The Implementation of Infant Anoesis and Adult Autonoesis in the Retrogenesis and Staging System of the Neurocognitive Disorders: A Proposal for a Multidimensional Person-Centered Model
Abstract
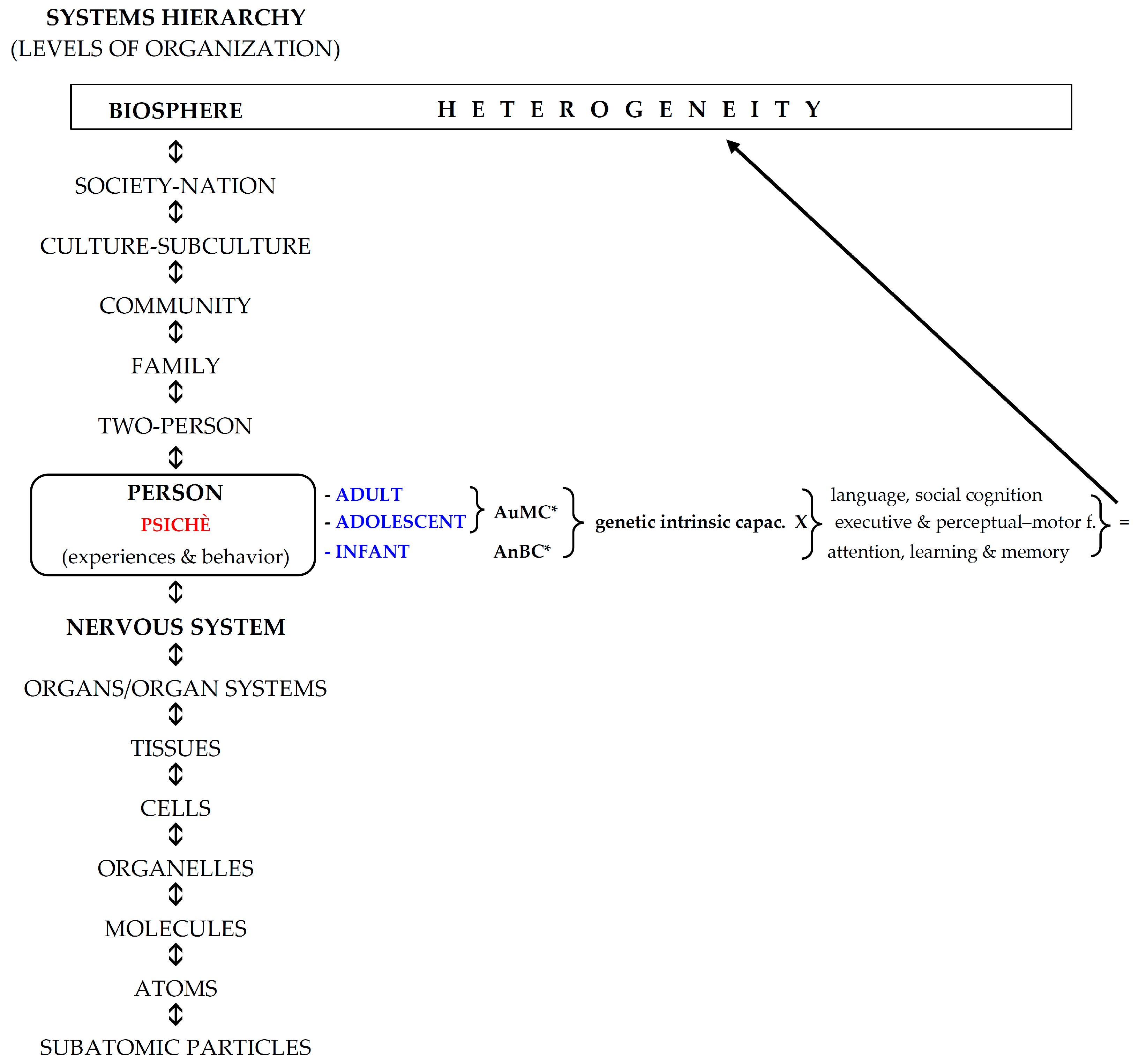
1. Introduction
2. Methods
- (a)
- Infancy;
- (b)
- Childhood–adolescence–adulthood;
- (c)
- Retrogenesis.
3. Results
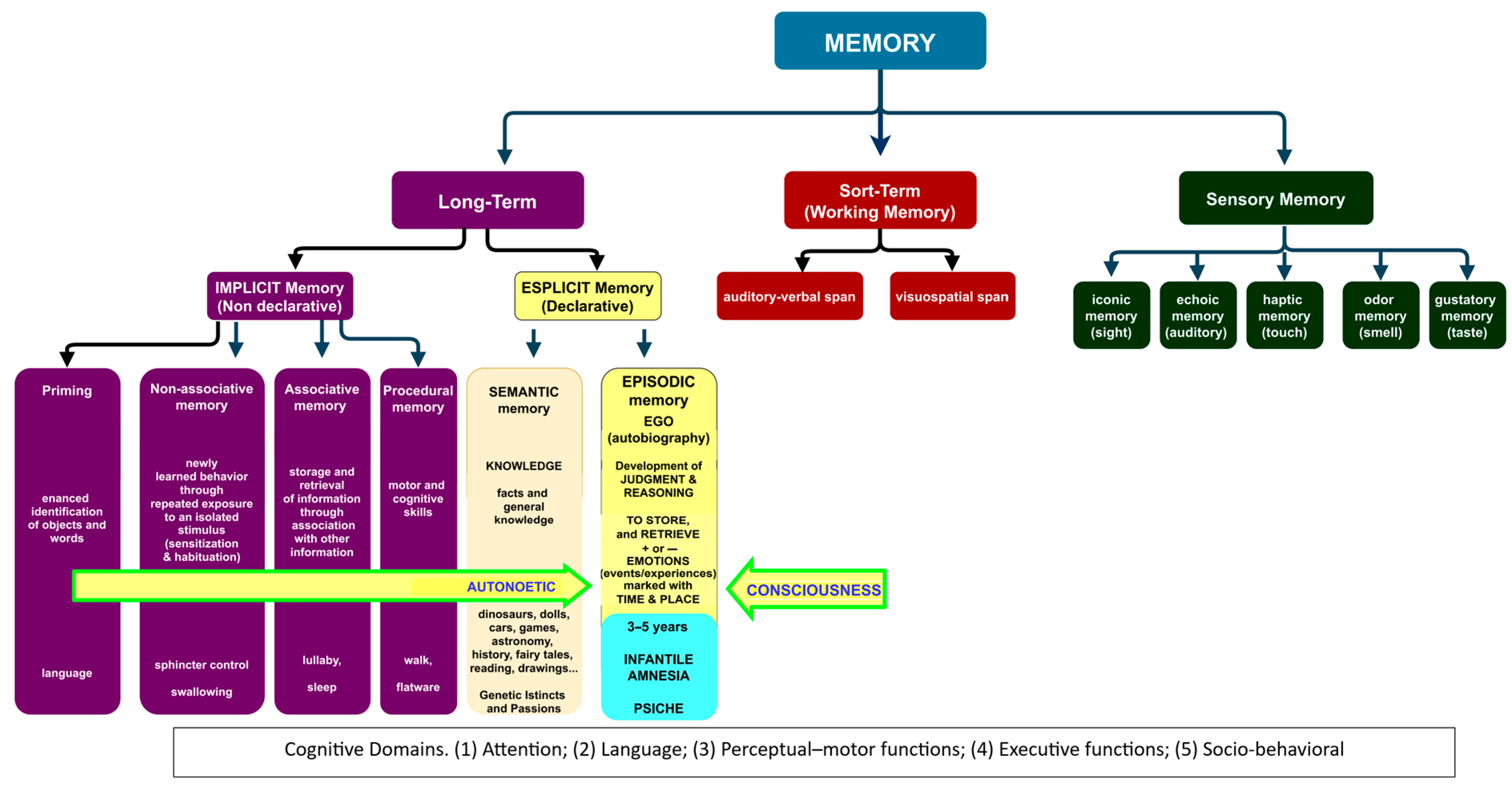
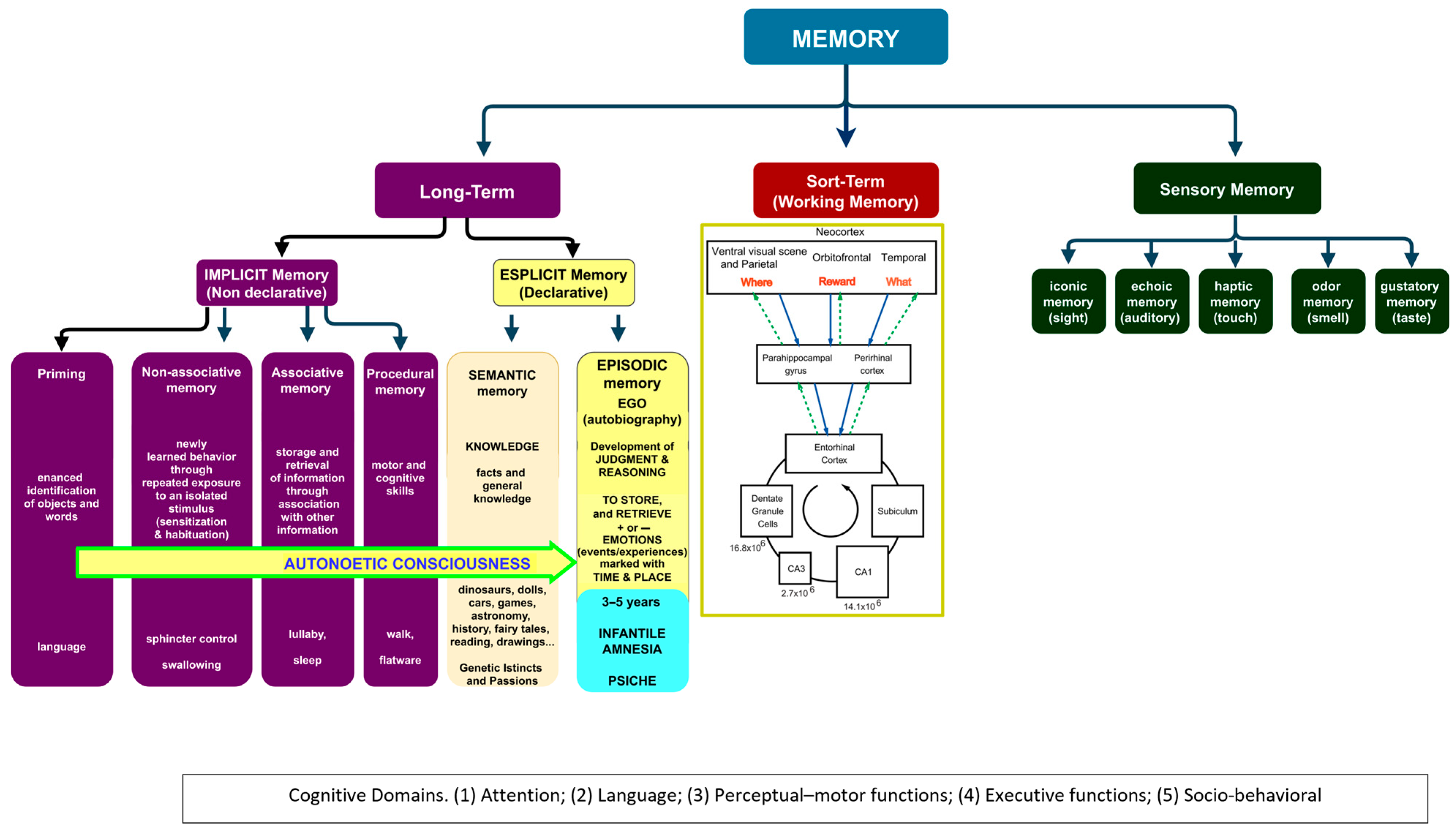
3.1. Infantile Amnesia
3.2. The Development of Knowledge in Infancy
3.2.1. Genetic Socio-Functional Goals: To Conquer the Body
3.2.2. Psychological Infantile Strategies: Background, Inputs/Outputs
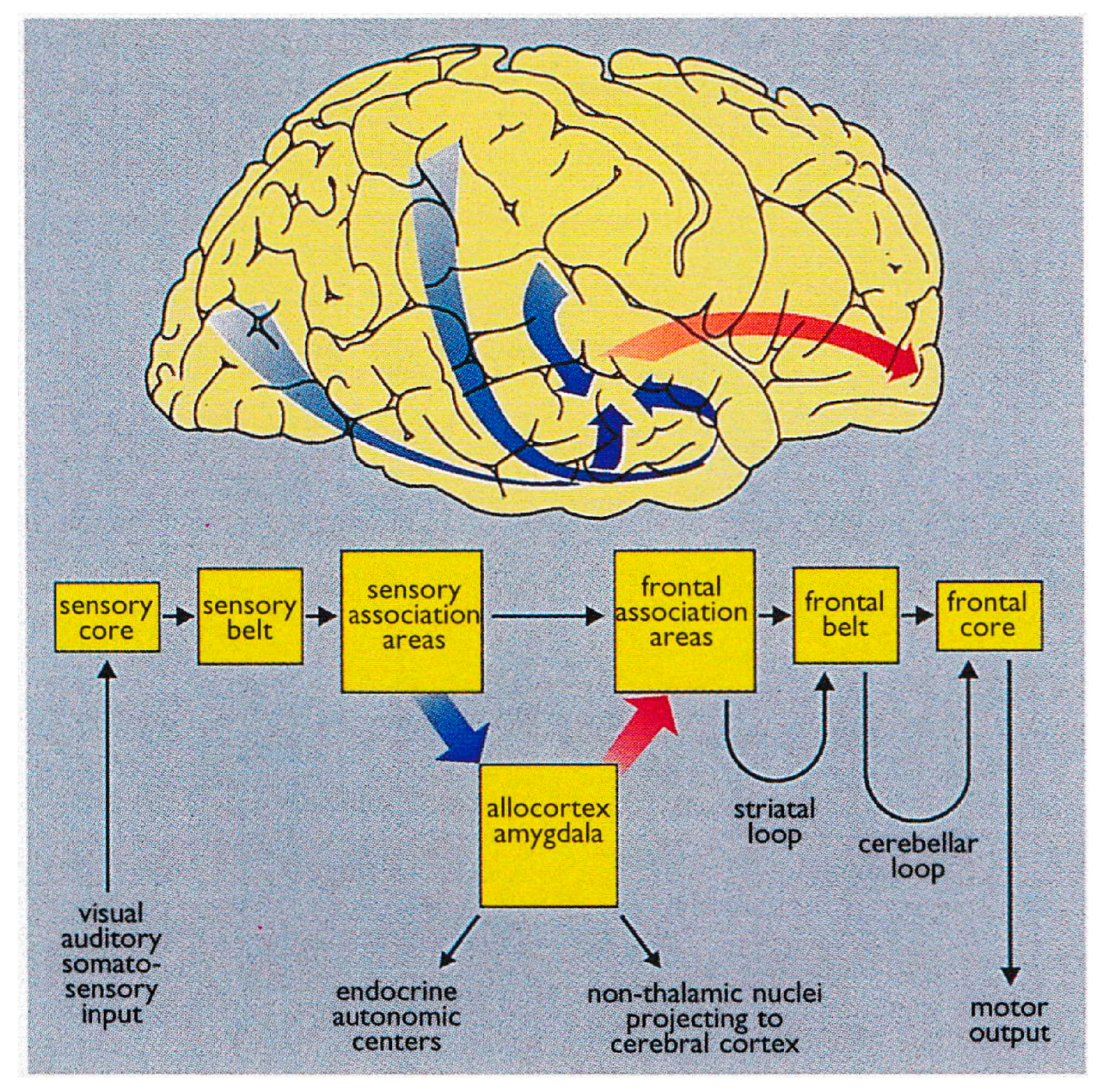
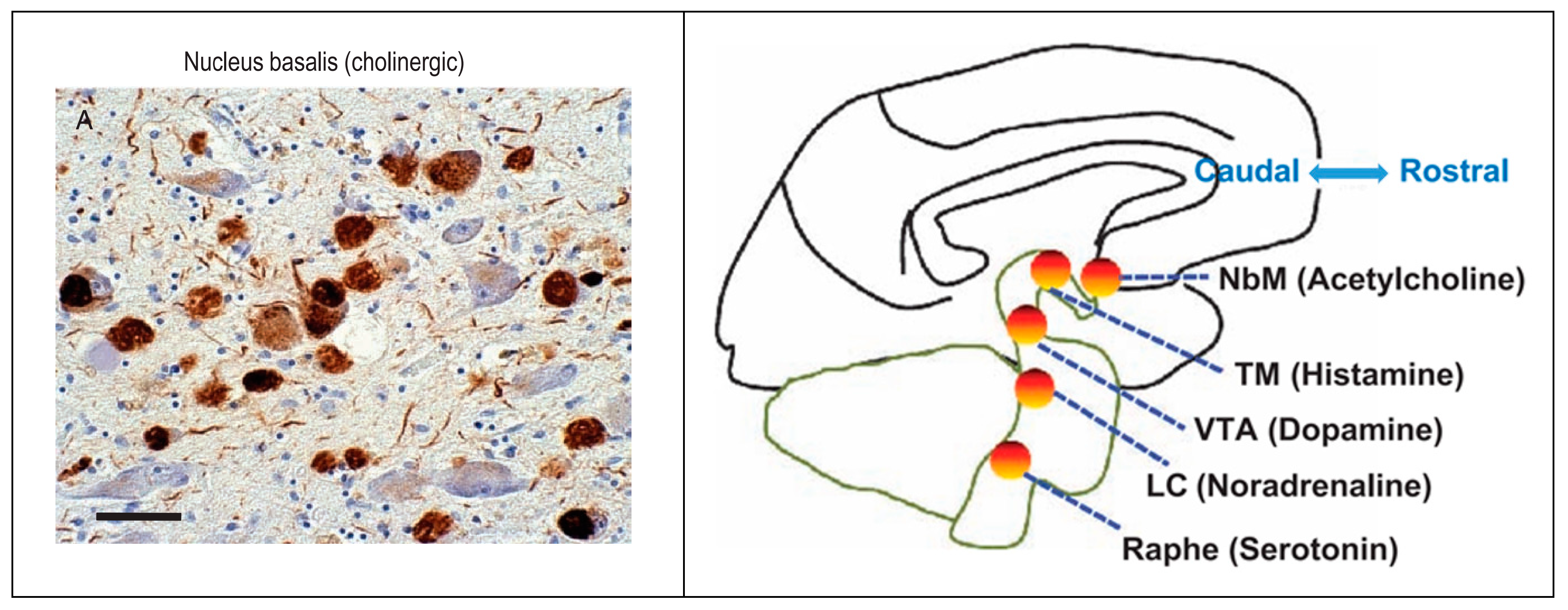
3.2.3. Neurobiology and Neurocircuitry
3.3. The Development of Knowledge in Childhood–Adolescence–Adulthood
3.3.1. Genetic Socio-Functional Goals: To Conquer the Mind/World
3.3.2. Psychological Strategies: Background, Inputs/Outputs
3.3.3. Neurobiology and Neurocircuitry
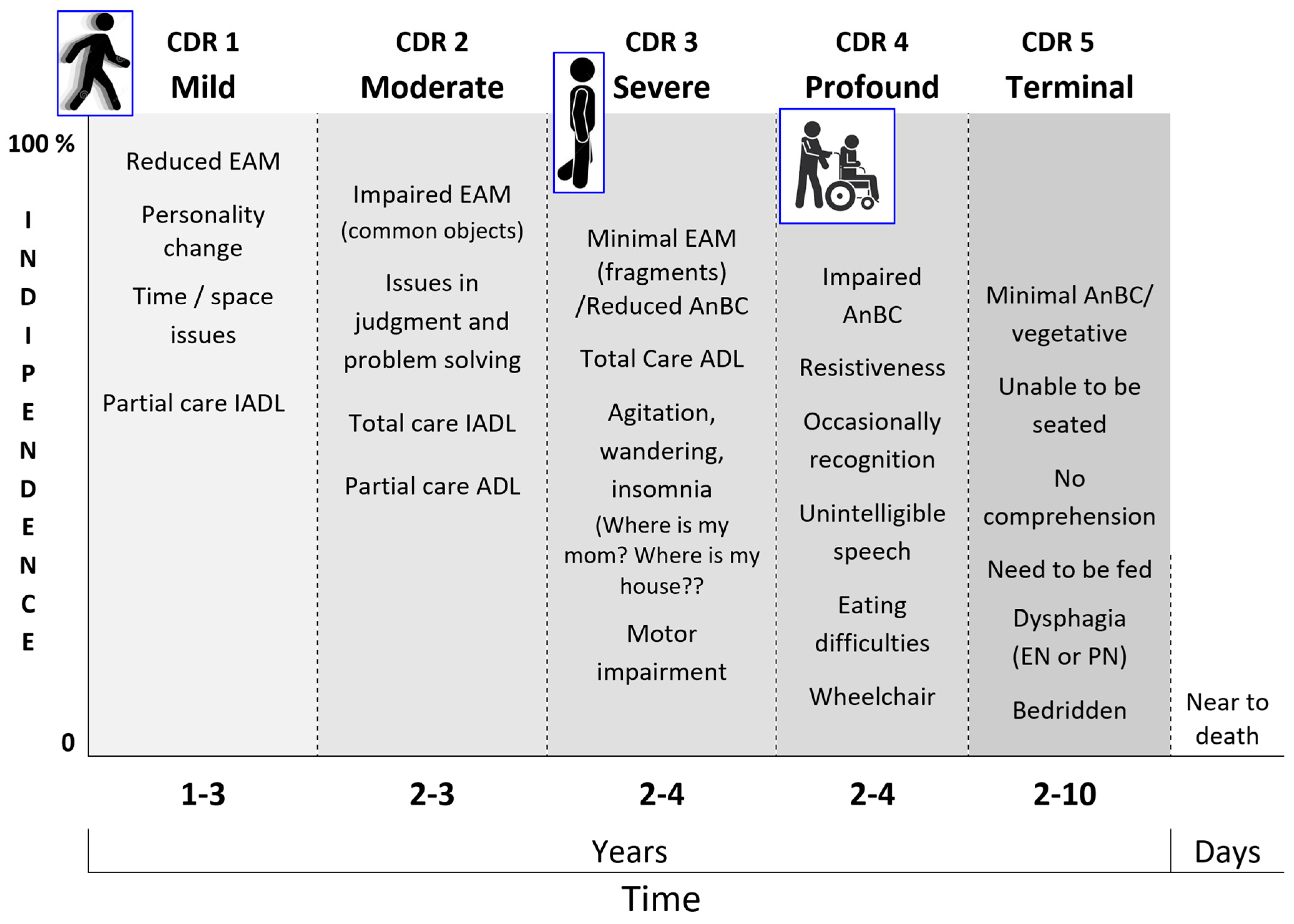
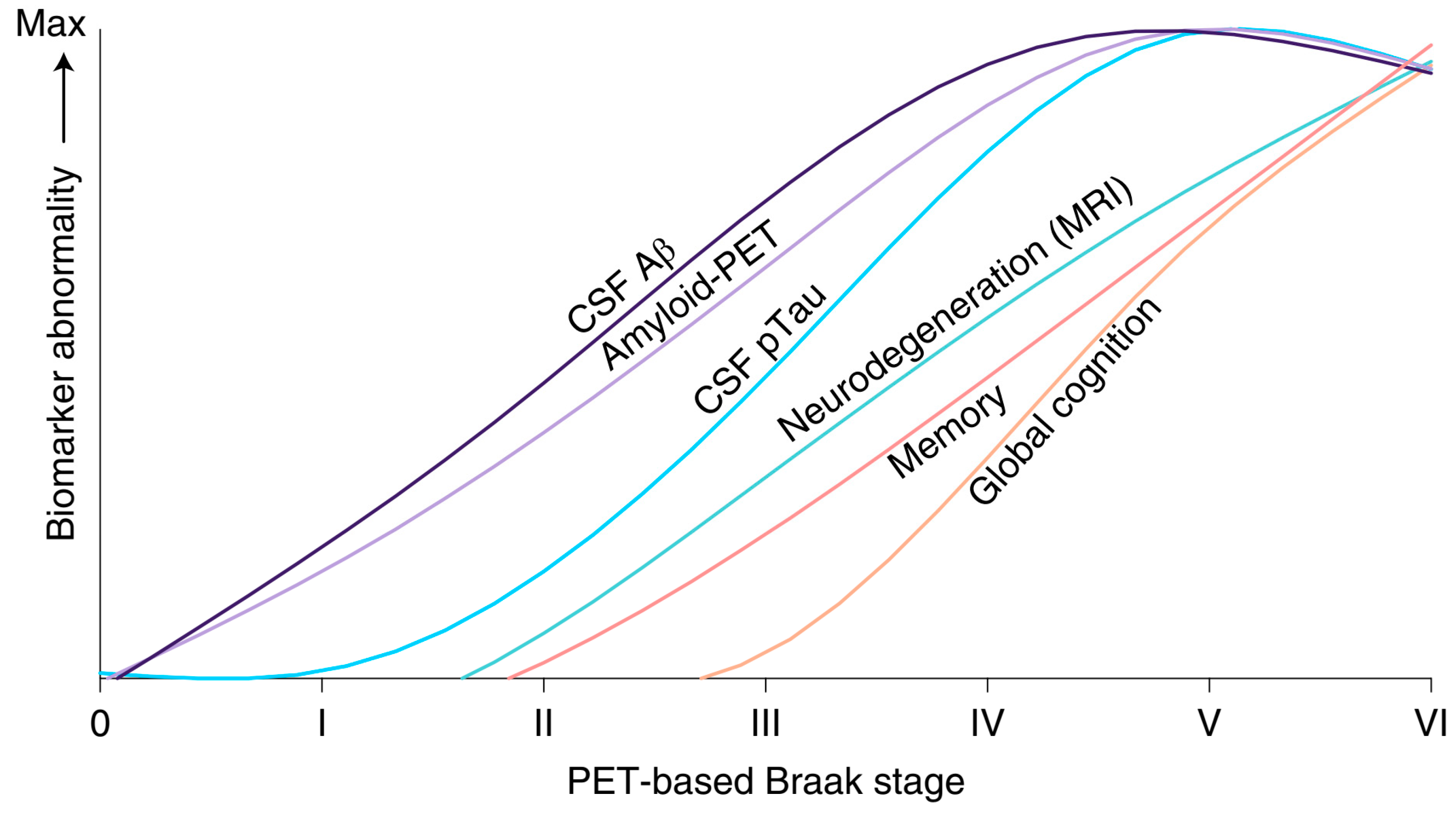
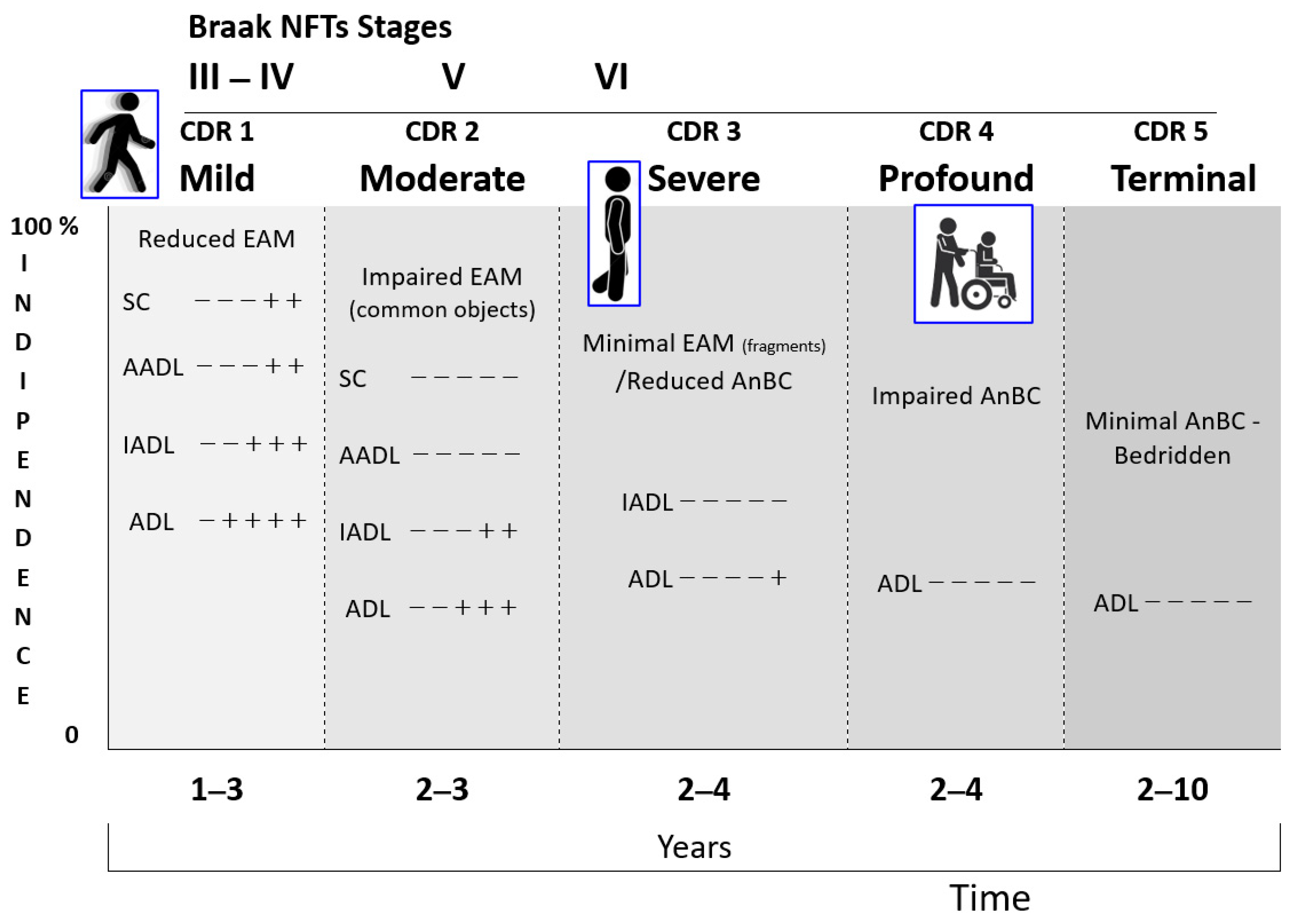
3.4. The Biopsychosocial and Functional Characteristics of the Retrogenesis
3.4.1. The Biological Domain
3.4.2. The Socio-Psychological Domain
- A PwMaNCD weighs, usually, more than 50 Kg and has the strength of an adult;
- The long-life developments and storage of millions of events, images, fantasies, thoughts, and behaviors that fill the empty bookcases of the infant mind, building the adult’s EAM, progressively overflow out of the control from the AuMC, impacting, often disastrously, on the psychic sphere of the PwMaNCDs, progressively deprived of critical abilities/judgment and management;
