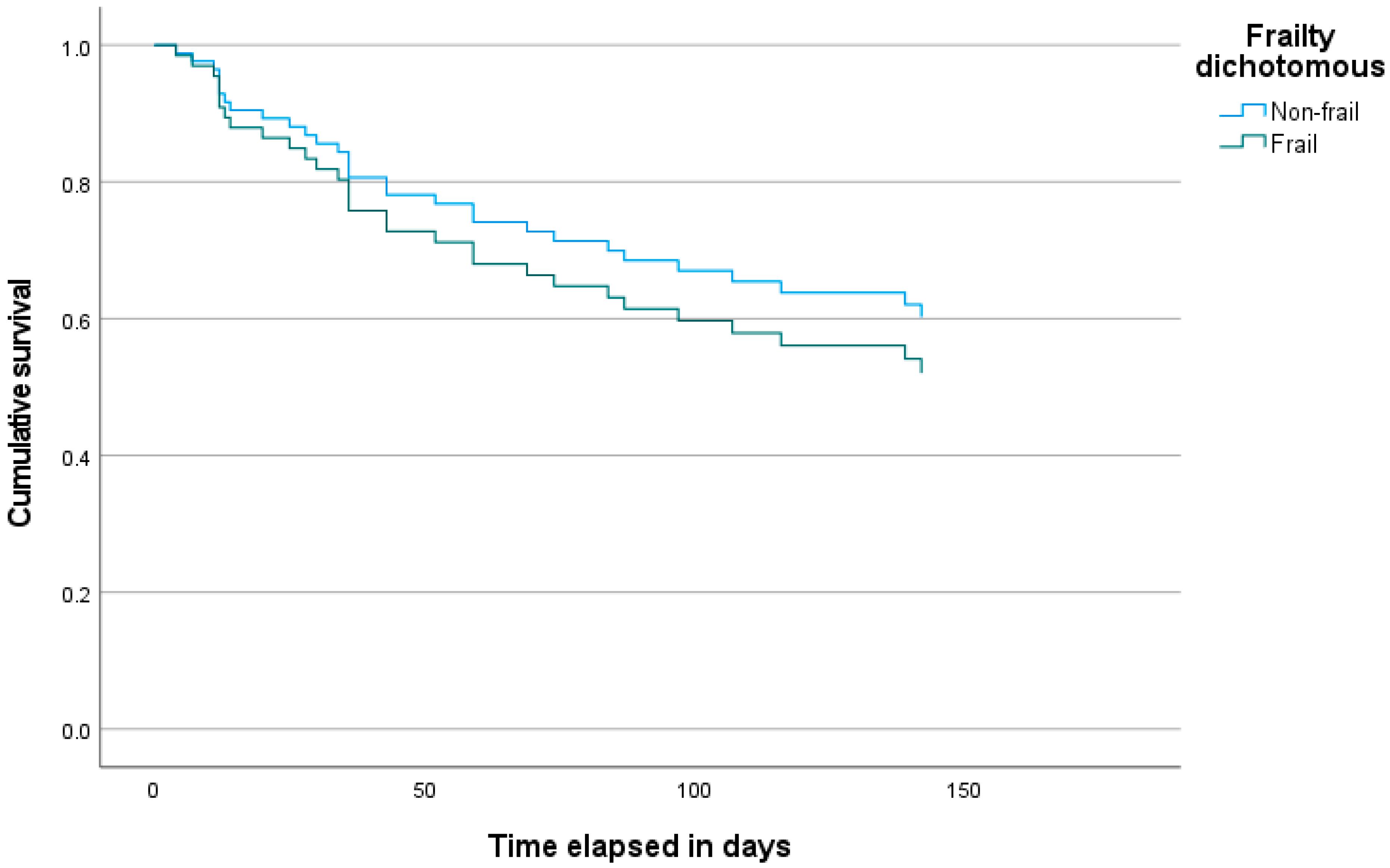
3.1. Time to Discontinuation
3.2. Number of Adverse Drug Reactions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADR | Adverse Drug Reaction |
| B | regression coefficient |
| CES-D | Center for Epidemiologic Studies Depression Scale |
| HR | hazard ratio |
| IPAQ | International Physical Activity Questionnaire |
| IQR | interquartile range |
| MMSE | Mini-Metal State Evaluation |
| N | number |
| OR | odds ratio |
| SD | standard deviation |
| SE | standard error |
| SNAQ | Short Nutritional Assessment Questionnaire |
| TTD | Time to Discontinuation |
References
- Morley, J.E.; Vellas, B.; Van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [PubMed]
- Rockwood, K.; Mitnitski, A. Frailty in relation to the accumulation of deficits. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar]
- Randles, M.A.; O’Mahony, D.; Gallagher, P.F. Frailty and potentially inappropriate prescribing in older people with polypharmacy: A bi-directional relationship? Drugs Aging 2022, 39, 597–606. [Google Scholar]
- Ye, L.; Nieboer, D.; Yang-Huang, J.; Borrás, T.A.; Garcés-Ferrer, J.; Verma, A.; van Grieken, A.; Raat, H. The association between frailty and the risk of medication-related problems among community-dwelling older adults in Europe. J. Am. Geriatr. Soc. 2023, 71, 2485–2494. [Google Scholar]
- Aprahamian, I.; Landowski, A.; Ahn, F.O.; Neves, B.A.; Rocha, J.T.; Strauss, J.; Borges, M.K.; Morley, J.E.; Oude Voshaar, R.C. Frailty in geriatric psychiatry inpatients: A retrospective cohort study. Int. Psychogeriatr. 2022, 34, 981–989. [Google Scholar]
- Benraad, C.E.; Haaksma, M.L.; Karlietis, M.H.; Oude Voshaar, R.C.; Spijker, J.; Melis, R.J.; Olde Rikkert, M.G. Frailty as a predictor of mortality in older adults within 5 years of psychiatric admission. Int. J. Geriatr. Psychiatry 2020, 35, 617–625. [Google Scholar]
- Benraad, C.E.; Disselhorst, L.; Laurenssen, N.C.; Hilderink, P.H.; Melis, R.J.; Spijker, J.; Olde Rikkert, M.G. Frailty, multimorbidity and functional status as predictors for health outcomes of acute psychiatric hospitalisation in older adults. Aging Ment. Health 2024, 24, 119–128. [Google Scholar]
- Stolz, E.; Rásky, É.; Jagsch, C. Frailty index predicts geriatric psychiatry inpatient mortality: A case–control study. Psychogeriatrics 2020, 20, 469–472. [Google Scholar]
