Atto488-Agitoxin 2—A Fluorescent Ligand with Increased Selectivity for Kv1.3 Channel Binding Site
Abstract
:1. Introduction
2. Materials and Methods
2.1. Peptide Toxins and GFP-Tagged AgTx2
2.2. Preparation of Spheroplasts
2.3. Microscopy
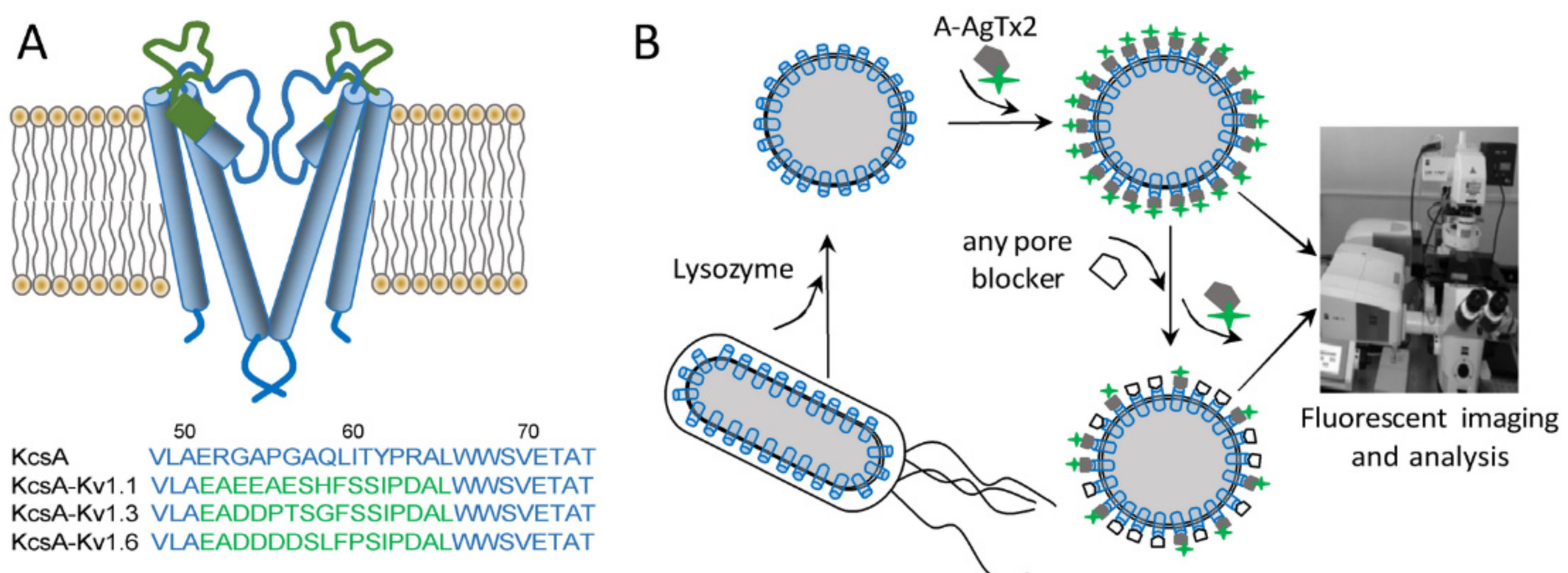
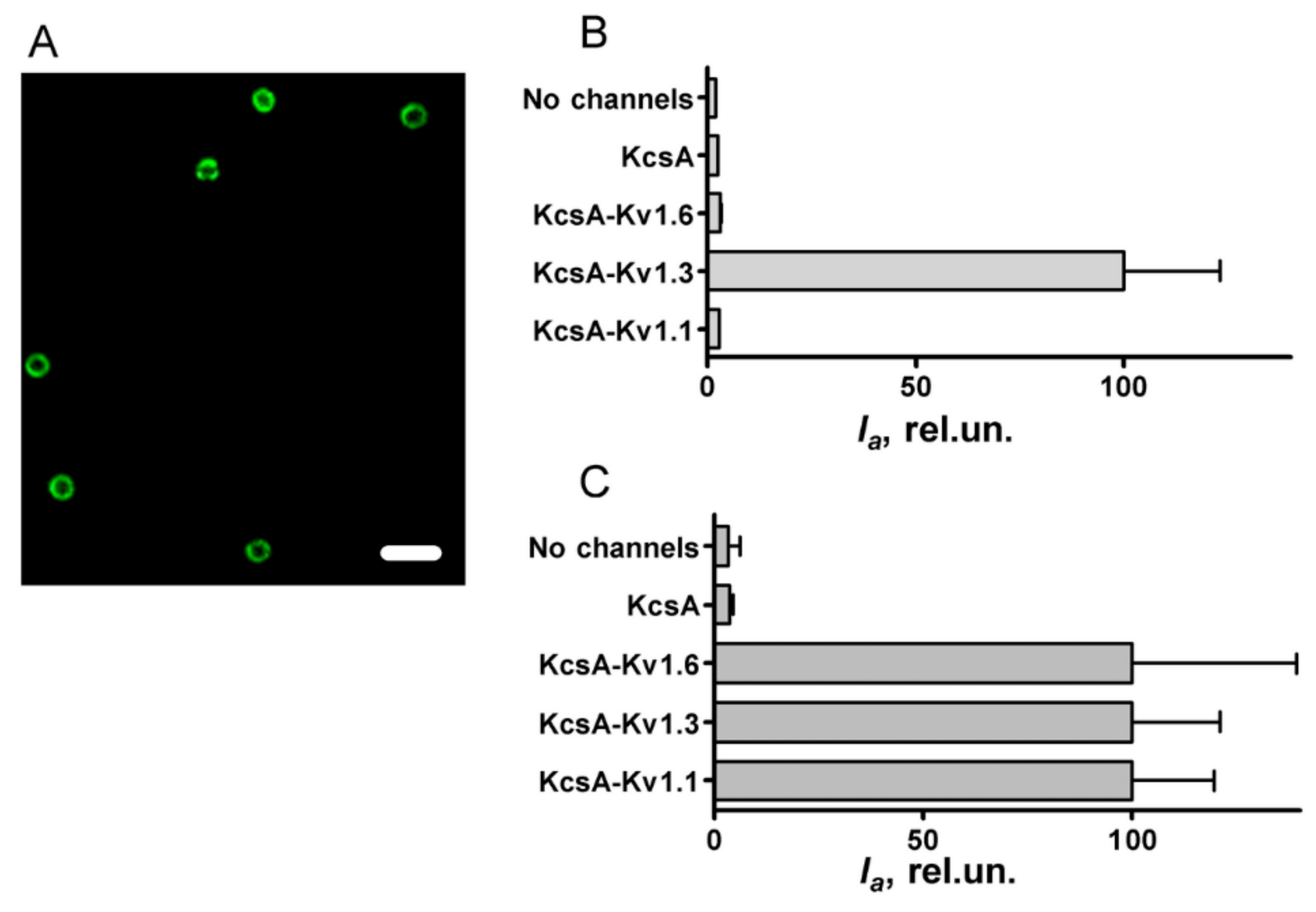
3. Results
3.1. Interaction of A-AgTx2 with Ligand-Binding Sites of Kv1.x (x = 1, 3, 6) Channels
3.2. Properties of A-AgTx2 as a Fluorescent Probe in the Bioengineering Ligand-Binding System with KcsA-Based Hybrid Channels
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia, M.L.; Garcia-Calvo, M.; Hidalgo, P.; Lee, A.; MacKinnon, R. Purification and Characterization of Three Inhibitors of Voltage-Dependent K+ Channels from Leiurus quinquestriatus var. hebraeus Venom. Biochemistry 1994, 33, 6834–6839. [Google Scholar] [CrossRef] [PubMed]
- Krezel, A.M.; Kasibhatla, C.; Hidalgo, P.; Mackinnon, R.; Wagner, G. Solution structure of the potassium channel inhibitor agitoxin 2: Caliper for probing channel geometry. Protein Sci. 1995, 4, 1478–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidalgo, P.; MacKinnon, R. Revealing the architecture of a K+ channel pore through mutant cycles with a peptide inhibitor. Science 1995, 268, 307–310. [Google Scholar] [CrossRef]
- Ranganathan, R.; Lewis, J.H.; MacKinnon, R. Spatial localization of the K+ channel selectivity filter by mutant cycle-based structure analysis. Neuron 1996, 16, 131–139. [Google Scholar] [CrossRef] [Green Version]
- MacKinnon, R.; Cohen, S.L.; Kuo, A.; Lee, A.; Chait, B.T. Structural conservation in prokaryotic and eukaryotic potassium channels. Science 1998, 280, 106–109. [Google Scholar] [CrossRef] [Green Version]
- Kudryashova, K.S.; Nekrasova, O.V.; Kirpichnikov, M.P.; Feofanov, A.V. Chimeras of KcsA and Kv1 as a bioengineering tool to study voltage-gated potassium channels and their ligands. Biochem. Pharmacol. 2021, 190, 114646. [Google Scholar] [CrossRef]
