Facial Pain: RCT between Conventional Treatment and Fascial Manipulation® for Temporomandibular Disorders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Procedures and Data Collection
2.2.1. Group 1
2.2.2. Group 2
2.3. Data Collection
2.3.1. VRS
2.3.2. RDC/TMD
2.3.3. EMG
2.3.4. Pression/Pain Evaluation
2.4. Statistical Analysis
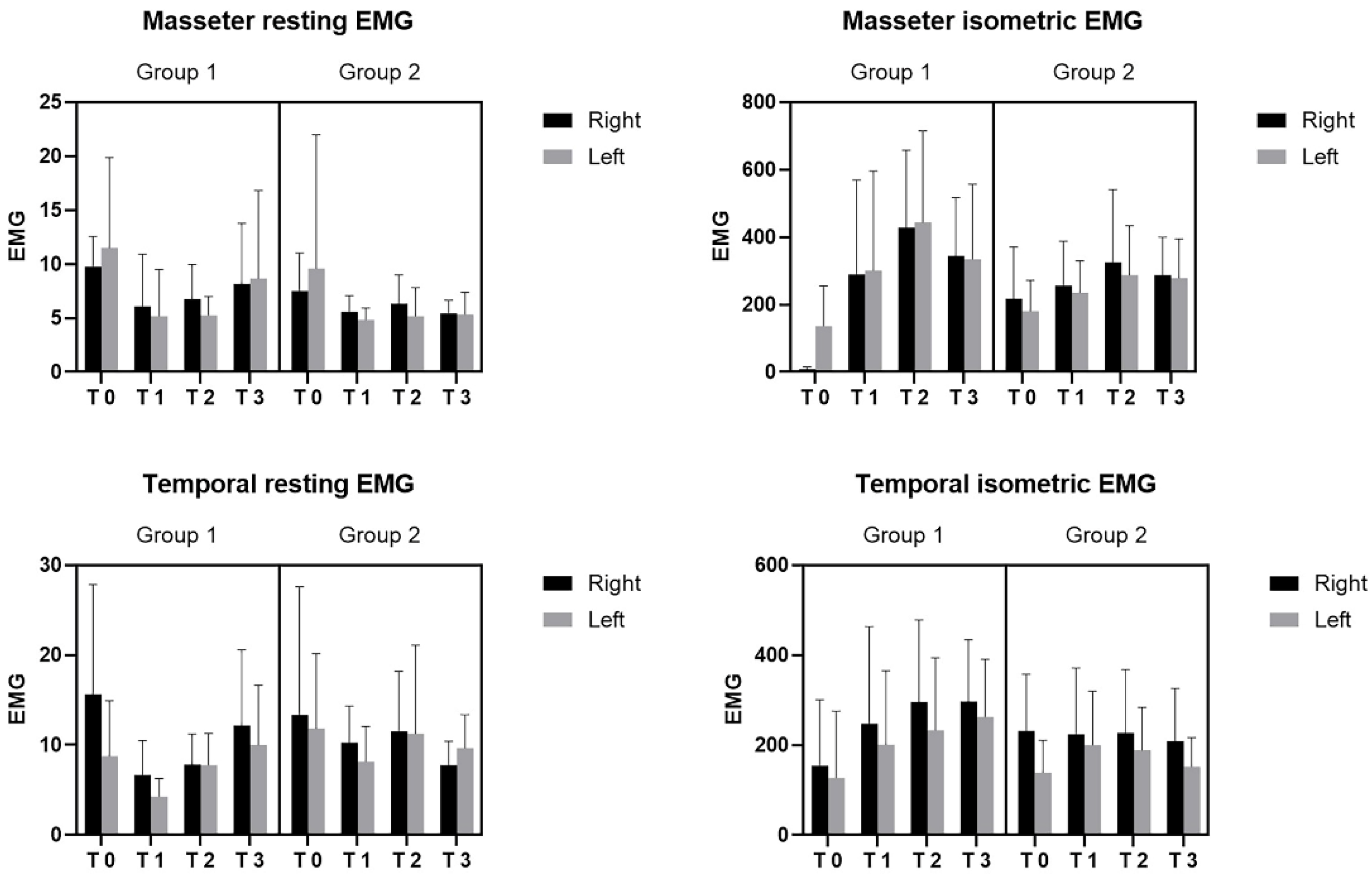
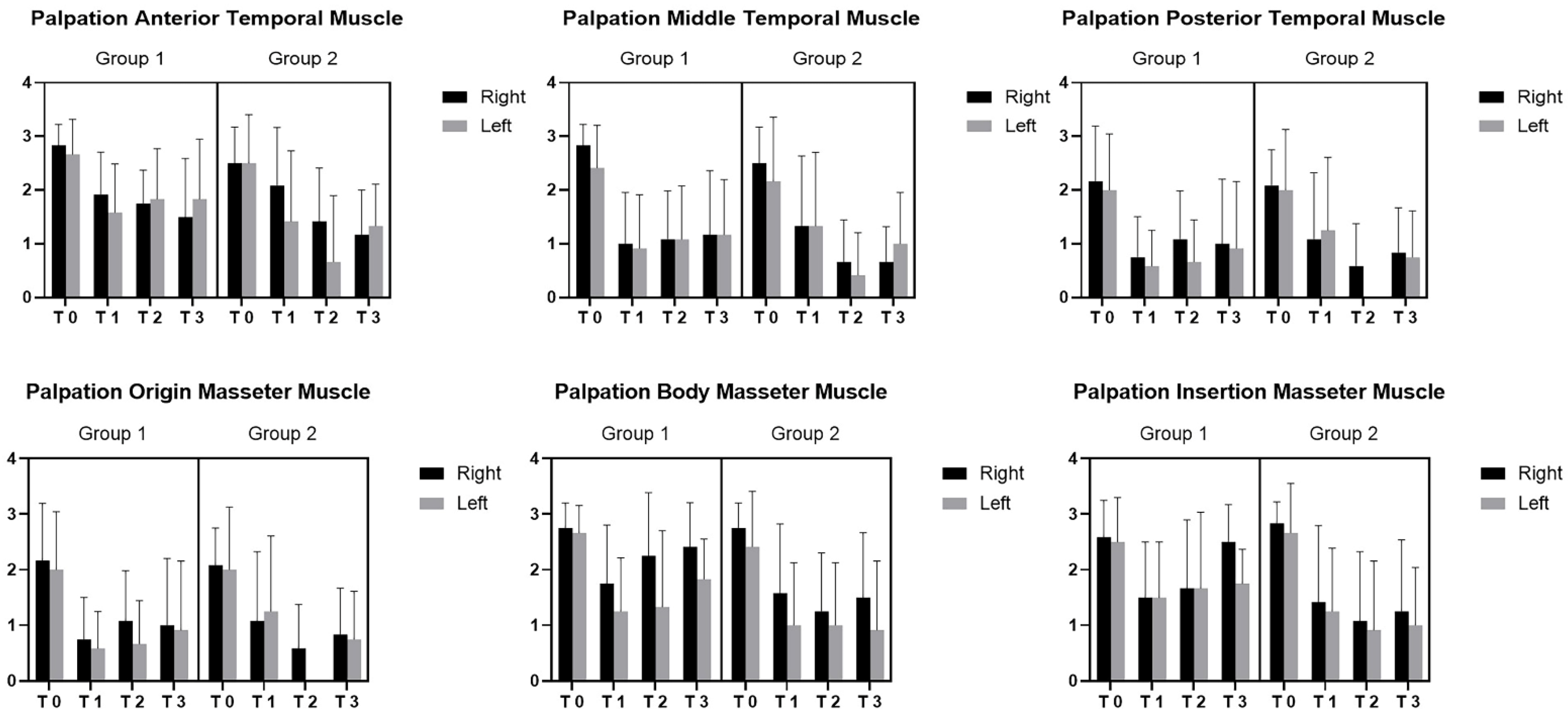
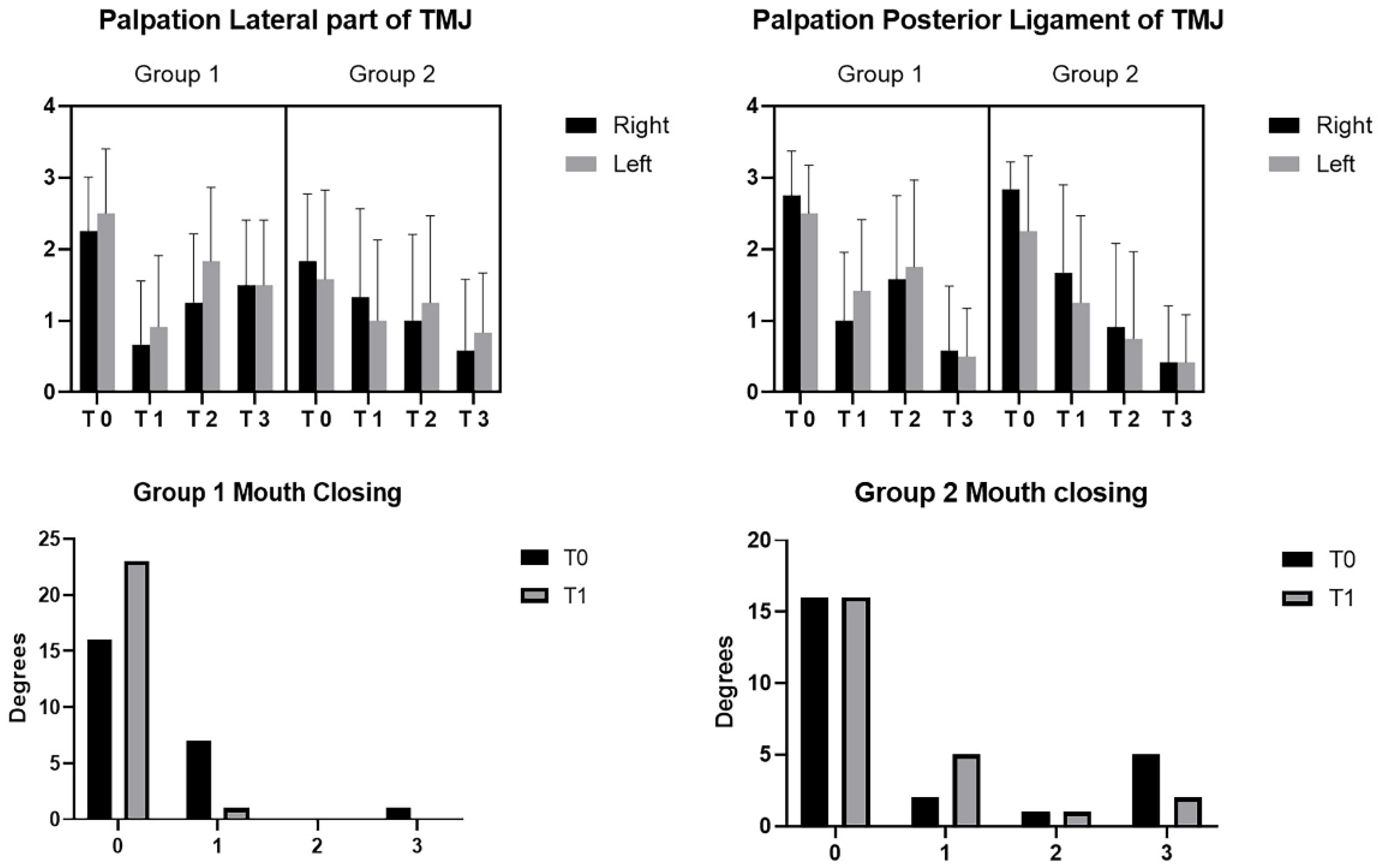
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okeson, J.P. Bell’s Orofacial Pains. The Clinical Management of Orofacial Pain, 6th ed.; Quintessence Publishing: Carol Stream, IL, USA, 2005. [Google Scholar]
- McNeill, C. Temporomandibular Disorders: Guidelines for Classification, Assesment, and Management, 2nd ed.; Quintessence Publishing: Chicago, IL, USA, 1993. [Google Scholar]
- Germain, L.; Malcmacher, L. Frontline Temporomandibular Joint/Orofacial Pain Therapy for Every Dental Practice. Compend. Contin. Educ. Dent. 2017, 38, 299–305. [Google Scholar] [PubMed]
- Fillingim, R.B.; Ohrbach, R.; Greenspan, J.D.; Knott, C.; Diatchenko, L.; Dubner, R.; Bair, E.; Baraian, C.; Mack, N.; Slade, G.D.; et al. Psychological factors associated with development of TMD: The OPPERA prospective cohort study. J. Pain 2013, 14 (Suppl. 12), T75–T90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simons, D.G.; Travell, J.G. Myofascial Pain and Dysfunction: The Trigger Point Manual; Williams & Wilkins: Baltimore, MD, USA, 1999; Volume 1. [Google Scholar]
- Lin, C.S. Brain signature of chronic orofacial pain: A systematic review and metaanalysis on neuroimaging research of trigeminal neuropathic pain and temporomandibular joint disorders. PLoS ONE. 2014, 9, e94300. [Google Scholar] [CrossRef] [PubMed]