- The time of the onset is different depending on the diverse etiologies of the NCDs, but the types and trajectories of the symptoms appear along an overlapping pattern;
- The overlapping pattern of socio-behavioral manifestations may be embodied in a time neurobiological algorithm:
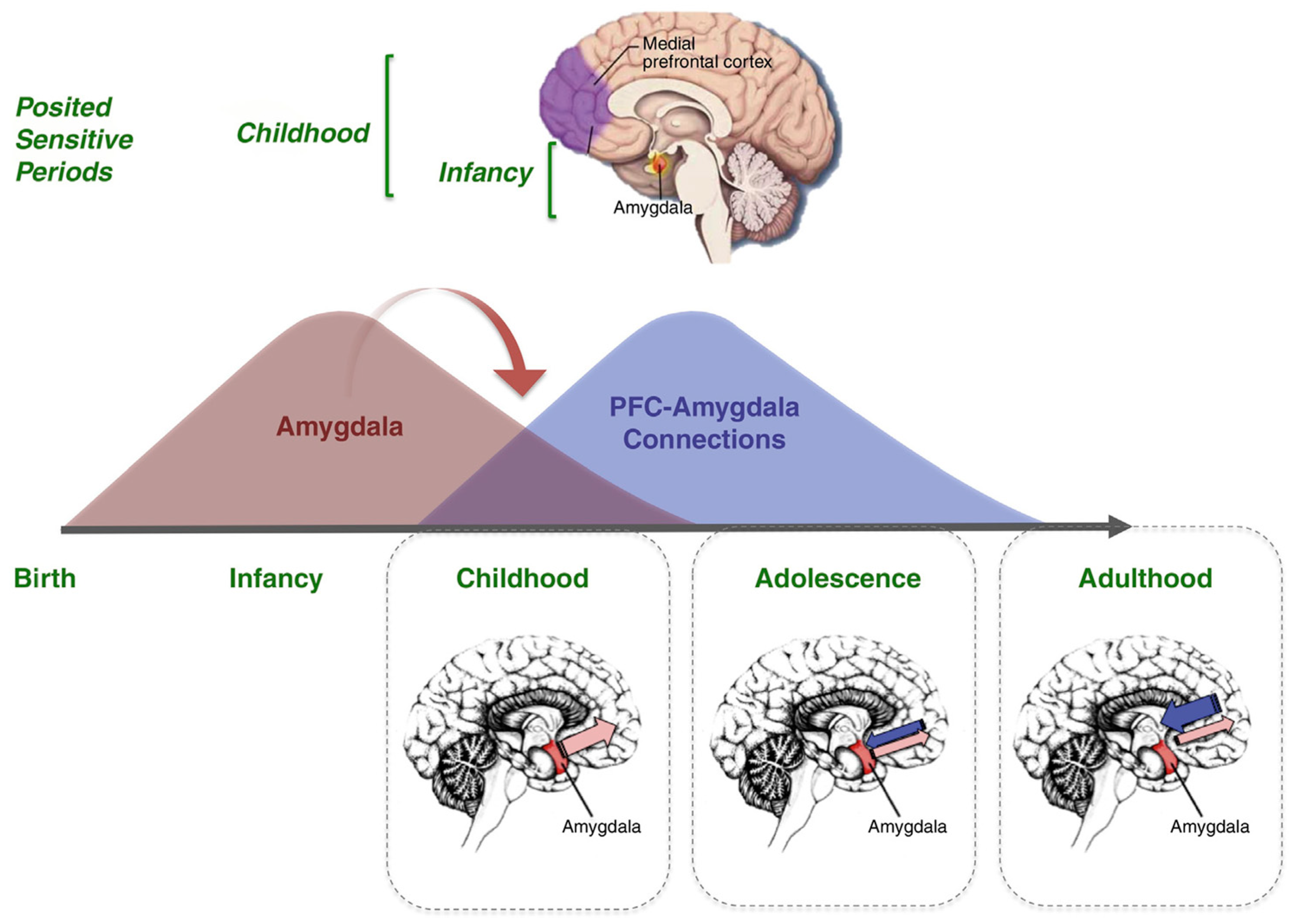
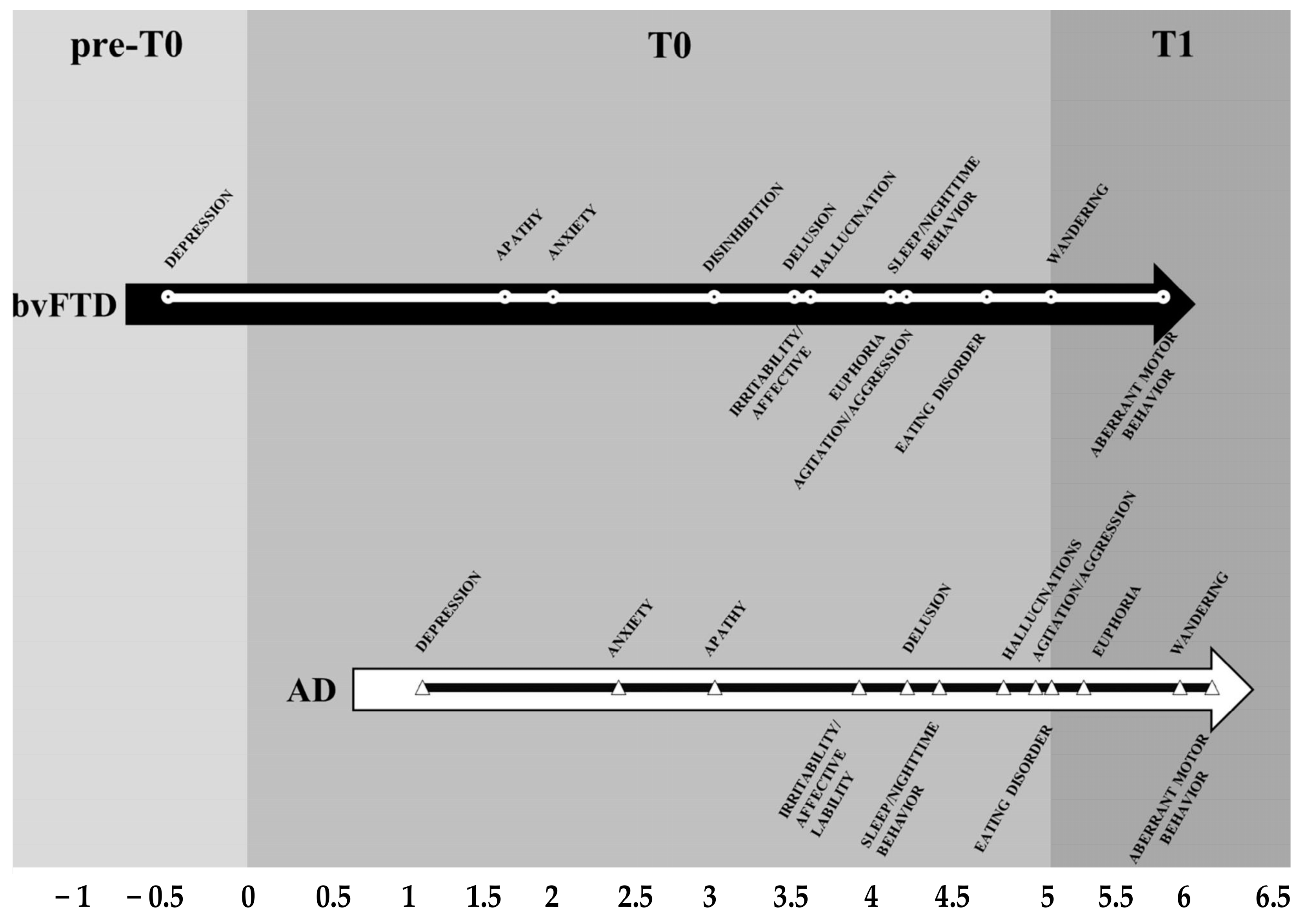
- I preclinical—3 years: symptoms are related, originally, to the involvement of the prefrontal–basal-forebrain–amygdala–HTPAG axis [184] circuit in mood alteration (depression, apathy, anxiety, euphoria) and then, after some years, the temperament alteration (irritability, disinhibition, agitation, aggression);
- II 3–5 years: the worsening of the prefrontal–basal-forebrain–amygdala–HTPAG axis circuit implies symptoms related to the progressive loss of control over the sensorial and semantic inputs (fears, delusion, hallucination) [110], while the detachment of the molecular clock and of the neurovisceral inputs of hungry and thirsty implies the disruption of the circadian rhythm for sleeping and eating;
- III > 5 years: the complete detachment of the prefrontal–basal-forebrain–amygdala–HTPAG axis circuit from the lower hippocampal circuit implies the resurfacing, from the “basement” of AnBC, of the aberrant motor behavior/wandering of the child, followed by the loss of gait and balance of the infant, and then by the chair-bounded period until the bedridden–vegetative state after the birth stage;