- Doyle, D.A.; Cabral, J.M.; Pfuetzner, R.A.; Kuo, A.; Gulbis, J.M.; Cohen, S.L.; Chait, B.T.; MacKinnon, R. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science 1998, 280, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Legros, C.; Pollmann, V.; Knaus, H.G.; Farrell, A.M.; Darbon, H.; Bougis, P.E.; Martin-Eauclaire, M.F.; Pongs, O. Generating a high affinity scorpion toxin receptor in KcsA-Kv1.3 chimeric potassium channels. J. Biol. Chem. 2000, 275, 16918–16924. [Google Scholar] [CrossRef] [Green Version]
- Legros, C.; Schulze, C.; Garcia, M.L.; Bougis, P.E.; Martin-Eauclaire, M.F.; Pongs, O. Engineering-specific pharmacological binding sites for peptidyl inhibitors of potassium channels into KcsA. Biochemistry 2002, 41, 15369–15375. [Google Scholar] [CrossRef]
- Kuzmenkov, A.I.; Vassilevski, A.A. Labelled animal toxins as selective molecular markers of ion channels: Applications in neurobiology and beyond. Neurosci. Lett. 2018, 679, 15–23. [Google Scholar] [CrossRef]
- Kudryashova, K.S.; Nekrasova, O.V.; Kuzmenkov, A.I.; Vassilevski, A.A.; Ignatova, A.A.; Korolkova, Y.V.; Grishin, E.V.; Kirpichnikov, M.P.; Feofanov, A.V. Fluorescent system based on bacterial expression of hybrid KcsA channels designed for Kv1.3 ligand screening and study. Anal. Bioanal. Chem. 2013, 405, 2379–2389. [Google Scholar] [CrossRef] [PubMed]
- Kuzmenkov, A.I.; Vassilevski, A.A.; Kudryashova, K.S.; Nekrasova, O.V.; Peigneur, S.; Tytgat, J.; Feofanov, A.V.; Kirpichnikov, M.P.; Grishin, E.V. Variability of potassium channel blockers in Mesobuthus eupeus scorpion venom with focus on Kv1.1: An integrated transcriptomic and proteomic study. J. Biol. Chem. 2015, 290, 12195–12209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nekrasova, O.V.; Volyntseva, A.D.; Kudryashova, K.S.; Novoseletsky, V.N.; Lyapina, E.A.; Illarionova, A.V.; Yakimov, S.A.; Korolkova, Y.V.; Shaitan, K.V.; Kirpichnikov, M.P.; et al. Complexes of Peptide Blockers with Kv1.6 Pore Domain: Molecular Modeling and Studies with KcsA-Kv1.6 Channel. J. Neuroimmune Pharmacol. 2017, 12, 260–276. [Google Scholar] [CrossRef] [PubMed]