- Romero-Reyes, M.; Uyanik, J.M. Orofacial pain management: Current perspectives. J. Pain Res. 2014, 7, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ervilha, U.F.; Graven-Nielsen, T.; Duarte, M. A simple test of muscle coactivation estimation using electromyography. Braz. J. Med. Biol. Res. 2012, 45, 977–981. [Google Scholar] [CrossRef] [Green Version]
- Kalamir, A.; Pollard, H.; Vitiello, A.; Bonello, R. Intra-oral myofascial therapy for chronic myogenous temporomandibular disorders: A randomized, controlled pilot study. J. Man. Manip. Ther. 2010, 18, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Bialosky, J.E.; Simon, C.B.; Bishop, M.D.; George, S.Z. Basis for spinal manipulative therapy: A physical therapist perspective. J. Electromyogr. Kinesiol. 2012, 22, 643–647. [Google Scholar] [CrossRef] [Green Version]
- Vigotsky, A.D.; Bruhns, R.P. The role of descending modulation in manual therapy and its analgesic implications: A narrative review. Pain Res. Treat. 2015, 2015, 292805. [Google Scholar] [CrossRef] [Green Version]
- Asquini, G.; Bianchi, A.E.; Heneghan, N.R.; Rushton, A.B.; Borromeo, G.; Locatelli, M.; Falla, D. Predictors of pain reduction following manual therapy in patients with temporomandibular disorders: A protocol for a prospective observational study. BMJ Open 2019, 9, e032113. [Google Scholar] [CrossRef] [Green Version]
- Medlicott, M.S.; Harris, S.R. A systematic review of the effectiveness of exercise, manual therapy, electrotherapy, relaxation training, and biofeedback in the management of temporomandibular disorder. Phys. Ther. 2006, 86, 955–973. [Google Scholar] [CrossRef]
- Asquini, G.; Pitance, L.; Michelotti, A.; Falla, D. Effectiveness of manual therapy applied to craniomandibular structures in temporomandibular disorders: A systematic review. J. Oral Rehabil. 