- The stressful and disabling socio-behavioral manifestations like agitation and sleep and eating disorders affect about 40–50% [43] of PwMaNCDs, but not all the PwMaNCDs as expected if they were entirely provoked by MaNCDs [185]. This aspect is likely due to the resurfacing of the infant’s original temperament in PwMaNCDs which is frequently quite different from the adult acquired behavior, as reported by the caregivers, both in males and females. It is a common experience that infant/child temperament expresses itself like a Gaussian curve whose extremes are: always calm and collaborative and always agitated and oppositive. Therefore, it may be reasonable to presume that PwMaNCDs with a “benign” calm and collaborative behavior or a “disruptive” behavior may recover their original infant/child’s one;
- The non-pharmacological therapies for the socio-behavioral manifestations of NCDs are applied following the decreasing level of cognitive functioning of PwMaNCDs. In the mild and moderate stages, it is possible to supply the stimulation of cognition, abilities, and games (reality orientation therapy, exercises, drawings, music therapy, …) [173,186,187], while, in the severe and profound stages, mainly games (dolls/pet therapy, elder-clowning, …) [188,189] and sensory memory stimuli (Snoezelen rooms) [190,191,192,193,194]. This pattern is the opposite of the infant/child development that starts with the sensory memory stimuli and games before acquiring the abilities (Figure 15).