- Kuzmenkov, A.I.; Nekrasova, O.V.; Kudryashova, K.S.; Peigneur, S.; Tytgat, J.; Stepanov, A.V.; Kirpichnikov, M.P.; Grishin, E.V.; Feofanov, A.V.; Vassilevski, A.A. Fluorescent protein-scorpion toxin chimera is a convenient molecular tool for studies of potassium channels. Sci. Rep. 2016, 6, 33314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nekrasova, O.V.; Primak, A.L.; Ignatova, A.A.; Novoseletsky, V.N.; Geras’kina, O.V.; Kudryashova, K.S.; Yakimov, S.A.; Kirpichnikov, M.P.; Arseniev, A.S.; Feofanov, A.V. N-Terminal Tagging with GFP Enhances Selectivity of Agitoxin 2 to Kv1.3-Channel Binding Site. Toxins 2020, 12, 802. [Google Scholar] [CrossRef]
- Nekrasova, O.; Kudryashova, K.; Fradkov, A.; Yakimov, S.; Savelieva, M.; Kirpichnikov, M.; Feofanov, A. Straightforward approach to produce recombinant scorpion toxins—Pore blockers of potassium channels. J. Biotechnol. 2017, 241, 127–135. [Google Scholar] [CrossRef]
- Kuipers, B.J.H.; Gruppen, H. Prediction of molar extinction coefficients of proteins and peptides using UV absorption of the constituent amino acids at 214 nm to enable quantitative reverse phase high-performance liquid chromatography-mass spectrometry analysis. J. Agric. Food Chem. 2007, 55, 5445–5451. [Google Scholar] [CrossRef]
- Shakkottai, V.G.; Regaya, I.; Wulff, H.; Fajloun, Z.; Tomita, H.; Fathallah, M.; Cahalan, M.D.; Gargus, J.J.; Sabatier, J.M.; Chandy, K.G. Design and Characterization of a Highly Selective Peptide Inhibitor of the Small Conductance Calcium-activated K+ Channel, SkCa2. J. Biol. Chem. 2001, 276, 43145–43151. [Google Scholar] [CrossRef] [Green Version]
- George Chandy, K.; Cahalan, M.; Pennington, M.; Norton, R.S.; Wulff, H.; Gutman, G.A. Potassium channels in T lymphocytes: Toxins to therapeutic immunosuppressants. Toxicon 2001, 39, 1269–1276. [Google Scholar] [CrossRef]
- Takacs, Z.; Toups, M.; Kollewe, A.; Johnson, E.; Cuello, L.G.; Driessens, G.; Biancalana, M.; Koide, A.; Ponte, C.G.; Perozo, E.; et al. A designer ligand specific for Kv1.3 channels from a scorpion neurotoxin-based library. Proc. Natl. Acad. Sci. USA 2009, 106, 22211–22216. [Google Scholar] [CrossRef] [Green Version]