2022, 49, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Asquini, G.; Rushton, A.; Pitance, L.; Heneghan, N.; Falla, D. The effectiveness of manual therapy applied to craniomandibular structures in the treatment of temporomandibular disorders: Protocol for a systematic review. Syst. Rev. 2021, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Branchini, M.; Lopopolo, F.; Andreoli, E.; Loreti, I.; Marchand, A.M.; Stecco, A. Fascial Manipulation® for chronic aspecific low back pain: A single blinded randomized controlled trial. Version 2. F1000Research 2015, 4, 1208. [Google Scholar] [CrossRef]
- Pratelli, E.; Pintucci, M.; Cultrera, P.; Baldini, E.; Stecco, A.; Petroncelli, A.; Pasquetti, P. Conservative treatment of carpal tunnel syndrome: Comparison between laser therapy and fascial manipulation. J. Bodyw. Mov. Ther. 2015, 19, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Stecco, A.; Meneghini, A.; Stern, R.; Stecco, C.; Imamura, M. Ultrasonography in myofascial neck pain: Randomized clinical trial for diagnosis and follow-up. Surg. Radiol. Anat. 2014, 36, 243–253. [Google Scholar] [CrossRef]
- Pintucci, M.; Imamura, M.; Thibaut, A.; de ExelNunes, L.M.; Mayumi Nagato, M.; Hideko Seguchi Kaziyama, H.; Tomikawa Imamura, S.; Stecco, A.; Fregni, F.; Rizzo Battistella, L. Evaluation of fascial manipulation in carpal tunnel syndrome: A pilot randomized clinical trial. Eur. J. Phys. Rehabil. Med. 2017, 53, 630–631. [Google Scholar] [CrossRef]
- Day, J.A.; Copetti, L.; Rucli, G. From clinical experience to a model for the human fascial system. J. Bodyw. Mov. Ther. 2012, 16, 372–380. [Google Scholar] [CrossRef]
- Armijo-Olivo, S.; Pitance, L.; Singh, V.; Neto, F.; Thie, N.; Michelotti, A. Effectiveness of Manual Therapy and Therapeutic Exercise for Temporomandibular Disorders: Systematic Review and Meta-Analysis. Phys. Ther. 2016, 96, 9–25. [Google Scholar] [CrossRef] [Green Version]
- Zafar, H.; Nordh, E.; Eriksson, P.O. Temporal coordination between mandibular and head-neck movements during jaw opening-closing tasks in man. Arch. Oral Biol. 2000, 45, 675–682. [Google Scholar] [CrossRef]