3.4.3. The Socio-Functional Domain
4. Discussion
- -
- Cultural: the lack of an interdisciplinary consensus among physicians, general practitioners, specialists, caregiver associations, nurses, psychologists, and social workers to consolidate definitively the model into a unique system of “science of NCD management” [6];
- -
- -
- Social: the presence of increasing and pervasive stigma toward PwMaNCDs, involving all the potential actors of caring and interesting the knowledge of dementia, attitudes and beliefs, and behaviors: 80% of the general public and 65% of health and care professionals think that NCDs are a normal part of ageing [196].
- -
- Structural: the availability of interdisciplinary teams (physician, nurse, nurses’ aid, caregiver, physiotherapist, psychologist, social worker, volunteers, and others) in the different settings where PwMaNCDs live or to which they are transferred (home, long-term care facility, nursing home, hospital, hospice, and other).
5. Conclusions
- (1)
- The quick diagnosis of symptoms related to the early involvement of the limbic system (mood and/or metamemory disorders) firstly in the general practice, as it has been undoubtedly demonstrated that, in AD, the deposition of tau and beta amyloid proteins in the brain begins many years before; for this purpose, the future availability of specific blood biomarkers should ameliorate the frequent diagnostic delay;
- (2)
- The development of new non-pharmacological strategies and pharmacological therapies primarily targeted at the involvement of the limbic system in the impairment of social cognition and the emergence of infantile uncontrolled behaviors in strong adults with MaNCDs;
- (3)
- The implementation of web technologies to ensure the needed online care, cure, rehabilitation, and support to PwMaNCDs.
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| ADL | Activities of Daily Living |
| AnBC | Anoetic Body Consciousness |
| ARAS | Ascending Reticular Activating System |
| AuMC | Autonoetic Mind Consciousness |
| BPSD | Behavioral and Psychiatric Symptoms of Dementia |
| BPS | Biopsychosocial (pathway) |
| bvFTD | behavioral variant of Frontotemporal Dementia |
| Braaks NFTs-ST | Braaks NFT Staging Tool |
| CDR | Clinical Dementia Rating scale |
| CNS | Central Nervous System |
| DAEs | Developmental Age Equivalents |
| EAM | Episodic Autobiographical Memory |
| EN | Enteral Nutrition |
| FAST | Functional Assessment Staging Tool |
| FTD | Frontotemporal Dementia |
| FOF | Fear of Falling |
| GDS | Global Deterioration Scale |
| HPTAG axis | Hypothalamic–Pituitary–Adrenal-Gonadal axis |
| IA | Infantile Amnesia |
| IADL | Instrumental Activities of Daily Living |
| Major NCD | MaNCD |
| NbM | Nucleus basalis of Meynert |
| NCD | Neurocognitive Disorder |
| NFTs | Neurofibrillary Tangles |
| NPI | Neuropsychiatric Inventory |
| NTs | Neuropil Threads |
| PN | Parenteral Nutrition |
| PwMaNCD | Person affected by a Major Neurocognitive Disorder |
| ROT | Reality Orientation Therapy |
| RSN | Resting-State Networks |
| SCN | Suprachiasmatic Nuclei |
References
- Reisberg, B.; Franssen, E.H.; Souren, L.E.; Auer, S.; Kenowsky, S. Progression of Alzheimer’s disease: Variability and consistency: Ontogenic models, their applicability and relevance. J. Neural. Transm. Suppl. 1998, 54, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Reisberg, B.; Franssen, E.H.; Souren, L.E.M.; Auer, S.R.; Akram, I.; Kenowsky, S. Evidence and mechanisms of retrogenesis in Alzheimer’s and other dementias: Management and treatment import. Am. J. Alzheimer’s Dis. Other Demen. 2002, 17, 202–212. [Google Scholar] [CrossRef]
- American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th ed.; APA: Washington, DC, USA, 2013. [Google Scholar]
- Reisberg, B.; Ferris, S.H.; Franssen, E.H. Functional degenerative stages in dementia of the Alzheimer’s type appear to reverse normal human development. In Biological Psychiatry 1985, Proceedings of the IVth World Congress of Biological Psychiatry Development in Psychiatry; Shagass, C., Josiassen, R.C., Bridger, W.H., Weiss, K.J., Stoff, D., Simpson, G.M., Eds.; Elsevier Science Publishing Company: Amsterdam, The Netherlands, 1986; Volume 7, pp. 1319–1321. [Google Scholar]
- Reisberg, B.; Franssen, E.H.; Hasan, S.M.; Monteiro, I.; Boksay, I.; Souren, L.E.M.; Kenowsky, S.; Auer, S.R.; Elahi, S.; Kluger, A. Retrogenesis: Clinical, physiologic, and pathologic mechanisms in brain aging, Alzheimer’s and other dementing processes. Eur. Arch. Psychiatry Clin. Neurosci. 1999, 249 (Suppl. S3), S28–S36. [Google Scholar] [CrossRef] [PubMed]
- Reisberg, B.; Kenowsky, S.; Franssen, E.H.; Auer, S.R.; Souren, L.E. Towards a science of Alzheimer’s disease management: A model based upon current knowledge of retrogenesis. Int. Psychogeriatr. 1999, 11, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Rubial-Álvarez, S.; De Sola, S.; Machado, M.C.; Sintas, E.; Böhm, P.; Sánchez-Benavides, G.; Langohr, K.; Muñiz, R.; Peña-Casanova, J. The comparison of cognitive and functional performance in children and Alzheimer’s Disease supports the Retrogenesis Model. J. Alzheimer’s Dis. 2013, 33, 191–203. [Google Scholar] [CrossRef]
- Thornbury, J.M. Cognitive performance on Piagetian tasks by Alzheimer’s disease patients. Res. Nurs. Health 1992, 15, 11–18. [Google Scholar] [CrossRef]
- Reisberg, B.; Franssen, E.; Souren, L.; Kenowsky, S.; Janjua, K.; Guillo Benarous, F.; Singh, S.; Khizar, A.; Shah, U.; Shah, R.; et al. Alzheimer’s disease. In Medical Aspects of Disability, 5th ed.; Moroz, A., Flanagan, S.R., Zaretsky, H., Eds.; Springer Publishing Company: New York, NY, USA, 2016; pp. 30–90. [Google Scholar] [CrossRef]
- Franssen, E.H.; Souren, L.E.; Torossian, C.L.; Reisberg, B. Utility of developmental reflexes in the differential diagnosis and prognosis of incontinence in Alzheimer’s disease. J. Geriatr. Psychiatry Neurol. 1997, 10, 22–28. [Google Scholar] [CrossRef]
- Souren, L.E.M.; Franssen, E.H.; Reisberg, B. Neuromotor changes in Alzheimer’s Disease: Implications for patient care. J. Geriatr. Psychiatry Neurol. 1997, 10, 93–98. [Google Scholar] [CrossRef]
- Engel, G.L. The clinical application of the biopsychosocial model. Am. J. Psychiatry 1980, 137, 535–544. [Google Scholar] [CrossRef]
- Engel, G.L. The Biopsychosocial Model and Medical Education. N. Eng. J. Med. 1982, 306, 802–805. [Google Scholar] [CrossRef]
- Muller, M.; Sigurdsson, S.; Kjartansson, O.; Jonsson, P.V.; Garcia, M.; von Bonsdorff, M.B.; Gunnarsdottir, I.; Thorsdottir, I.; Harris, T.B.; van Buchem, M.; et al. Birth Size and Brain Function 75 Years Later. Pediatrics 2014, 134, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Engel, G.L. From biomedical to biopsychosocial: I. Being scientific in the human domain. Fam. Syst. Health 1996, 14, 425–433. [Google Scholar] [CrossRef]
- Pally, R.; Olds, D.; Solms, M. The Mind-Brain Relationship; Routledge: Abingdon, UK, 2018. [Google Scholar] [CrossRef]
- Hering, E. Über das Gedächtniss als eine allgemeine Function der organisirten Materie: Vortrag, gehalten in der feierlichen Sitzung der Kaiserlichen Akademie der Wissenschaften am 30. Mai 1870; Open Court: Chicago, IL, USA, 1870. [Google Scholar]
- Spector, A.; Orrell, M. Using a biopsychosocial model of dementia as a tool to guide clinical practice. Int. Psychogeriat. 2010, 22, 957–965. [Google Scholar] [CrossRef] [PubMed]
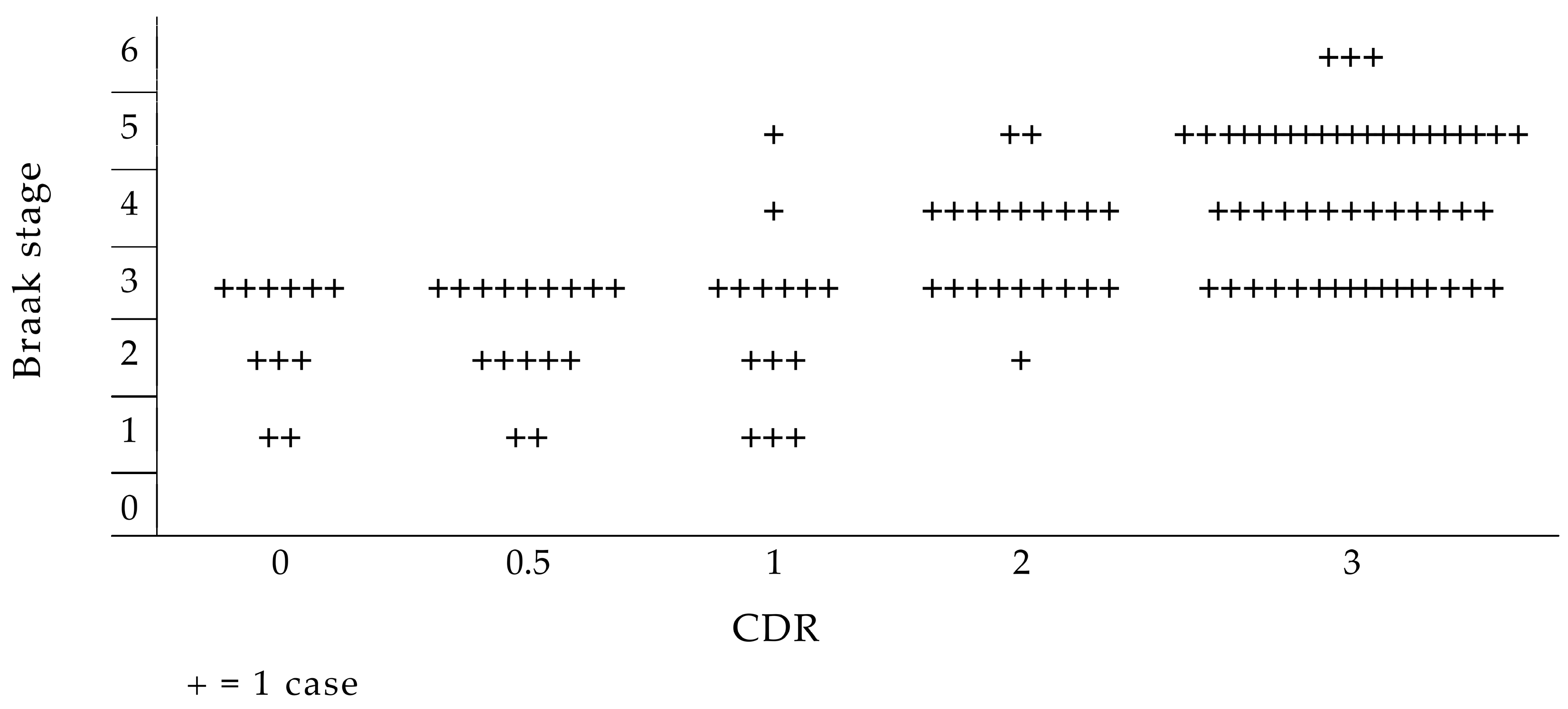
- Braak, H.; Braak, E.; Yilmazer, D.; Bohl, J. Functional anatomy of human hippocampal formation and related structures. J. Child Neurol. 1996, 11, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 2011, 121, 171–181. [Google Scholar] [CrossRef]
- Alves, G.S.; Oertel Knöchel, V.; Knöchel, C.; Carvalho, A.F.; Pantel, J.; Engelhardt, E.; Laks, J. Integrating retrogenesis theory to Alzheimer’s disease pathology: Insight from DTI-TBSS investigation of the white matter microstructural integrity. BioMed Res. Int. 2015, 2015, 291658. [Google Scholar] [CrossRef]
- Ewers, M.; Frisoni, G.B.; Teipel, S.J.; Grinberg, L.T.; Amaro, E.; Heinsen, H.; Thompson, P.M.; Hampel, H. Staging Alzheimer’s disease progression with multimodality neuroimaging. Prog. Neurobiol. 2011, 95, 535–546. [Google Scholar] [CrossRef]