- Wulff, H.; Castle, N.A.; Pardo, L.A. Voltage-gated potassium channels as therapeutic targets. Nat. Rev. Drug Discov. 2009, 8, 982–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Li, G.; Guo, J.; Zhang, Z.; Zhang, S.; Zhu, Y.; Cheng, J.; Yu, L.; Ji, Y.; Tao, J. Kv1.3 Channel as a Key Therapeutic Target for Neuroinflammatory Diseases: State of the Art and Beyond. Front. Neurosci. 2020, 13, 1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varga, Z.; Gurrola-Briones, G.; Papp, F.; Rodriguez De La Vega, R.C.; Pedraza-Alva, G.; Tajhya, R.B.; Gaspar, R.; Cardenas, L.; Rosenstein, Y.; Beeton, C.; et al. Vm24, a natural immunosuppressive peptide, potently and selectively blocks Kv1.3 potassium channels of human T cells. Mol. Pharmacol. 2012, 82, 372–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuzmenkov, A.I.; Nekrasova, O.V.; Peigneur, S.; Tabakmakher, V.M.; Gigolaev, A.M.; Fradkov, A.F.; Kudryashova, K.S.; Chugunov, A.O.; Efremov, R.G.; Tytgat, J.; et al. K V 1.2 channel-specific blocker from Mesobuthus eupeus scorpion venom: Structural basis of selectivity. Neuropharmacology 2018, 143, 228–238. [Google Scholar] [CrossRef]
- Cañas, C.A.; Castaño-Valencia, S.; Castro-Herrera, F. Pharmacological blockade of KV1.3 channel as a promising treatment in autoimmune diseases. J. Transl. Autoimmun. 2022, 5, 100146. [Google Scholar] [CrossRef]
- Zhou, Y.-Y.; Hou, G.-Q.; He, S.-W.; Xiao, Z.; Xu, H.-J.; Qiu, Y.-T.; Jiang, S.; Zheng, H.; Li, Z.-Y. Psora-4, a Kv1.3 Blocker, Enhances Differentiation and Maturation in Neural Progenitor Cells. CNS Neurosci. Ther. 2015, 21, 558–567. [Google Scholar] [CrossRef]
- Mouhat, S.; Visan, V.; Ananthakrishnan, S.; Wulff, H.; Andreotti, N.; Grissmer, S.; Darbon, H.; De Waard, M.; Sabatier, J.-M. K+ channel types targeted by synthetic OSK1, a toxin from Orthochirus scrobiculosus scorpion venom. Biochem. J. 2005, 385, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Kalman, K.; Pennington, M.W.; Lanigan, M.D.; Nguyen, A.; Rauer, H.; Mahnir, V.; Paschetto, K.; Kem, W.R.; Grissmer, S.; Gutman, G.A.; et al. ShK-Dap22, a potent Kv1.3-specific immunosuppressive polypeptide. J. Biol. Chem. 1998, 273, 32697–32707. [Google Scholar] [CrossRef] [Green Version]