- Buigues, C.; Padilla-Sánchez, C.; Garrido, J.F.; Navarro-Martínez, R.; Ruiz-Ros, V.; Cauli, O. The relationship between depression and frailty syndrome: A systematic review. Aging Ment. Health 2015, 19, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Soysal, P.; Veronese, N.; Thompson, T.; Kahl, K.G.; Fernandes, B.S.; Prina, A.M.; Solmi, M.; Schofield, P.; Koyanagi, A.; Tseng, P.T.; et al. Relationship between depression and frailty in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2017, 36, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Lam JY, J.; Barras, M.; Scott, I.A.; Long, D.; Shafiee Hanjani, L.; Falconer, N. Scoping review of studies evaluating frailty and its association with medication harm. Drugs Aging 2022, 39, 333–353. [Google Scholar] [CrossRef]
- Gnjidic, D.; Hilmer, S.N.; Blyth, F.M.; Naganathan, V.; Cumming, R.G.; Handelsman, D.J.; McLachlan, A.J.; Abernethy, D.R.; Banks, E.; Le Couteur, D.G. High-risk prescribing and incidence of frailty among older community-dwelling men. Clin. Pharmacol. Ther. 2012, 91, 521–528. [Google Scholar] [CrossRef]
- Walsh, C.A.; Cahir, C.; Tecklenborg, S.; Byrne, C.; Culbertson, M.A.; Bennett, K.E. The association between medication non-adherence and adverse health outcomes in ageing populations: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2019, 85, 2464–2478. [Google Scholar] [CrossRef]
- Barbosa da Silva, A.; Queiroz de Souza, I.; Da Silva, I.K.; Borges Lopes Tavares da Silva, M.; Oliveira Dos Santos, A.C. Factors associated with frailty syndrome in older adults. J. Nutr. Health Aging 2020, 24, 218–222. [Google Scholar] [CrossRef]
- Chudiak, A.; Jankowska-Polańska, B.; Uchmanowicz, I. Effect of frailty syndrome on treatment compliance in older hyper-tensive patients. Clin. Interv. Aging 2017, 12, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Jankowska-Polańska, B.; Dudek, K.; Szymanska-Chabowska, A.; Uchmanowicz, I. The influence of frailty syndrome on medication adherence among elderly patients with hypertension. Clin. Interv. Aging 2016, 11, 1781–1790. [Google Scholar] [CrossRef]
- Qiao, X.; Tian, X.; Liu, N.; Dong, L.; Jin, Y.; Si, H.; Liu, X.; Wang, C. The association between frailty and medication adherence among community-dwelling older adults with chronic diseases: Medication beliefs acting as mediators. Patient Educ. Couns. 2020, 103, 2548–2554. [Google Scholar] [CrossRef]