- Gogtay, N.; Giedd, J.N.; Lusk, L.; Hayashi, K.M.; Greenstein, D.; Vaituzis, A.C.; Nugent, T.F.; Herman, D.H.; Clasen, L.S.; Toga, A.W.; et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. USA 2004, 101, 8174–8179. [Google Scholar] [CrossRef]
- Gogtay, N.; Thompson, P.M. Mapping gray matter development: Implications for typical development and vulnerability to psychopathology. Brain Cogn. 2010, 72, 6–15. [Google Scholar] [CrossRef]
- Thompson, P.M.; Hayashi, K.M.; de Zubicaray, G.; Janke, A.L.; Rose, S.E.; Semple, J.; Herman, D.; Hong, M.S.; Dittmer, S.S.; Doddrell, D.M.; et al. Dynamics of gray matter loss in Alzheimer’s disease. J. Neurosci. 2003, 23, 994–1005. [Google Scholar] [CrossRef]
- Wegiel, J.; Flory, M.; Kuchna, I.; Nowicki, K.; Ma, S.Y.; Wegiel, J.; Badmaev, E.; de Leon, M.; Wisniewski, T.; Reisberg, B. Clinicopathological staging of dynamics of neurodegeneration and neuronal loss in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2021, 80, 21–44. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Mansfield, J. Heterogeneity in Dementia: Challenges and Opportunities. Alzheimer Dis. Assoc. Disord. 2000, 14, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Reisberg, B.; Ferris, S.H.; de Leon, M.J.; Crook, T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am. J. Psychiatry 1982, 139, 1136–1139. [Google Scholar] [CrossRef]
- Reisberg, B. Functional assessment staging (FAST). Psychopharmacol. Bull. 1988, 24, 653–659. [Google Scholar] [PubMed]
- Auer, S.; Reisberg, B. The GDS/FAST staging system. Int. Psychogeriatr. 1997, 9 (Suppl. S1), 167–171. [Google Scholar] [CrossRef]
- Reisberg, B.; Jamil, I.A.; Khan, S.; Monteiro, I.; Torossian, C.; Ferris, S.; Sabbagh, M.; Gauthier, S.; Auer, S.; Shulman, M.B.; et al. Staging Dementia. In Principles and Practice of Geriatric Psychiatry, 3rd ed.; Abou-Saleh, M.T.C., Katona, L.E., Kumar, A., Eds.; Wiley: Hoboken, NJ, USA, 2011; pp. 162–169. [Google Scholar]
- Morris, J.C. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 1993, 43, 2412–2414. [Google Scholar] [CrossRef]
- Dooneief, G.; Marder, K.; Tang, M.X.; Stern, Y. The Clinical Dementia Rating scale: Community-based validation of “profound” and “terminal” stages. Neurology 1996, 46, 1746–1749. [Google Scholar] [CrossRef]
- Odenheimer, G.; Borson, S.; Sanders, A.E.; Swain-Eng, R.J.; Kyomen, H.H.; Tierney, S.; Gitlin, L.; Forciea, M.A.; Absher, J.; Shega, J.; et al. Quality improvement in neurology: Dementia management quality measures. J. Am. Geriatr. Soc. 2014, 62, 558–561. [Google Scholar] [CrossRef]
- Becker, P.M.; Cohen, H.J. The functional approach to the care of the elderly. J. Am. Geriat. Soc. 1984, 32, 923–929. [Google Scholar] [CrossRef]
- Rubenstein, L.Z. The clinical effectiveness of multidimensional geriatric assessment. J. Am. Geriat. Soc. 1983, 31, 758–762. [Google Scholar] [CrossRef]
- Rubenstein, L.Z.; Josephson, K.R.; Wieland, G.D.; English, P.A.; Sayre, J.A.; Kane, R.L. Effectiveness of a geriatric evaluation unit. A randomized clinical trial. N. Engl. J. Med. 1984, 311, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Bautmans, I.; Knoop, V.; Amuthavalli Thiyagarajan, J.; Maier, A.B.; Beard, J.R.; Freiberger, E.; Belsky, D.; Aubertin-Leheudre, M.; Mikton, C.; Cesari, M.; et al. WHO working definition of vitality capacity for healthy longevity monitoring. Lancet Healthy Longev. 2022, 3, e789–e796. [Google Scholar] [CrossRef]
- Cesari, M.; De Carvalho, I.A.; Thiyagarajan, J.A.; Cooper, C.; Martin, F.C.; Reginster, J.Y.; Vellas, B.; Beard, J.R. Evidence for the domains supporting the construct of intrinsic capacity. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, P.S.; Blacker, D.; Blazer, D.G.; Ganguli, M.; Jeste, D.V.; Paulsen, J.S.; Petersen, R.C. Classifying neurocognitive disorders: The DSM-5 approach. Nat. Rev. Neurol. 2014, 10, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L. The Neuropsychiatric Inventory: Assessing psychopathology in dementia patients. Neurology 1997, 48 (Suppl. S6), S10–S16. [Google Scholar] [CrossRef] [PubMed]
- Watt, J.A.; Porter, J.; Tavilsup, P.; Chowdhury, M.; Hatch, S.; Ismail, Z.; Kumar, S.; Kirkham, J.; Goodarzi, Z.; Seitz, D. Guideline Recommendations on Behavioral and Psychological Symptoms of Dementia: A Systematic Review. J. Am. Med. Dir. Assoc. 2024, 25, 837–846.e21. [Google Scholar] [CrossRef]
- Sano, M.; Cummings, J.; Auer, S.; Bergh, S.; Fischer, C.E.; Gerritsen, D.; Grossberg, G.; Ismail, Z.; Lanctôt, K.; Lapid, M.I.; et al. Agitation in cognitive disorders: Progress in the International Psychogeriatric Association consensus clinical and research definition. Int. Psychogeriat. 2024, 36, 238–250. [Google Scholar] [CrossRef]
- Lyketsos, C.G.; Lopez, O.; Jones, B.; Fitzpatrick, A.L.; Breitner, J.; DeKosky, S. Prevalence of Neuropsychiatric Symptoms in Dementia and Mild Cognitive Impairment. JAMA 2002, 288, 1475. [Google Scholar] [CrossRef]
- Selbæk, G.; Engedal, K.; Benth, J.Š.; Bergh, S. The course of neuropsychiatric symptoms in nursing-home patients with dementia over a 53-month follow-up period. Int. Psychogeriat. 2014, 26, 81–91. [Google Scholar] [CrossRef]
- Tustin, K.; Hayne, H. Defining the boundary: Age-related changes in childhood amnesia. Dev. Psychol. 2010, 46, 1049–1061. [Google Scholar] [CrossRef]
- Dafni-Merom, A.; Arzy, S. The radiation of autonoetic consciousness in cognitive neuroscience: A functional neuroanatomy perspective. Neuropsychologia 2020, 143, 107477. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, B.C.; Eichenbaum, H. The episodic memory system: Neurocircuitry and disorders. Neuropsychopharmacology 2010, 35, 86–104. [Google Scholar] [CrossRef] [PubMed]
- Alberini, C.M.; Travaglia, A. Infantile Amnesia: A critical period of learning to learn and remember. J. Neurosci. 2017, 37, 5783–5795. [Google Scholar] [CrossRef]
- Travaglia, A.; Bisaz, R.; Sweet, E.S.; Blitzer, R.D.; Alberini, C.M. Infantile amnesia reflects a developmental critical period for hippocampal learning. Nat. Neurosci. 2016, 19, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Grayson, D.S.; Fair, D.A. Development of large-scale functional networks from birth to adulthood: A guide to the neuroimaging literature. NeuroImage 2017, 160, 15–31. [Google Scholar] [CrossRef]
- Braun, U.; Schäfer, A.; Walter, H.; Erk, S.; Romanczuk-Seiferth, N.; Haddad, L.; Schweiger, J.I.; Grimm, O.; Heinz, A.; Tost, H.; et al. Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proc. Natl. Acad. Sci. USA 2015, 112, 11678–11683. [Google Scholar] [CrossRef]
- Marek, S.; Hwang, K.; Foran, W.; Hallquist, M.N.; Luna, B. The contribution of network organization and integration to the development of cognitive control. PLoS Biol. 2015, 13, e1002328. [Google Scholar] [CrossRef]
- Akhtar, S.; Conway, M.A.; Justice, L.V.; Morrison, C.M. In my life: Memory, self and The Beatles. Memory 2024, 32, 296–307. [Google Scholar] [CrossRef]
- Loveday, C.; Woy, A.; Conway, M.A. The self-defining period in autobiographical memory: Evidence from a long-running radio show. Q. J. Exp. Psychol. 2020, 73, 1969–1976. [Google Scholar] [CrossRef]
- Curci, A.; Battista, F.; Lanciano, T.; d’Ovidio, F.D.; Conway, M.A. The reminiscence bump and the self: Evidence from five studies on positive and negative memories. Memory 2024, 32, 757–775. [Google Scholar] [CrossRef]
- Aydin, C.; Conway, M.A. Cultural self-goals influence how much is remembered from early childhood events. J. Pers. 2020, 88, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Galvin, J.E. The Quick Dementia Rating System (QDRS): A rapid dementia staging tool. Alzheimer’s Dement. 2015, 1, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Markowitsch, H.J.; Staniloiu, A. Memory, autonoetic consciousness, and the self. Conscious Cogn. 2011, 20, 16–39. [Google Scholar] [CrossRef] [PubMed]
- Occhionero, M.; Tonetti, L.; Giovagnoli, S.; Natale, V. The Infantile Amnesia phenomenon and the beginning of Autobiographical Memories. Appl. Sci. 2023, 13, 1158. [Google Scholar] [CrossRef]
- Souchay, C.; Guillery-Girard, B.; Pauly-Takacs, K.; Wojcik, D.Z.; Eustache, F. Subjective experience of episodic memory and metacognition: A neurodevelopmental approach. Front. Behav. Neurosci. 2013, 7, 212. [Google Scholar] [CrossRef][Green Version]
