Cognitive Performance Among Older Adults with Subjective Cognitive Decline
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Instruments
2.3. Procedure
2.4. Data Analysis
3. Results
3.1. Subjects’ Classification
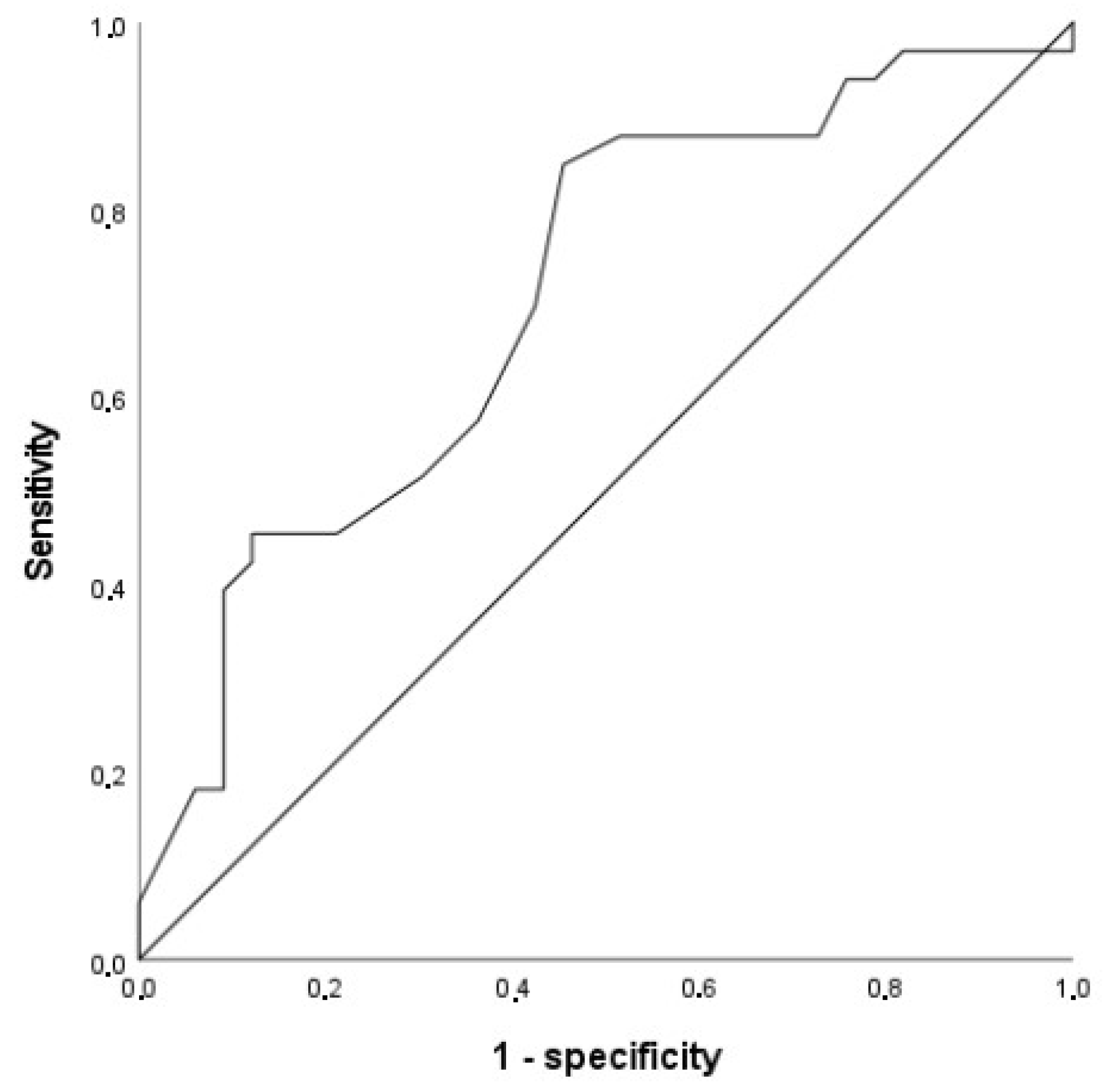
3.2. ROC Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jessen, F.; Amariglio, R.E.; Van Boxtel, M.; Breteler, M.; Ceccaldi, M.; Chételat, G.; Dubois, B.; Dufouil, C.; Ellis, K.A.; Van Der Flier, W.M. Subjective Cognitive Decline Initiative. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014, 10, 844–852. [Google Scholar] [CrossRef]
- Petersen, R.C.; Caracciolo, B.; Brayne, C.; Gauthier, S.; Jelic, V.; Fratiglioni, L. Mild cognitive impairment: A concept in evolution. J. Intern. Med. 2014, 275, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Jessen, F.; Amariglio, R.E.; Buckley, R.F.; van der Flier, W.M.; Han, Y.; Molinuevo, J.L.; Rabin, L.; Rentz, D.M.; Rodriguez-Gomez, O.; Saykin, A.J. The characterization of subjective cognitive decline. Lancet Neurol. 2020, 19, 271–278. [Google Scholar] [CrossRef]
- Chen, S.T.; Siddarth, P.; Ercoli, L.M.; Merrill, D.A.; Torres-Gil, F.; Small, G.W. Modifiable risk factors for Alzheimer’s disease and subjective memory impairment across age groups. PLoS ONE 2014, 9, e98630. [Google Scholar] [CrossRef]
- Rabin, L.A.; Smart, C.M.; Crane, P.K.; Amariglio, R.E.; Berman, L.M.; Boada, M.; Buckley, R.F.; Chételat, G.; Dubois, B.; Ellis, K.A.; et al. Subjective cognitive decline in older adults: An overview of self-report measures used across 19 international research studies. J. Alzheimer’s Dis. 2015, 48, S63–S86. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Chen, T.F.; Chiu, M.J. From mild cognitive impairment to subjective cognitive decline: Conceptual and methodological evolution. Neuropsychiatr. Dis. Treat. 2017, 13, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Beaumont, H.; Ferguson, D.; Yadegarfar, M.; Stubbs, B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: Meta-analysis. Acta Psychiatr. Scand. 2014, 130, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Rotenberg, S.; Maeir, A.; Dawson, D.R. Changes in Activity Participation Among Older Adults with Subjective Cognitive Decline or Objective Cognitive Deficits. Front. Neurol. 2020, 10, 1393. [Google Scholar] [CrossRef]
- Li, H.; Tan, C.C.; Tan, L.; Xu, W. Predictors of cognitive deterioration in subjective cognitive decline: Evidence from longitudinal studies and implications for SCD-plus criteria. J. Neurol. Neurosurg. Psychiatry 2023, 94, 844–854. [Google Scholar] [CrossRef]
- Roehr, S.; Villringer, A.; Angermeyer, M.C.; Luck, T.; Riedel-Heller, S.G. Outcomes of stable and unstable patterns of subjective cognitive decline—Results from the Leipzig Longitudinal Study of the Aged (LEILA75+). BMC Geriatr. 2016, 16, 180. [Google Scholar] [CrossRef]