- Beeton, C.; Pennington, M.W.; Wulff, H.; Singh, S.; Nugent, D.; Crossley, G.; Khaytin, I.; Calabresi, P.A.; Chen, C.Y.; Gutman, G.A.; et al. Targeting effector memory T cells with a selective peptide inhibitor of Kv1.3 channels for therapy of autoimmune diseases. Mol. Pharmacol. 2005, 67, 1369–1381. [Google Scholar] [CrossRef]
- Pennington, M.W.; Beeton, C.; Galea, C.A.; Smith, B.J.; Chi, V.; Monaghan, K.P.; Garcia, A.; Rangaraju, S.; Giuffrida, A.; Plank, D.; et al. Engineering a stable and selective peptide blocker of the Kv1.3 channel in T lymphocytes. Mol. Pharmacol. 2009, 75, 762–773. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denisova, K.R.; Orlov, N.A.; Yakimov, S.A.; Kirpichnikov, M.P.; Feofanov, A.V.; Nekrasova, O.V. Atto488-Agitoxin 2—A Fluorescent Ligand with Increased Selectivity for Kv1.3 Channel Binding Site. Bioengineering 2022, 9, 295. https://doi.org/10.3390/bioengineering9070295
Denisova KR, Orlov NA, Yakimov SA, Kirpichnikov MP, Feofanov AV, Nekrasova OV. Atto488-Agitoxin 2—A Fluorescent Ligand with Increased Selectivity for Kv1.3 Channel Binding Site. Bioengineering. 2022; 9(7):295. https://doi.org/10.3390/bioengineering9070295
Chicago/Turabian StyleDenisova, Kristina R., Nikita A. Orlov, Sergey A. Yakimov, Mikhail P. Kirpichnikov, Alexey V. Feofanov, and Oksana V. Nekrasova. 2022. "Atto488-Agitoxin 2—A Fluorescent Ligand with Increased Selectivity for Kv1.3 Channel Binding Site" Bioengineering 9, no. 7: 295. https://doi.org/10.3390/bioengineering9070295
APA StyleDenisova, K. R., Orlov, N. A., Yakimov, S. A., Kirpichnikov, M. P., Feofanov, A. V., & Nekrasova, O. V. (2022). Atto488-Agitoxin 2—A Fluorescent Ligand with Increased Selectivity for Kv1.3 Channel Binding Site. Bioengineering, 9(7), 295. https://doi.org/10.3390/bioengineering9070295