- Madsen, H.B.; Kim, J.H. Ontogeny of memory: An update on 40 years of work on infantile amnesia. Behav. Brain Res. 2016, 298, 4–14. [Google Scholar] [CrossRef]
- Freud, S. Psychopathology of Everyday Life; The Macmillan Company: New York, NY, USA, 1914. [Google Scholar]
- Denton, D. The Primordial Emotions. The Dawning of Consciousness; Oxford University Press: Oxford, UK, 2006. [Google Scholar] [CrossRef]
- Hood, T.; Price, J. Infantile amnesia in human and nonhuman animals. In The Nature of Early Memory: An Adaptive Theory of the Genesis and Development of Memory; Howe, M.L., Ed.; Oxford University Press, Inc.: Oxford, UK, 2011; pp. 47–66. [Google Scholar]
- Amrein, I.; Isler, K.; Lipp, H. Comparing adult hippocampal neurogenesis in mammalian species and orders: Influence of chronological age and life history stage. Eur. J. Neurosci. 2011, 34, 978–987. [Google Scholar] [CrossRef]
- Bath, K.G.; Manzano-Nieves, G.; Goodwill, H. Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Horm. Behav. 2016, 82, 64–71. [Google Scholar] [CrossRef]
- Deal, A.L.; Erickson, K.J.; Shiers, S.I.; Burman, M.A. Limbic system development underlies the emergence of classical fear conditioning during the third and fourth weeks of life in the rat. Behav. Neurosci. 2016, 130, 212–230. [Google Scholar] [CrossRef]
- Ramsaran, A.I.; Schlichting, M.L.; Frankland, P.W. The ontogeny of memory persistence and specificity. Dev. Cogn. Neurosci. 2019, 36, 100591. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Evolution of the neuropathology of Alzheimer’s disease. Acta Neurol. Scand. 1996, 94 (Suppl. S165), 3–12. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. The hippocampus, ventromedial prefrontal cortex, and episodic and semantic memory. Prog. Neurobiol. 2022, 217, 102334. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.T.; Skalaban, L.J.; Yates, T.S.; Bejjanki, V.R.; Córdova, N.I.; Turk-Browne, N.B. Evidence of hippocampal learning in human infants. Curr. Biol. 2021, 31, 3358–3364.e4. [Google Scholar] [CrossRef] [PubMed]
- Bessières, B.; Travaglia, A.; Mowery, T.M.; Zhang, X.; Alberini, C.M. Early life experiences selectively mature learning and memory abilities. Nat. Commun. 2020, 11, 628. [Google Scholar] [CrossRef]
- Denton, D.A.; McKinley, M.J.; Farrell, M.; Egan, G.F. The role of primordial emotions in the evolutionary origin of consciousness. Conscious Cogn. 2009, 18, 500–514. [Google Scholar] [CrossRef]
- Denton, D.; Shade, R.; Zamarippa, F.; Egan, G.; Blair-West, J.; McKinley, M.; Lancaster, J.; Fox, P. Neuroimaging of genesis and satiation of thirst and an interoceptor-driven theory of origins of primary consciousness. Proc. Natl. Acad. Sci. USA 1999, 96, 5304–5309. [Google Scholar] [CrossRef]
- Piaget, J. La Naissance de l’Intelligence chez l’Enfant; Delachaux & Niestlè: Paris, France, 1936. [Google Scholar]
- Belsky, J.; Caspi, A.; Moffit, T.E.M.; Poulton, R. The Origins of You; Harvard University Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Edelman, G.M.; Tononi, G. A Universe of Consciousness: How Matter Becomes Imagination; Basic Books: New York, NY, USA, 2000. [Google Scholar]
- Hume, D. A Treatise of Human Nature: An Attempt to Introduce the Experimental Method of Reasoning into Moral Subjects; Noon, J., Ed.; White-Hart: Buckingham, UK, 1739; Volumes 1–3. [Google Scholar]
- Jiang, J.; Liu, T.; Crawford, J.D.; Kochan, N.A.; Brodaty, H.; Sachdev, P.S.; Wen, W. Stronger bilateral functional connectivity of the frontoparietal control network in near-centenarians and centenarians without dementia. NeuroImage 2020, 215, 116855. [Google Scholar] [CrossRef]
- Dykiert, D.; Der, G.; Starr, J.M.; Deary, I.J. Why is Mini-Mental state examination performance correlated with estimated premorbid cognitive ability? Psychol. Med. 2016, 46, 2647–2654. [Google Scholar] [CrossRef]
- Gow, A.J.; Johnson, W.; Pattie, A.; Whiteman, M.C.; Starr, J.; Deary, I.J. Mental ability in childhood and cognitive aging. Gerontology 2008, 54, 177–186. [Google Scholar] [CrossRef]
- Murray, C.; Johnson, W.; Wolf, M.S.; Deary, I.J. The association between cognitive ability across the lifespan and health literacy in old age: The Lothian Birth Cohort 1936. Intelligence 2011, 39, 178–187. [Google Scholar] [CrossRef]
- Ouvrier, R.; Goldsmith, R.; Ouvrier, S.; Williams, I. The Value of the Mini-Mental State Examination in childhood: A preliminary study. J. Child Neurol. 2015, 8, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Rubial-Álvarez, S.; Machado, M.-C.; Sintas, E.; de Sola, S.; Böhm, P.; Peña-Casanova, J. A Preliminary Study of the Mini-Mental State Examination in a Spanish Child Population. J. Child Neurol. 2007, 22, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, K.; Sheya, A. Ontogenesis of learning and memory: Biopsychosocial and dynamical systems perspectives. Dev. Psychobiol. 2019, 61, 402–415. [Google Scholar] [CrossRef] [PubMed]
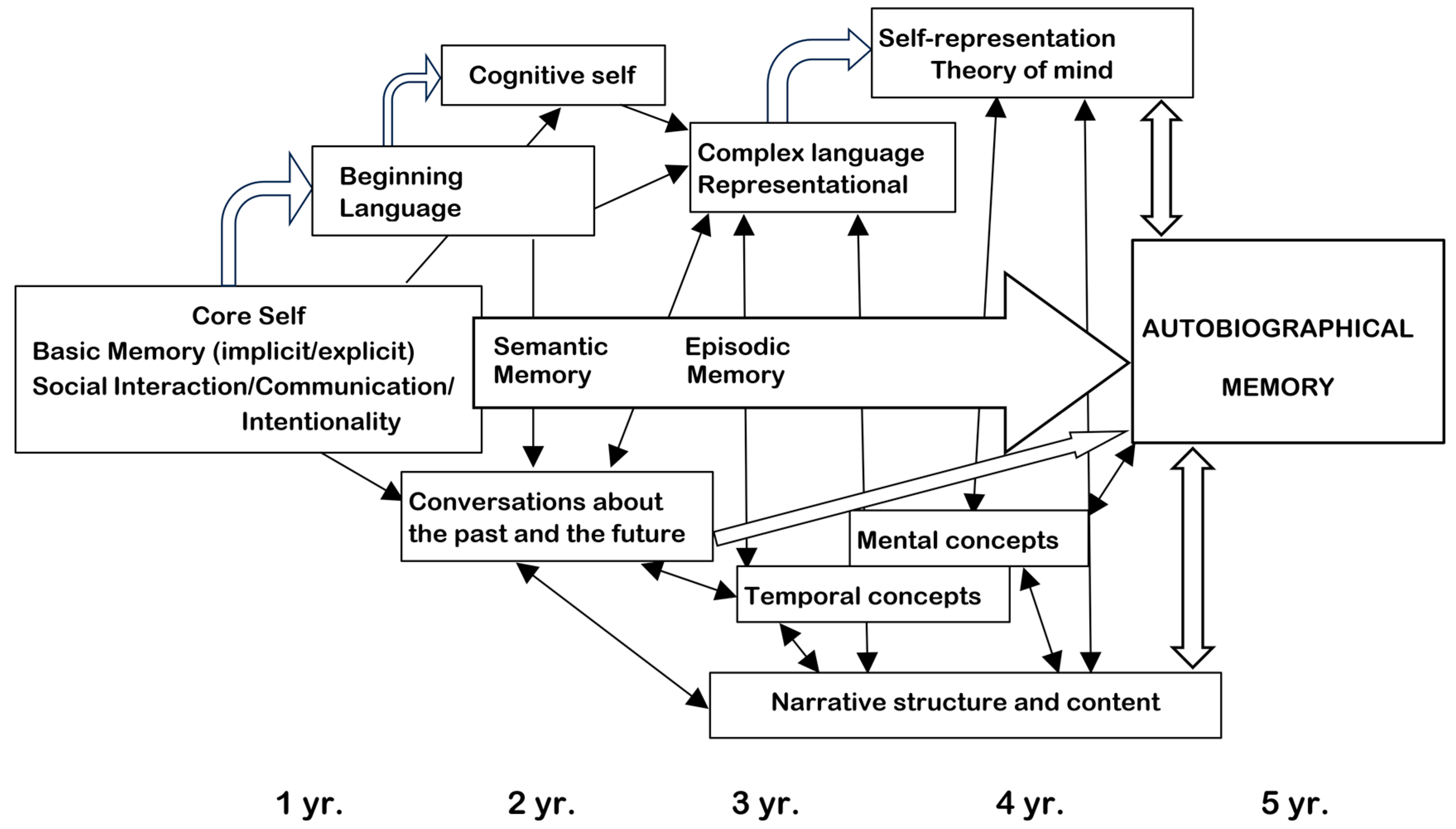
- Nelson, K.; Fivush, R. The Emergence of Autobiographical Memory: A social cultural developmental theory. Psychol. Rev. 2004, 111, 486–511. [Google Scholar] [CrossRef]
- Fivush, R.; Nelson, K. Culture and Language in the Emergence of Autobiographical Memory. Psychol. Sci. 2004, 15, 573–577. [Google Scholar] [CrossRef]
- Des Cartes, R. Discours de la methode. In De l’Imprimeire de Jan Maire; Leide: Leiden, The Netherlands, 1637. [Google Scholar]
- Camina, E.; Güell, F. The neuroanatomical, neurophysiological and psychological basis of memory: Current models and their origins. Front. Pharmacol. 2017, 8, 438. [Google Scholar] [CrossRef]
- Darwin, C. The Descent of Man, and Selection in Relation to Sex; John Murray: London, UK, 1871. [Google Scholar]
- Darwin, C. The Expression of the Emotions in Man and Animals; John Murray: London, UK, 1872. [Google Scholar]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of Illness in the Aged: The Index of ADL: A standardized measure of biological and psychosocial function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef]
- Cappellini, G.; Sylos-Labini, F.; Dewolf, A.H.; Solopova, I.A.; Morelli, D.; Lacquaniti, F.; Ivanenko, Y. Maturation of the locomotor circuitry in children with cerebral palsy. Front. Bioeng. Biotechnol. 2020, 8, 998. [Google Scholar] [CrossRef]
- Sheng, J.A.; Bales, N.J.; Myers, S.A.; Bautista, A.I.; Roueinfar, M.; Hale, T.M.; Handa, R.J. The Hypothalamic-Pituitary-Adrenal Axis: Development, programming actions of hormones, and maternal-fetal interactions. Front. Behav. Neurosci. 2021, 14, 601939. [Google Scholar] [CrossRef]
- Stiles, J.; Jernigan, T.L. The basics of brain development. Neuropsychol. Rev. 2010, 20, 327–348. [Google Scholar] [CrossRef]
- Piolino, P.; Desgranges, B.; Eustache, F. Episodic autobiographical memories over the course of time: Cognitive, neuropsychological and neuroimaging findings. Neuropsychologia 2009, 47, 2314–2329. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Pruitt, P.J.; Yu, Q.; Homayouni, R.; Daugherty, A.M.; Damoiseaux, J.S.; Ofen, N. Differential functional connectivity in anterior and posterior hippocampus supporting the development of memory formation. Front. Hum. Neurosci. 2020, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Panksepp, J.; Clarici, A.; Vandekerckhove, M.; Yovell, Y. Neuro-Evolutionary foundations of infant minds: From psychoanalytic visions of how primal emotions guide constructions of human minds toward affective neuroscientific understanding of emotions and their disorders. Psychoanal. Inq. 2019, 39, 36–51. [Google Scholar] [CrossRef]
- Godfrey, M.; Casnar, C.; Stolz, E.; Ailion, A.; Moore, T.; Gioia, G. A review of procedural and declarative metamemory development across childhood. Child Neuropsychol. 2023, 29, 183–212. [Google Scholar] [CrossRef]
- Chaudhary, S.; Figueroa, J.; Shaikh, S.; Mays, E.W.; Bayakly, R.; Javed, M.; Smith, M.L.; Moran, T.P.; Rupp, J.; Nieb, S. Pediatric falls ages 0–4: Understanding demographics, mechanisms, and injury severities. Inj. Epidemiol. 2018, 5, 77–87. [Google Scholar] [CrossRef]
- Lach, H.W.; Parsons, J.L. Impact of fear of falling in long term care: An integrative review. J. Am. Med. Dir. Assoc. 2013, 14, 573–577. [Google Scholar] [CrossRef]