- Yu, H.H.; Tan, C.C.; Huang, S.J.; Zhang, X.H.; Tan, L.; Xu, W.; Alzheimer’s Disease Neuroimaging Initiative. Predicting the reversion from mild cognitive impairment to normal cognition based on magnetic resonance imaging, clinical, and neuropsychological examinations. J. Affect. Disord. 2024, 353, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Jonker, C.; Geerlings, M.I.; Schmand, B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int. J. Geriatr. Psychiatry 2000, 15, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ptacek, S.; Cavallin, L.; Kåreholt, I.; Kramberger, M.G.; Winblad, B.; Jelic, V.; Eriksdotter, M. Subjective cognitive impairment subjects in our clinical practice. Dement. Geriatr. Cogn. Disord. Extra 2014, 4, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Rijs, K.J.; Van den Kommer, T.N.; Comijs, H.C.; Deeg, D.J. Prevalence and incidence of memory complaints in employed compared to non-employed aged 55–64 years and the role of employment characteristics. PLoS ONE 2015, 10, e0119192. [Google Scholar] [CrossRef]
- Zhou, C.; Jeryous Fares, B.; Thériault, K.; Trinh, B.; Joseph, M.; Jauhal, T.; Sheppard, C.; Labelle, P.R.; Krishnan, A.; Rabin, L.; et al. Subjective cognitive decline and objective cognitive performance in older adults: A systematic review of longitudinal and cross-sectional studies. J. Neuropsychol. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Costa, A.N.; Nowakowski, L.M.; McCrae, C.S.; Cowan, N.; Curtis, A.F. Discrepancies in Objective and Subjective Cognition in Middle-Aged and Older Adults: Does Personality Matter? Gerontol. Geriatr. Med. 2023, 9, 23337214221146663. [Google Scholar] [CrossRef]
- Nuzum, H.; Dorociak, K.; Kamil-Rosenberg, S.; Louras, P.; Mostofi, M.; Fairchild, J.K. Objective and Subjective Cognitive Function, and Relations with Quality of Life and Psychological Distress. Innov. Aging 2020, 4, 306–307. [Google Scholar] [CrossRef]
- Balash, Y.; Mordechovich, M.; Shabtai, H.; Giladi, N.; Gurevich, T.; Korczyn, A.D. Subjective memory complaints in elders: Depression, anxiety, or cognitive decline? Acta Neurol. Scand. 2013, 127, 344–350. [Google Scholar] [CrossRef]
- Mulligan, B.P.; Smart, C.M.; Ali, J.I. Relationship of subjective and objective performance indicators in subjective cognitive decline. Psychol. Neurosci. 2016, 9, 362–378. [Google Scholar] [CrossRef]
- Wolfsgruber, S.; Kleineidam, L.; Guski, J.; Polcher, A.; Frommann, I.; Roeske, S.; Spruth, E.J.; Franke, C.; Priller, J.; Kilimann, I.; et al. DELCODE Study Group. Minor neuropsychological deficits in patients with subjective cognitive decline. Neurology 2020, 95, e1134–e1143. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhao, X.; Cui, X.; Liu, X.; Zhu, X.; Jiang, Y.; Li, J. Subtle Pathophysiological Changes in Working Memory-Related Potentials and Intrinsic Theta Power in Community-Dwelling Older Adults with Subjective Cognitive Decline. Innov. Aging 2023, 7, igad004. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.J.; Park, J.W.; Lee, S.B.; Kim, S.H.; Kim, Y.; Ryu, D.W.; Park, K.W.; Yang, D.W. The influence of amyloid burden on cognitive decline over 2 years in older adults with subjective cognitive decline: A prospective cohort study. Dement. Geriatr. Cogn. Disord. 2022, 50, 437–445. [Google Scholar] [CrossRef]