- Guarda-Nardini, L.; Stecco, A.; Stecco, C.; Masiero, S.; Manfredini, D. Myofascial Pain of Jaw Muscles: Comparison of Short-Term Effectiveness of Botulinum Toxin Injections and Fascial manipulation Tehnique. J. Craniomandib. Pract. 2012, 30, 95–102. [Google Scholar] [CrossRef]
- Graff-Radford, S.B. Regional myofascial pain syndrome and headache: Principles of diagnosis and management. Curr. Pain Headache Rep. 2001, 5, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Fricton, J.; Look, J.O.; Wright, E.; Alencar, F.G., Jr.; Chen, H.; Lang, M.; Ouyang, W.; Velly, A.M. Systematic review and meta- analysis of randomized controlled trials evaluating intraoral ortho- pedic appliances for temporomandibular disorders. J. Orofac. Pain. 2010, 24, 237–254. [Google Scholar] [PubMed]
- Klasser, G.D.; Greene, C.S. Oral appliances in the management of temporomandibular disorders. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 107, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Berni, K.C.; Dibai-Filho, A.V.; Pires, P.F.; Rodrigues-Bigaton, D. Accuracy of the surface electromyography RMS processing for the diagnosis of myogenous temporomandibular disorder. J. Electromyogr. Kinesiol. 2015, 25, 596–602. [Google Scholar] [CrossRef]
- Packer, A.C.; Pires, P.F.; Dibai-Filho, A.V.; Rodrigues-Bigaton, D. Effect of upper thoracic manipulation on mouth opening and electromyographic activity of masticatory muscles in women with temporomandibular disorder: A randomized clinical trial. J. Manip. Physiol. Ther. 2015, 38, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Koho, P.; Borodulin, K.; Kautiainen, H.; Kujala, U.; Pohjolainen, T.; Hurri, H. Finnish version of the Tampa Scale of Kinesiophobia: Reference values in the Finnish general population and associations with leisure-time physical activity. J. Rehabil. Med. 2015, 47, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Gupta, B.D.; Aggarwal, S.; Gupta, B.; Gupta, M.; Gupta, N. Effect of deep cervical flexor training vs. conventional isometric training on forward head posture, pain, neck disability index in dentists suffering from chronic neck pain. J. Clin. Diagn. Res. 2013, 7, 2261–2264. [Google Scholar] [CrossRef]