- Gambaro, E.; Gramaglia, C.; Azzolina, D.; Campani, D.; Dal Molin, A.; Zeppegno, P. The complex associations between late life depression, fear of falling and risk of falls. A systematic review and meta-analysis. Ageing Res. Rev. 2022, 73, 101532. [Google Scholar] [CrossRef]
- Hutson, M. Basic instincts. Science 2018, 360, 845–847. [Google Scholar] [CrossRef]
- Gartstein, M.A.; Rothbart, M.K. Studying infant temperament via the Revised Infant Behavior Questionnaire. Infant. Behav. Develop. 2003, 26, 64–86. [Google Scholar] [CrossRef]
- Liu, J.; Lewis, G.; Evans, L. Understanding aggressive behaviour across the lifespan. J. Psychiatr. Ment. Health Nurs. 2013, 20, 156–168. [Google Scholar] [CrossRef]
- O’Leary, D.; Jyringi, D.; Sedler, M. Childhood conduct problems, stages of Alzheimer’s disease, and physical aggression against caregivers. Int. J. Geriatr. Psychiatry 2005, 20, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Tao, D.; He, Z.; Lin, Y.; Liu, C.; Tao, Q. Where does fear originate in the brain? A coordinate-based meta-analysis of explicit and implicit fear processing. NeuroImage 2021, 227, 117686. [Google Scholar] [CrossRef] [PubMed]
- Low, L.-F.; Brodaty, H.; Goodenough, B.; Spitzer, P.; Bell, J.-P.; Fleming, R.; Casey, A.-N.; Liu, Z.; Chenoweth, L. The Sydney Multisite Intervention of LaughterBosses and ElderClowns (SMILE) study: Cluster randomised trial of humour therapy in nursing homes. BMJ Open 2013, 3, e002072. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, R.T.; Stern, Y. Behavioral and psychiatric symptoms of dementia and rate of decline in Alzheimer’s disease. Front. Pharmacol. 2019, 10, 1062. [Google Scholar] [CrossRef] [PubMed]
- Ray, W.A.; Taylor, J.A.; Liechtenstein, M.J.; Meador, K.G. The Nursing Home Behavior Problem Scale. J. Gerontol. 1992, 47, M9–M16. [Google Scholar] [CrossRef] [PubMed]
- Gómez, D.M.; Berent, I.; Benavides-Varela, S.; Bion, R.A.H.; Cattarossi, L.; Nespor, M.; Mehler, J. Language universals at birth. Proc. Natl. Acad. Sci. USA 2014, 111, 5837–5841. [Google Scholar] [CrossRef]
- Staniloiu, A.; Markowitsch, H.J. Episodic memory is emotionally laden memory, requiring amygdala involvement. Behav. Brain Sci. 2019, 42, e299. [Google Scholar] [CrossRef]
- Robinson, G.E.; Barron, A.B. Epigenetics and the evolution of instincts. Science 2017, 356, 26–27. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Development of Alzheimer-related neurofibrillary changes in the neocortex inversely recapitulates cortical myelogenesis. Acta Neuropathol. 1996, 92, 197–201. [Google Scholar] [CrossRef]
- Vandekerckhove, M.; Panksepp, J. A neurocognitive theory of higher mental emergence: From anoetic affective experiences to noetic knowledge and autonoetic awareness. Neurosci. Biobehav. Rev. 2011, 35, 2017–2025. [Google Scholar] [CrossRef]
- Hortensius, R.; Terburg, D.; Morgan, B.; Stein, D.J.; van Honk, J.; de Gelder, B. The basolateral amygdalae and frontotemporal network functions for threat perception. eNeuro 2017, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J. The amygdala. Curr. Biol. 2007, 17, R868–R874. [Google Scholar] [CrossRef] [PubMed]
- Tottenham, N.; Gabard-Durnam, L.J. The developing amygdala: A student of the world and a teacher of the cortex. Curr. Opin. Psychol. 2017, 17, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Neuropathological stages of Alzheimer’s disease. In An Atlas of Alzheimer’s Disease; de Leon, M.J., Ed.; The Parthenon Publishing Group: Carnforth, UK, 1999; pp. 57–74. [Google Scholar]
- Janak, P.H.; Tye, K.M. From circuits to behaviour in the amygdala. Nature 2015, 517, 284–292. [Google Scholar] [CrossRef]
- Markowitsch, H.J.; Staniloiu, A. Amygdala in action: Relaying biological and social significance to autobiographical memory. Neuropsychologia 2011, 49, 718–733. [Google Scholar] [CrossRef]
- Carter, B.; Justin, H.S.; Gulick, D.; Gamsby, J.J. The Molecular Clock and Neurodegenerative Disease: A stressful time. Front. Mol. Biosci. 2021, 8, 644747. [Google Scholar] [CrossRef]
- Alexander, G.M. Postnatal testosterone concentrations and male social development. Front. Endocrinol. 2014, 5, 15. [Google Scholar] [CrossRef]
- Cacciatore, R.; Korteniemi-Poikela, E.; Kaltiala, R. The Steps of Sexuality—A developmental, emotion-focused, child-centered model of sexual development and sexuality education from birth to adulthood. Int. J. Sex. Health 2019, 31, 319–338. [Google Scholar] [CrossRef]
- Kuiri-Hänninen, T.; Haanpää, M.; Turpeinen, U.; Hämäläinen, E.; Seur, R.; Tyrväinen, E.; Sankilampi, U.; Dunkel, L. Postnatal ovarian activation has effects in estrogen target tissues in infant girls. J. Clin. Endocrinol. Metab. 2013, 98, 4709–4716. [Google Scholar] [CrossRef]
- Lamminmäki, A.; Hines, M.; Kuiri-Hänninen, T.; Kilpeläinen, L.; Dunkel, L.; Sankilampi, U. Testosterone measured in infancy predicts subsequent sex-typed behavior in boys and in girls. Horm. Behav. 2012, 61, 611–616. [Google Scholar] [CrossRef]
- Scarf, D.; Gross, J.; Colombo, M.; Hayne, H. To have and to hold: Episodic memory in 3- and 4-year-old children. Dev. Psychobiol. 2013, 55, 125–132. [Google Scholar] [CrossRef]
- Sheridan, M.D.; Sharma, A.; Cockerill, H.; Sanctuary, L. From Birth to Five Years, 5th ed.; Routledge: Abingdon, UK, 2022. [Google Scholar]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontol. 1969, 9, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Carta, E.; Riccardi, A.; Marinetto, S.; Mattivi, S.; Selini, E.; Pucci, V.; Mondini, S. Over ninety years old: Does high cognitive reserve still help brain efficiency? Psychol. Res. 2023, 88, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Sikkes, S.A.M.; Knol, D.L.; Pijnenburg, Y.A.L.; De Lange-De Klerk, E.S.M.; Uitdehaag, B.M.J.; Scheltens, P. Validation of the Amsterdam IADL questionnaire©, a new tool to measure instrumental activities of daily living in dementia. Neuroepidemiology 2013, 41, 35–41. [Google Scholar] [CrossRef] [PubMed]
- De Vriendt, P.; Gorus, E.; Cornelis, E.; Bautmans, I.; Petrovic, M.; Mets, T. The advanced activities of daily living: A tool allowing the evaluation of subtle functional decline in mild cognitive impairment. J. Nutr. Health Aging 2013, 17, 64–71. [Google Scholar] [CrossRef]
- LaFreniere, J.R. Defining and discussing independence in emerging adult college students. J. Adult Dev. 2024, 31, 293–303. [Google Scholar] [CrossRef]
- Mlinac, M.E.; Feng, M.C. Assessment of Activities of Daily Living, Self-Care, and Independence. Arch. Clin. Neuropsychol. 2016, 31, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Vandekerckhove, M.; Bulnes, L.C.; Panksepp, J. The emergence of primary anoetic consciousness in episodic memory. Front. Behav. Neurosci. 2014, 7, 210. [Google Scholar] [CrossRef][Green Version]
- Tulving, E. Precis of Elements of episodic memory. Behav. Brain Sci. 1984, 7, 223–268. [Google Scholar] [CrossRef]
- Tulving, E. Episodic memory: From mind to brain. Annu. Rev. Psychol. 2002, 53, 1–25. [Google Scholar] [CrossRef]
- Sutin, A.R.; Robins, R.W. When the “I” looks at the “Me”: Autobiographical memory, visual perspective, and the self. Conscious Cogn. 2008, 17, 1386–1397. [Google Scholar] [CrossRef]
- Klein, S.B.; Gangi, C.E. The multiplicity of self: Neuropsychological evidence and its implications for the self as a construct in psychological research. Ann. N. Y. Acad. Sci. 2010, 1191, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Neisser, U. Five Kinds of Self-knowledge. Philos. Psychol. 1988, 1, 35–59. [Google Scholar] [CrossRef]
- Rivkees, S.A. Developing circadian rhythmicity in infants. Pediatrics 2003, 112, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Flinn, M.V.; Nepomnaschy, P.A.; Muehlenbein, M.P.; Ponzi, D. Evolutionary functions of early social modulation of hypothalamic-pituitary-adrenal axis development in humans. Neurosci. Biobehav. Rev. 2011, 35, 1611–1629. [Google Scholar] [CrossRef] [PubMed]
- McCormick, C.M.; Mathews, I.Z. Adolescent development, hypothalamic-pituitary-adrenal function, and programming of adult learning and memory. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Child Sexual Abuse Committee. Information for Parents and Caregivers Sexual Development and Behavior in Children. National Child Traumatic Stress Network. 2009. Available online: https://nctsn.org/nctsn_assets/pdfs/caring/sexualbehaviorproblems.pdf (accessed on 21 November 2024).