- Corlier, F.W.; Shaw, C.; Hayes-Larson, E.; Mungas, D.; Farias, S.T.; Glymour, M.M.; Whitmer, M.A.; Mayeda, E.R. Association Between Cognitive Test Performance and Subjective Cognitive Decline in a Diverse Cohort of Older Adults: Findings from the KHANDLE Study. Alzheimer Dis. Assoc. Disord. 2020, 34, 198–205. [Google Scholar] [CrossRef]
- López-Higes, R.; Prados, J.M.; Rubio, S.; Montejo, P.; Del Río, D. Executive functions and linguistic performance in SCD older adults and healthy controls. Aging Neuropsychol. Cogn. 2017, 24, 717–734. [Google Scholar] [CrossRef] [PubMed]
- López-Higes, R.; Rubio-Valdehita, S.; Fernandes, S.M.; Rodrigues, P.F. Differentiation between Normal Cognition and Subjective Cognitive Decline in Older Adults Using Discrepancy Scores Derived from Neuropsychological Tests. Geriatrics 2024, 9, 83. [Google Scholar] [CrossRef]
- Lobo, A.; Saz, P.; Marcos, G.; Día, J.L.; De La Cámara, C.; Ventura, T.; Morales Asín, F.; Fernando Pascual, L.; Montañés, J.A.; Aznar, S. Revalidation and standardization of the cognition mini-exam (first Spanish version of the Mini-Mental Status Examination) in the general geriatric population. Med. Clínica 1999, 112, 767–774. [Google Scholar]
- Sheikh, J.; Yesavage, J. Geriatric Depression Scale (GDS): Recent findings and development of a shorter version. In Clinical Gerontology: A Guide to Assessment and Intervention; Brink, T., Ed.; The Haworth Press: New York, NY, USA, 1986; pp. 165–174. [Google Scholar]
- Wechsler, D. WMS-III Escala de Memoria de Wechsler-III; TEA: Madrid, Spain, 2004. [Google Scholar]
- Rami, L.; Valls-Pedret, C.; Bartres-Faz, D.; Caprile, C.; Sole-Padulles, C.; Castellvi, M.; Olives, B.; Bosch, J.L.; Molinuevo, J.L. Cognitive Reserve Questionnaire. Values obtained in a healthy elderly population and in Alzheimer’s disease. Rev. De Neurol. 2011, 52, 195–201. [Google Scholar]
- MacDonald, M.C.; Almor, A.; Henderson, V.W.; Kempler, D.; Andersen, E.S. Assessing working memory and language comprehension in Alzheimer’s disease. Brain Lang. 2001, 78, 17–42. [Google Scholar] [CrossRef]
- Golden, C.J. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses; Stoelting Co.: Chicago, IL, USA, 1978. [Google Scholar]
- Reitan, R.M. Trail Making Test: Manual for Administration and Scoring; Reitan Neuropsychology Laboratory: Tucson, AZ, USA, 1992. [Google Scholar]
- García-Albea, J.E.; Sánchez-Bernardos, M.L.; del Viso-Pabón, S. Boston Naming Test for Aphasia Diagnosis: Spanish version. In Assessment of Aphasia and Related Disorders; Goodglass, H., Kaplan, E., Eds.; Editorial Medica Panamericana: Madrid, Spain, 1986; pp. 129–198. [Google Scholar]
- López-Higes, R.; Rubio, S.; Martín-Aragoneses, M.T.; Del Río, D.; Mejuto, G. Assessment of grammatical comprehension in normal and pathological aging: A summary of the results obtained with ECCO and ECCO_Senior tests. Int. J. Psychol. Res. 2012, 5, 96–108. [Google Scholar] [CrossRef]