- Chao, C.T.; Huang, J.W. Geriatric syndromes are potential determinants of the medication adherence status in prevalent dialysis patients. PeerJ 2016, 4, e2122. [Google Scholar] [CrossRef]
- Semahegn, A.; Torpey, K.; Manu, A.; Assefa, N.; Tesfaye, G.; Ankomah, A. Psychotropic medication non-adherence and its associated factors among patients with major psychiatric disorders: A systematic review and meta-analysis. Syst. Rev. 2020, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2003; pp. 1–28. Available online: https://apps.who.int/iris/bitstream/handle/10665/42682/9241545992.pdf (accessed on 25 November 2024).
- Kato, M.; Hori, H.; Inoue, T.; Iga, J.; Iwata, M.; Inagaki, T.; Shinohara, K.; Imai, H.; Murata, A.; Mishima, K.; et al. Discontinuation of antidepressants after remission with antidepressant medication in major depressive disorder: A systematic review and meta-analysis. Mol. Psychiatry 2021, 26, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Suzuki, T.; Uchida, H.; Watanabe, K.; Mimura, M. Antipsychotic treatment for schizophrenia in the maintenance phase: A systematic review of the guidelines and algorithms. Schizoph. Res. 2012, 134, 219–225. [Google Scholar]
- Brown, P.J.; Ciarleglio, A.; Roose, S.P.; Garcia, C.M.; Chung, S.; Alvarez, J.; Stein, A.; Gomez, S.; Rutherford, B.R. Frailty worsens antidepressant treatment outcomes in late life depression. Am J Geriatr. Psychiatry 2021, 29, 944–955. [Google Scholar]
- Benraad, C.E.; Kamerman-Celie, F.; van Munster, B.C.; Oude Voshaar, R.C.; Spijker, J.; Olde Rikkert, M.G. Geriatric characteristics in randomised controlled trials on antidepressant drugs for older adults: A systematic review. Int. J. Geriatr. Psychiatry 2016, 31, 990–1003. [Google Scholar]
- Comijs, H.C.; van Marwijk, H.W.; van der Mast, R.C.; Naarding, P.; Oude Voshaar, R.C.; Beekman, A.T.; Boshuisen, M.; Dekker, J.; Kok, R.; de Waal, M.W.; et al. The Netherlands study of depression in older persons (NESDO); A prospective cohort study. BMC Res. Notes 2011, 4, 524. [Google Scholar] [CrossRef]
- Radloff, L.S. A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar]
- Kruizenga, H.M.; Seidell, J.C.; de Vet, H.C.; Wierdsma, N.J. Development and validation of a hospital screening tool for malnutrition: The short nutritional assessment questionnaire (SNAQ©). Clin. Nutr. 2005, 24, 75–82. [Google Scholar] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar]