- Vignozzi, L.; Maggi, M.; Corona, G. Sexuality and childhood. In The EFS and ESSM Syllabus of Clinical Sexuology; Kirana, P.S., Tripodi, F., Reisman, Y., Porst, H., Eds.; Medix: Amsterdam, The Netherlands, 2015; pp. 135–159. [Google Scholar]
- Anderson, P.B.; Struckman-Johnson, C. Sexually Aggressive Women: Current Perspectives and Controversies; Guilford Press: New York, NY, USA, 1998. [Google Scholar]
- Evans, H.R.; Lankford, A. Femcel discussions of sex, frustration, power, and revenge. Arch. Sex Behav. 2024, 53, 917–930. [Google Scholar] [CrossRef]
- Fisher, T.D.; Moore, Z.T.; Pittenger, M.J. Sex on the brain? An examination of frequency of sexual cognitions as a function of gender, erotophilia, and social desirability. J. Sex Res. 2012, 49, 69–77. [Google Scholar] [CrossRef]
- Ryan, K.M. Further evidence for a cognitive component of rape. Aggress. Violent Behav. 2004, 9, 579–604. [Google Scholar] [CrossRef]
- Lankford, A. A sexual frustration theory of aggression, violence, and crime. J. Crim. Justice 2021, 77, 10186. [Google Scholar] [CrossRef]
- Lussier, P.; Cale, J. Understanding the origins and the development of rape and sexual aggression against women: Four generations of research and theorizing. Aggress. Violent Behav. 2016, 31, 66–81. [Google Scholar] [CrossRef]
- Pirani, A.; Belloi, L.; Neri, M.; Francia, R.; Gobbi, G.; Vecchi, G.P. The use of computerized multidimensional geriatric assessment in epidemiological research on mental health and aging in community-based services. In Psychogeriatrics: Biomedical and Social Advances. Selected Proceedings of the 4th Congress of the International Psychogeriatric Association, Tokyo; Hasegawa, K., Homma, A., Eds.; Excerpta Medica: Amsterdam, The Netherlands, 1990; pp. 196–201. [Google Scholar]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici-Braak, K. Alzheimer’s Disease, Neural Basis of. In International Encyclopedia of the Social & Behavioral Sciences, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 591–596. [Google Scholar] [CrossRef]
- Braak, H.; Thal, D.R.; Ghebremedhin, E.; Del Tredici, K. Stages of the pathologic process in Alzheimer disease: Age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 2011, 70, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Alafuzoff, I.; Arzberger, T.; Kretzschmar, H.; Del Tredici, K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006, 112, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Jicha, G.A.; Parisi, J.E.; Dickson, D.W.; Johnson, K.; Cha, R.; Ivnik, R.J.; Tangalos, E.G.; Boeve, B.F.; Knopman, D.S.; Braak, H.; et al. Neuropathologic outcome of Mild Cognitive Impairment following progression to clinical dementia. Arch. Neurol. 2006, 63, 674–681. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Therriault, J.; Pascoal, T.A.; Lussier, F.Z.; Tissot, C.; Chamoun, M.; Bezgin, G.; Servaes, S.; Benedet, A.L.; Ashton, N.J.; Karikari, T.K.; et al. Biomarker modeling of Alzheimer’s disease using PET-based Braak staging. Nat. Aging 2022, 2, 526–535. [Google Scholar] [CrossRef]
- Gold, G.; Bouras, C.; Kövari, E.; Canuto, A.; González Glaría, B.; Malky, A.; Hof, P.R.; Michel, J.-P.; Giannakopoulos, P. Clinical validity of Braak neuropathological staging in the oldest-old. Acta Neuropathol. 2000, 99, 579–582. [Google Scholar] [CrossRef]
- Grober, E.; Qi, Q.; Kuo, L.; Hassenstab, J.; Perrin, R.J.; Lipton, R.B. Stages of objective memory impairment predict Alzheimer’s Disease neuropathology: Comparison with the Clinical Dementia Rating Scale–Sum of Boxes. J. Alzheimer’s Dis. 2021, 80, 185–195. [Google Scholar] [CrossRef]
- Qian, J.; Hyman, B.T.; Betensky, R.A. Neurofibrillary tangle stage and the rate of progression of Alzheimer symptoms. JAMA Neurol. 2017, 74, 540. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.T.; Ames, D.; Burns, A. Dementia, 5th ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Schönheit, B.; Zarski, R.; Ohm, T.G. Spatial and temporal relationships between plaques and tangles in Alzheimer-pathology. Neurobiol. Aging 2004, 25, 697–711. [Google Scholar] [CrossRef] [PubMed]
- Markowitsch, H.J.; Staniloiu, A. Amnesic disorders. Lancet 2012, 380, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, N.; Fonov, V.; Dadar, M.; Spreng, R.N.; Collins, D.L. Degeneration in Nucleus basalis of Meynert signals earliest stage of Alzheimer’s disease progression. Neurobiol. Aging 2024, 139, 54–63. [Google Scholar] [CrossRef]
- Nelson, P.T.; Braak, H.; Markesbery, W.R. Neuropathology and Cognitive Impairment in Alzheimer Disease: A complex but coherent relationship. J. Neuropathol. Exp. Neurol. 2009, 68, 1–14. [Google Scholar] [CrossRef]
- Aghourian, M.; Legault-Denis, C.; Soucy, J.P.; Rosa-Neto, P.; Gauthier, S.; Kostikov, A.; Gravel, P.; Bedard, M.A. Quantification of brain cholinergic denervation in Alzheimer’s disease using PET imaging with [18F]-FEOBV. Mol. Psychiatry 2017, 22, 1531–1538. [Google Scholar] [CrossRef]
- Pessoa, L. Emotion and cognition and the amygdala: From “what is it?” to “what’s to be done?”. Neuropsychologia 2010, 48, 3416–3429. [Google Scholar] [CrossRef]
- Šimić, G.; Tkalčić, M.; Vukić, V.; Mulc, D.; Španić, E.; Šagud, M.; Olucha-Bordonau, F.E.; Vukšić, M.; Hof, P.R. Understanding emotions: Origins and roles of the amygdala. Biomolecules 2021, 11, 823. [Google Scholar] [CrossRef]
- Baldelli, M.V.; Pirani, A.; Motta, M.; Abati, E.; Mariani, E.; Manzi, V. Effects of reality orientation therapy on elderly patients in the community. Arch. Gerontol. Geriatr. 1993, 17, 211–218. [Google Scholar] [CrossRef]
- Paggetti, A.; Druda, Y.; Sciancalepore, F.; Della Gatta, F.; Ancidoni, A.; Locuratolo, N.; Piscopo, P.; Vignatelli, L.; Sagliocca, L.; Guaita, A.; et al. The efficacy of cognitive stimulation, cognitive training, and cognitive rehabilitation for people living with dementia: A systematic review and meta-analysis. GeroScience 2024, ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Pillai, J.A.; Bena, J.; Rothenberg, K.; Boron, B.; Leverenz, J.B. Association of variation in behavioral symptoms with initial cognitive phenotype in adults with dementia confirmed by neuropathology. JAMA Netw. Open 2022, 5, e220729. [Google Scholar] [CrossRef] [PubMed]
- Resnick, B.; Galik, E.; Kolanowski, A.; VanHaitsma, K.; Boltz, M.; Zhu, S.; Ellis, J.; Behrens, L.; Eshraghi, K. Gender differences in presentation and management of behavioral and psychological symptoms associated with dementia among nursing home residents with moderate to severe dementia. J. Women Aging 2021, 33, 635–652. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.-W.; Chen, T.-F.; Yip, P.-K.; Hua, M.-S.; Yang, C.-C.; Chiu, M.-J. Comparison of behavioral and psychological symptoms of Alzheimer’s disease among institution residents and memory clinic outpatients. Int. Psychogeriatr. 2009, 21, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.S.; Abner, E.L.; Jicha, G.A.; Schmitt, F.A.; Di, J.; Wilcock, D.M.; Barber, J.M.; Van Eldik, L.J.; Katsumata, Y.; Fardo, D.W.; et al. Neurodegenerative pathologies associated with behavioral and psychological symptoms of dementia in a community-based autopsy cohort. Acta Neuropathol. Commun. 2023, 11, 89. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.; Brodaty, H.; Trollor, J.; Sachdev, P. Behavioral and psychological symptoms associated with dementia subtype and severity. Int. Psychogeriat. 2010, 22, 300–305. [Google Scholar] [CrossRef]
- Kabeshita, Y.; Adachi, H.; Matsushita, M.; Kanemoto, H.; Sato, S.; Suzuki, Y.; Yoshiyama, K.; Shimomura, T.; Yoshida, T.; Shimizu, H.; et al. Sleep disturbances are key symptoms of very early-stage Alzheimer disease with behavioral and psychological symptoms: A Japan multi-center cross-sectional study (J-BIRD). Int. J. Geriatr. Psychiatry 2017, 32, 222–230. [Google Scholar] [CrossRef]
- Laganà, V.; Bruno, F.; Altomari, N.; Bruni, G.; Smirne, N.; Curcio, S.; Mirabelli, M.; Colao, R.; Puccio, G.; Frangipane, F.; et al. Neuropsychiatric or Behavioral and Psychological Symptoms of Dementia (BPSD): Focus on prevalence and natural history in Alzheimer’s Disease and Frontotemporal Dementia. Front. Neurol. 2022, 13, 832199. [Google Scholar] [CrossRef]
- Pfeiffer, E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J. Am. Geriatr. Soc. 1975, 23, 433–441. [Google Scholar] [CrossRef]
- Rowe, R.; Costello, E.J.; Angold, A.; Copeland, W.E.; Maughan, B. Developmental pathways in oppositional defiant disorder and conduct disorder. J. Abnorm. Psychol. 2010, 119, 726–738. [Google Scholar] [CrossRef]
- Pecher, H.; Storch, M.; Beyer, F.; Witte, V.; Baasner, C.-F.; Schönknecht, P.; Weise, C.M. Hypothalamic atrophy and structural covariance in amnestic mild cognitive impairment and Alzheimer’s dementia. NeuroImage Clin. 2024, 44, 103687. [Google Scholar] [CrossRef]
- Kwon, C.-Y.; Lee, B. Prevalence of Behavioral and Psychological Symptoms of Dementia in community-dwelling dementia patients: A systematic review. Front. Psychiatry. 2021, 12, 741059. [Google Scholar] [CrossRef] [PubMed]
- Bleibel, M.; El Cheikh, A.; Sadier, N.S.; Abou-Abbas, L. The effect of music therapy on cognitive functions in patients with Alzheimer’s disease: A systematic review of randomized controlled trials. Alzheimer’s Res. Ther. 2023, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, D.; Di Rosa, E.; Nocita, R.; Sava, D. Cognitive Stimulation in Patients with Dementia: Randomized Controlled Trial. Dement. Geriatr. Cogn. Dis. Extra 2013, 3, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Kontos, P.; Miller, K.L.; Colobong, R.; Palma Lazgare, L.I.; Binns, M.; Low, L.F.; Surr, C.; Naglie, G. Elder-clowning in long-term dementia care: Results of a pilot study. J. Am. Geriatr. Soc. 2016, 64, 347–353. [Google Scholar] [CrossRef]
- Pezzati, R.; Molteni, V.; Bani, M.; Settanta, C.; Di Maggio, M.G.; Villa, I.; Poletti, B.; Ardito, R.B. Can Doll therapy preserve or promote attachment in people with cognitive, behavioral, and emotional problems? A pilot study in institutionalized patients with dementia. Front. Psychol. 2014, 5, 342. [Google Scholar] [CrossRef]
- Berkheimer, S.D.; Qian, C.; Malmstrom, T.K. Snoezelen Therapy as an intervention to reduce agitation in Nursing Home patients with dementia: A pilot study. J. Am. Med. Dir. Assoc. 2017, 18, 1089–1091. [Google Scholar] [CrossRef]
- Hulsegge, J.; Verheul, A. Snoezelen: Another World; ROMPA: Boxtel, The Netherlands, 1987. [Google Scholar]
- Lotan, M.; Shapiro, M. Management of young children with Rett disorder in the controlled multi-sensory (Snoezelen) environment. Brain Devel. 2005, 27 (Suppl. S1), S88–S94. [Google Scholar] [CrossRef]
- Staal, J.A.; Amanda, S.; Matheis, R.; Collier, L.; Calia, T.; Hanif, H.; Kofman, E.S. The Effects of Snoezelen (Multi-Sensory Behavior Therapy) and Psychiatric Care on Agitation, Apathy, and Activities of Daily Living in Dementia Patients on a Short Term Geriatric Psychiatric Inpatient Unit. Int. J. Psychiatry Med. 2007, 37, 357–370. [Google Scholar] [CrossRef]
- Testerink, G.; ten Brug, A.; Douma, G.; van der Putten, A. Snoezelen in people with intellectual disability or dementia: A systematic review. Int. J. Nurs. Stud. Adv. 2023, 5, 100152. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Liu, K.Y.; Costafreda, S.G.; Selbæk, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Sommerlad A, Mukadam N. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef]
- Alzheimer’s Disease International. World Alzheimer Report 2024: Global Changes in Attitudes to Dementia; Alzheimer’s Disease International: London, UK, 2024. [Google Scholar]




| Functions | Inborn | Learned |
|---|---|---|
| 1. Swallowing solids | + | |
| 2. Language | + | |
| 3. Napping/sleep | + | |
| 4. Nictemeral sleep | + | |
| 5. Balance | + | |
| 6. Gait, walk, run | + | |
| 7. Sphincteric control | + | |
| 8. Dressing | + | |
| 9. Hygiene | + |
| ISTINCTS AND AFFECTS | ||||||
|---|---|---|---|---|---|---|
| ISTINCTIVE | EMOTIONAL | SENSORY | HOMEOSTATIC | |||
| self-preservation (survival) | crying-smiling | pleasure | air | independent |  | Experiences and Behaviors—AnBC |
| creativity | fear | sweet | thirst | maternal bond | ||
| curiosity | play | grainy | hunger | maternal bond | ||
| passions | seeking | pain | light | maternal bond | ||
| wills | lust | salty | cold | maternal bond | ||
| rage/anger | sharp | heat | maternal bond | |||
| care | noise | |||||
| panic | quietness | |||||
|
| MTL | D | BF | |
|---|---|---|---|
| Capabilities of conscious reflection and self-awareness | + | +/− | −/+ |
| Difficulties with time relationships | − | −/+ | − |
| Problems with attention and concentration | − | − | + |
| Unbalanced impairment of recall as opposed to recognition | − | − | + |
| Emotional instability and mood disorders | − | −/+ | + |
| Impairment in executive functions | − | +/− | + |
| Manifestations of disinhibition and perseveration | − | +/− | −/+ |
| Anosognosia | − | −/+ | + |
| Confabulation | − | −/+ | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirani, A. The Implementation of Infant Anoesis and Adult Autonoesis in the Retrogenesis and Staging System of the Neurocognitive Disorders: A Proposal for a Multidimensional Person-Centered Model. Geriatrics 2025, 10, 20. https://doi.org/10.3390/geriatrics10010020
Pirani A. The Implementation of Infant Anoesis and Adult Autonoesis in the Retrogenesis and Staging System of the Neurocognitive Disorders: A Proposal for a Multidimensional Person-Centered Model. Geriatrics. 2025; 10(1):20. https://doi.org/10.3390/geriatrics10010020
Chicago/Turabian StylePirani, Alessandro. 2025. "The Implementation of Infant Anoesis and Adult Autonoesis in the Retrogenesis and Staging System of the Neurocognitive Disorders: A Proposal for a Multidimensional Person-Centered Model" Geriatrics 10, no. 1: 20. https://doi.org/10.3390/geriatrics10010020
APA StylePirani, A. (2025). The Implementation of Infant Anoesis and Adult Autonoesis in the Retrogenesis and Staging System of the Neurocognitive Disorders: A Proposal for a Multidimensional Person-Centered Model. Geriatrics, 10(1), 20. https://doi.org/10.3390/geriatrics10010020