- Hao, L.; Xing, Y.; Li, X.; Mu, B.; Zhao, W.; Wang, G.; Wang, T.; Jia, J.; Han, Y. Risk factors and neuropsychological assessments of subjective cognitive decline (plus) in Chinese memory clinic. Front. Neurosci. 2019, 13, 846. [Google Scholar] [CrossRef]
- Jessen, F.; Jessen, F.; Wolfsgruber, S.; Kleineindam, L.; Spottke, A.; Altenstein, S.; Bartels, C.; Berger, M.; Brosseron, F.; Daamen, M.; et al. Subjective cognitive decline and stage 2 of Alzheimer’s disease in patients from memory centers. Alzheimer’s Dement. 2023, 19, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.; Oliver, M.D. Subjective cognitive decline is associated with lower baseline cognition and increased rate of cognitive decline. J. Gerontol. Ser. B 2023, 78, 573–584. [Google Scholar] [CrossRef]
- Seo, E.H.; Kim, H.; Lee, K.H.; Choo, I.L.H. Altered executive function in pre-mild cognitive impairment. J. Alzheimer’s Dis. 2016, 54, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Ebenau, J.L.; Timmers, T.; Wesselman, L.M.; Verberk, I.M.; Verfaillie, S.C.; Slot, R.E.; van Harten, A.C.; Teunissen, C.E.; Barkhof, F.; Bosch, K.A.v.D.; et al. ATN classification and clinical progression in subjective cognitive decline: The SCIENCe project. Neurology 2020, 95, e46–e58. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A. Executive Functions. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 173, pp. 225–240. [Google Scholar]
- Rey-Mermet, A.; Gade, M. Inhibition in Aging: What Is Preserved? What Declines? A Meta-Analysis. Psychon. Bull. Rev. 2018, 25, 1695–1716. [Google Scholar] [CrossRef]
- Forte, G.; Troisi, G.; Favieri, F.; Casagrande, M. Inhibition changes across the lifespan: Experimental evidence from the Stroop task. BMC Psychol. 2024, 12, 336. [Google Scholar] [CrossRef]
- Campbell, K.L.; Lustig, C.; Hasher, L. Aging and inhibition: Introduction to the special issue. Psychol. Aging 2020, 35, 605. [Google Scholar] [CrossRef]
- Macoir, J.; Tremblay, P.; Hudon, C. The use of executive fluency tasks to detect cognitive impairment in individuals with subjective cognitive decline. Behav. Sci. 2022, 12, 491. [Google Scholar] [CrossRef]
- Troyer, A.K.; Leach, L.; Strauss, E. Aging and response inhibition: Normative data for the Victoria Stroop Test. Aging Neuropsychol. Cogn. 2006, 13, 20–35. [Google Scholar] [CrossRef]
- Saari, T.; Smith, E.E.; Ismail, Z. Network analysis of impulse dyscontrol in mild cognitive impairment and subjective cognitive decline. Int. Psychogeriatr. 2022, 34, 553–562. [Google Scholar] [CrossRef]
- Stenbäck, V.; Marsja, E.; Hällgren, M.; Lyxell, B.; Larsby, B. The contribution of age, working memory capacity, and inhibitory control on speech recognition in noise in young and older adult listeners. J. Speech Lang. Hear. Res. 2021, 64, 4513–4523. [Google Scholar] [CrossRef] [PubMed]