- Harada, T.; Ichiki, R.; Tsukiyama, Y.; Koyano, K. The effect of oral splint devices on sleep bruxism: A 6-week observation with an ambulatory electromyographic recording device. J. Oral Rehabil. 2006, 33, 482–488. [Google Scholar] [CrossRef]
- Ovalle, W.K.; Dow, P.R. Comparative ultrastructure of the inner capsule of the muscle spindle and the tendon organ. Am. J. Anat. 1983, 166, 343–357. [Google Scholar] [CrossRef]
- Cowman, M.K.; Schmidt, T.A.; Raghavan, P.; Stecco, A. Viscoelastic Properties of Hyaluronan in Physiological Conditions. F1000Research 2015, 4, 622. [Google Scholar] [CrossRef] [Green Version]
- Kordaß, B.; Fasold, A. Manual Techniques for Structural Analysis of TMJ Part I: Basics and Clinical Examination [ManuelleStrukturanalyse. Teil 1: Grundlagen und klinische Untersuchung]. ZWR 2012, 212, 8–11. [Google Scholar]



| Interventions | Fascial Manipulation® | TMDT | ||
|---|---|---|---|---|
| Fascial Manipulation® | Michigan Occlusal Appliance | Anesthetic Injection (Lidocaine 0.5%) | Dry Needling of Muscle Trigger Points | |
| Duration | 1 h (3–5 min for each point) | Every night + 3 daytime h for 5 weeks. Then only at night for 6 months | 0.3–0.8 mL | - |
| Quantity | 6 points | - | 8 points | 8 points |
| Frequency | Weekly | Weekly adjustment | Weekly | Weekly |
| Number of sessions | 5 | 5 | 3 | 3 |
| Group 1 | Group 2 | |
|---|---|---|
| T0 vs. T1 | * <0.0001 | * 0.0001 |
| T0 vs. T2 | * <0.0001 | * <0.0001 |
| T0 vs. T3 | * <0.0001 | * <0.0001 |
| T1 vs. T2 | 0.9553 | 0.8622 |
| T1 vs. T3 | 0.8245 | 0.7372 |
| T2 vs. T3 | 0.5482 | >0.9999 |
| Group 1 | Group 2 | |
|---|---|---|
| T0 vs. T1 | * 0.0026 | 0.0738 |
| T0 vs. T2 | * 0.0149 | 0.1304 |
| T0 vs. T3 | 0.1214 | 0.7188 |