- Naranjo, C.A.; Busto, U.; Sellers, E.M.; Sandor, P.; Ruiz, I.; Roberts, E.A.; Janecek, E.; Domecq, C.; Greenblatt, D.J. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981, 30, 239–245. [Google Scholar]
| Characteristics | |
|---|---|
| Age, mean (SD), range | 73.9 (6.4), 65–93 |
| Female sex, n (%) | 56 (72.7) |
| Inpatient, n (%) | 62 (80.5) |
| Psychotropic drug class | |
| Antidepressant | 55 |
| Antipsychotic | 39 |
| Mood stabilizer | 25 |
| Number of drugs, median (IQR), range | 5 (3–7), 1–13 |
| MMSE-score, median (IQR), range | 27 (26–29), 19–30 |
| Frail according to Fried criteria, n (%) | 27 (35.1%) |
| Number of frailty components, median (IQR) | 2 (1–3) |
| Grip strength in kilograms, median (IQR), range | 47.5 (37.5–62.5), 14.5–104 |
| Gait speed, median (IQR), range in seconds | 4.5 (3.4–6.3), 1.8–9.4 |
| Variable | Dichotomous Frailty | Number of Frailty Components | Grips Strength | Gait Speed | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Entire cohort | ||||||||||||
| Frailty variable | 1.08 | 0.48–2.43 | 0.148 | 1.12 | 0.84–1.49 | 0.457 | 1.0 | 0.98–1.04 | 0.513 | 1.23 | 1.0–1.50 | 0.049 |
| Female sex | 0.85 | 0.37–2.0 | 0.427 | 0.87 | 0.38–2.0 | 0.737 | 1.01 | 0.30–3.46 | 0.987 | 0.72 | 0.30–1.72 | 0.460 |
| Age | 1.05 | 0.99–1.11 | 0.029 | 1.05 | 0.99–1.11 | 0.091 | 1.06 | 0.99–1.12 | 0.052 | 1.04 | 0.98–1.11 | 0.203 |
| Outpatient | 0.75 | 0.28–2.0 | 0.505 | 0.79 | 0.29–2.12 | 0.683 | 0.82 | 0.31–2.18 | 0.693 | 0.93 | 0.33–2.58 | 0.886 |
| Polypharmacy | 1.02 | 0.89–1.17 | 0.074 | 1.00 | 0.87–1.16 | 0.998 | 1.04 | 0.90–1.19 | 0.620 | 0.99 | 0.85–1.15 | 0.868 |
| Post-baseline group | ||||||||||||
| Frailty variable | 2.05 | 0.71–5.94 | 0.188 | 1.40 | 0.97–2.02 | 0.070 | 1.00 | 0.97–1.03 | 0.792 | 1.39 | 1.00–1.92 | 0.047 |
| Female sex | 1.19 | 0.42–3.35 | 0.744 | 1.52 | 0.50–4.61 | 0.459 | 0.84 | 0.20–3.51 | 0.808 | 0.99 | 0.33–2.93 | 0.981 |
| Age | 1.07 | 0.98–1.1.6 | 0.137 | 1.07 | 0.97–117 | 0.102 | 1.07 | 0.98–1.17 | 0.133 | 1.02 | 0.93–1.12 | 0.739 |
| Outpatient | 0.59 | 0.19–1.82 | 0.355 | 0.60 | 0.19–1.91 | 0.385 | 0.55 | 0.16–1.90 | 0.346 | 0.81 | 0.25–2.66 | 0.734 |
| Polypharmacy | 0.98 | 0.81–1.18 | 0.839 | 0.95 | 0.78–1.16 | 0.609 | 1.00 | 0.83–1.22 | 0.979 | 0.95 | 0.77–1.17 | 0.594 |
| Variable | Number of Frailty Components a | Grip Strength b | Gait Speed b | |||
|---|---|---|---|---|---|---|
| H | p | H | p | H | p | |
| Entire cohort | 4.077 | 0.538 | 2.114 | 0.347 | 1.260 | 0.533 |