- De Klerk, M.T.; Smeets, P.A.M.; La Fleur, S.E. Inhibitory control as a potential treatment target for obesity. Nutr. Neurosci. 2023, 26, 429–444. [Google Scholar] [CrossRef] [PubMed]
| GROUP | F (1,63)= | Significance p= | Partial Eta Square | Observed Power | ||
|---|---|---|---|---|---|---|
| Controls Mean (SD) | SCD Mean (SD) | |||||
| Age | 70.39 (4.31) | 70.30 (4.33) | 0.00 | 0.96 | 0.000 | 0.050 |
| Years of education | 14.58 (5.64) | 14.85 (5.31) | 0.09 | 0.75 | 0.002 | 0.061 |
| Cognitive reserve | 15.30 (3.59) | 15.18 (3.24) | 0.01 | 0.92 | 0.000 | 0.051 |
| MMSE | 29.13 (1.10) | 28.67 (1.19) | 2.07 | 0.15 | 0.032 | 0.294 |
| MEMORY | ||||||
| Digit reordering | 12.75 (1.80) | 12.00 (2.16) | 2.38 | 0.13 | 0.036 | 0.331 |
| Digits: backward | 5.63 (1.79) | 5.87 (2.31) | 0.21 | 0.65 | 0.003 | 0.074 |
| Word List—immediate recall | 31.30 (5.56) | 29.58 (6.86) | 0.11 | 0.74 | 0.002 | 0.063 |
| Word List—delayed recall | 7.94 (2.39) | 5.94 (2.97) | 9.06 | 0.004 | 0.126 | 0.843 |
| EXECUTIVE FUNCTIONS | ||||||
| FAS | 41.03 (14.08) | 39.79 (12.84) | 0.14 | 0.71 | 0.002 | 0.066 |
| Semantic Verbal Fluency (animals + fruits) | 33.55 (6.57) | 30.94 (6.62) | 2.28 | 0.14 | 0.035 | 0.318 |
| TMT-A time | 50.94 (16.64) | 54.36 (17.44) | 0.85 | 0.36 | 0.013 | 0.148 |
| Stroop’s interference condition | 49.34 (7.99) | 43.13 (8.69) | 9.50 | 0.003 | 0.131 | 0.859 |
| LANGUAGE | ||||||
| BNT | 53.44 (4.72) | 52.44 (5.78) | 1.32 | 0.25 | 0.021 | 0.205 |
| ECCO: non-canonical sentences | 14.72 (1.98) | 13.57 (2.25) | 4.02 | 0.049 | 0.060 | 0.506 |
| ECCO: sentences with 2 propositions | 15.69 (2.24) | 14.57 (2.55) | 2.93 | 0.09 | 0.044 | 0.392 |
| B | Standard Error | Wald | df | Sig. | Exp(B) | |
|---|---|---|---|---|---|---|
| Delayed recall | −0.200 | 0.112 | 3.22 | 1 | 0.073 | 0.818 |
| Stroop’s interference | −0.090 | 0.033 | 7.35 | 1 | 0.007 | 0.914 |
| ECCO non-canonical sentences | 0.025 | 0.162 | 0.026 | 1 | 0.873 | 1.026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Higes, R.; Rubio-Valdehita, S.; López-Sanz, D.; Fernandes, S.M.; Rodrigues, P.F.S.; Delgado-Losada, M.L. Cognitive Performance Among Older Adults with Subjective Cognitive Decline. Geriatrics 2025, 10, 39. https://doi.org/10.3390/geriatrics10020039
López-Higes R, Rubio-Valdehita S, López-Sanz D, Fernandes SM, Rodrigues PFS, Delgado-Losada ML. Cognitive Performance Among Older Adults with Subjective Cognitive Decline. Geriatrics. 2025; 10(2):39. https://doi.org/10.3390/geriatrics10020039
Chicago/Turabian StyleLópez-Higes, Ramón, Susana Rubio-Valdehita, David López-Sanz, Sara M. Fernandes, Pedro F. S. Rodrigues, and María Luisa Delgado-Losada. 2025. "Cognitive Performance Among Older Adults with Subjective Cognitive Decline" Geriatrics 10, no. 2: 39. https://doi.org/10.3390/geriatrics10020039
APA StyleLópez-Higes, R., Rubio-Valdehita, S., López-Sanz, D., Fernandes, S. M., Rodrigues, P. F. S., & Delgado-Losada, M. L. (2025). Cognitive Performance Among Older Adults with Subjective Cognitive Decline. Geriatrics, 10(2), 39. https://doi.org/10.3390/geriatrics10020039