| T1 vs. T2 | 0.9968 | 0.9846 |
| T1 vs. T3 | 0.4949 | 0.8486 |
| T2 vs. T3 | 0.283 | 0.5873 |
| Group 1 | Group 2 | |
|---|---|---|
| T0 vs. T1 | * 0.001 | 0.61 |
| T0 vs. T2 | 0.1349 | 0.1416 |
| T0 vs. T3 | 0.7031 | 0.9995 |
| T1 vs. T2 | 0.4181 | 0.825 |
| T1 vs. T3 | 0.1505 | 0.9056 |
| T2 vs. T3 | 0.5879 | 0.3008 |
| Group 1 | Group 2 | |
|---|---|---|
| T0 vs. T1 | * 0.0031 | 0.9605 |
| T0 vs. T2 | 0.221 | * 0.0281 |
| T0 vs. T3 | 0.9877 | 0.4979 |
| T1 vs. T2 | 0.8384 | 0.5106 |
| T1 vs. T3 | 0.116 | 0.4291 |
| T2 vs. T3 | 0.3314 | * 0.0144 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekito, F.; Pintucci, M.; Pirri, C.; Ribeiro de Moraes Rego, M.; Cardoso, M.; Soares Paixão, K.; Ribeiro da Silva, V.; Stecco, A. Facial Pain: RCT between Conventional Treatment and Fascial Manipulation® for Temporomandibular Disorders. Bioengineering 2022, 9, 279. https://doi.org/10.3390/bioengineering9070279
Sekito F, Pintucci M, Pirri C, Ribeiro de Moraes Rego M, Cardoso M, Soares Paixão K, Ribeiro da Silva V, Stecco A. Facial Pain: RCT between Conventional Treatment and Fascial Manipulation® for Temporomandibular Disorders. Bioengineering. 2022; 9(7):279. https://doi.org/10.3390/bioengineering9070279
Chicago/Turabian StyleSekito, Florence, Marco Pintucci, Carmelo Pirri, Mariana Ribeiro de Moraes Rego, Mayra Cardoso, Kenia Soares Paixão, Valquiria Ribeiro da Silva, and Antonio Stecco. 2022. "Facial Pain: RCT between Conventional Treatment and Fascial Manipulation® for Temporomandibular Disorders" Bioengineering 9, no. 7: 279. https://doi.org/10.3390/bioengineering9070279
APA StyleSekito, F., Pintucci, M., Pirri, C., Ribeiro de Moraes Rego, M., Cardoso, M., Soares Paixão, K., Ribeiro da Silva, V., & Stecco, A. (2022). Facial Pain: RCT between Conventional Treatment and Fascial Manipulation® for Temporomandibular Disorders. Bioengineering, 9(7), 279. https://doi.org/10.3390/bioengineering9070279