| Post-baseline group | 4.720 | 0.451 | 2.196 | 0.333 | 0.266 | 0.876 |
| Variable | Dichotomous Frailty | Number of Frailty Components | Grips Strength | Gait Speed | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| Entire cohort | ||||||||||||
| Frailty variable | 1.34 | 0.52–3.49 | 0.544 | 1.12 | 0.80–1.57 | 0.503 | 0.99 | 0.96–1.02 | 0.509 | 1.18 | 0.91–1.51 | 0.207 |
| Female sex | 0.71 | 0.27–1.84 | 0.477 | 0.68 | 0.26–1.75 | 0.436 | 0.48 | 0.11–2.02 | 0.318 | 0.58 | 0.22–1.55 | 0.277 |
| Age | 1.27 | 0.96–1.10 | 0.503 | 1.02 | 0.95–1.09 | 0.651 | 1.03 | 0.96–1.10 | 0.435 | 1.01 | 0.94–1.09 | 0.791 |
| Outpatient | 0.62 | 0.19–2.13 | 0.431 | 0.65 | 0.20–2.13 | 0.481 | 0.66 | 0.20–2.12 | 0.499 | 0.69 | 0.21–2.28 | 0.538 |
| Polypharmacy | 0.71 | 0.83–1.13 | 0.674 | 0.96 | 0.82–1.13 | 0.601 | 0.97 | 0.83–1.14 | 0.724 | 0.94 | 0.79–1.11 | 0.438 |
| Post-baseline group | ||||||||||||
| Frailty variable | 1.41 | 0.35–5.75 | 0.631 | 1.14 | 0.71–1.82 | 0.595 | 1.00 | 0.95–1.04 | 0.814 | 1.18 | 0.79–1.75 | 0.417 |
| Female sex | 0.48 | 0.13–1.79 | 0.276 | 0.50 | 0.13–1.85 | 0.297 | 0.29 | 0.04–2.11 | 0.223 | 0.41 | 0.11–1.59 | 0.196 |
| Age | 0.99 | 0.90–1.11 | 0.957 | 1.00 | 0.90–1.11 | 0.960 | 1.00 | 0.90–1.11 | 0.979 | 0.98 | 0.87–1.10 | 0.713 |
| Outpatient | 0.41 | 0.09–1.82 | 0.239 | 0.41 | 0.09–1.86 | 0.246 | 0.39 | 0.08–1.94 | 0.247 | 0.41 | 0.09–1.90 | 0.254 |
| Polypharmacy | 0.95 | 0.75–1.18 | 0.618 | 0.94 | 0.75–1.18 | 0.577 | 0.93 | 0.73–1.18 | 0.554 | 0.89 | 0.70–1.14 | 0.361 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, T.A.; Kok, R. The Influence of Frailty on Pharmacotherapy Adherence and Adverse Drug Reactions in Older Psychiatric Patients. Geriatrics 2025, 10, 57. https://doi.org/10.3390/geriatrics10020057
Phan TA, Kok R. The Influence of Frailty on Pharmacotherapy Adherence and Adverse Drug Reactions in Older Psychiatric Patients. Geriatrics. 2025; 10(2):57. https://doi.org/10.3390/geriatrics10020057
Chicago/Turabian StylePhan, Tuan Anh, and Rob Kok. 2025. "The Influence of Frailty on Pharmacotherapy Adherence and Adverse Drug Reactions in Older Psychiatric Patients" Geriatrics 10, no. 2: 57. https://doi.org/10.3390/geriatrics10020057
APA StylePhan, T. A., & Kok, R. (2025). The Influence of Frailty on Pharmacotherapy Adherence and Adverse Drug Reactions in Older Psychiatric Patients. Geriatrics, 10(2), 57. https://doi.org/10.3390/geriatrics10020057