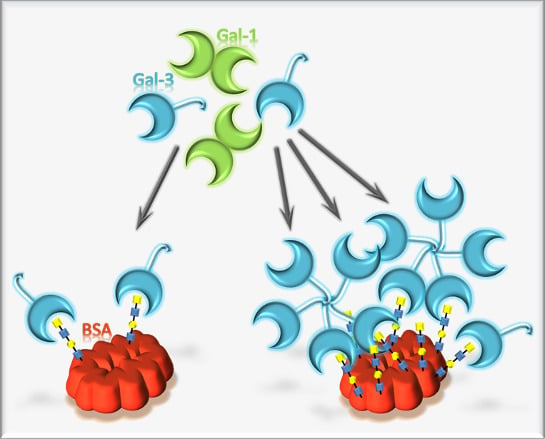
Biotinylated N-Acetyllactosamine- and N,N-Diacetyllactosamine-Based Oligosaccharides as Novel Ligands for Human Galectin-3
Abstract
:1. Introduction
2. Materials and Methods
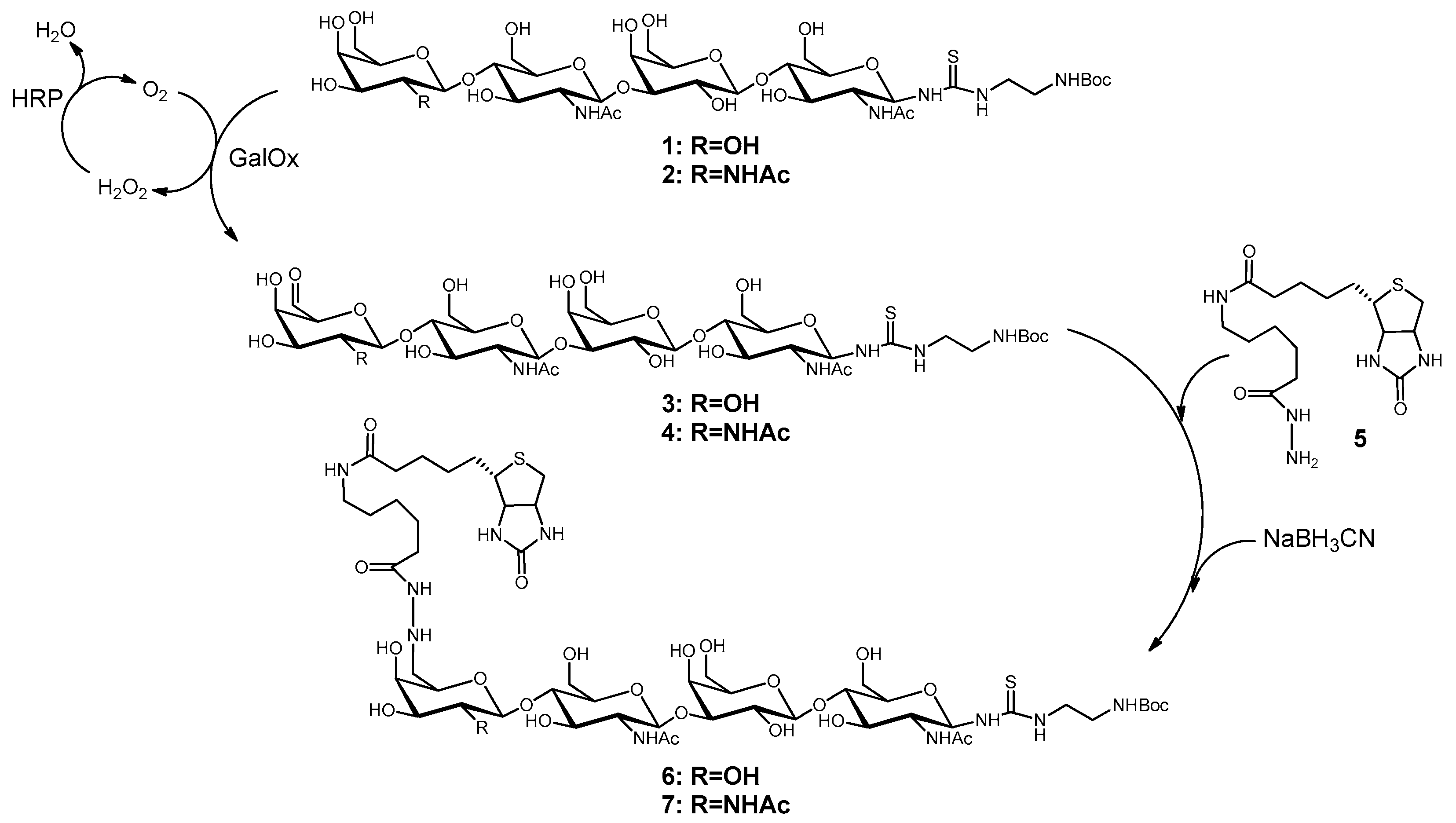
2.1. Synthesis of Biotinylated Glycans (6 and 7)
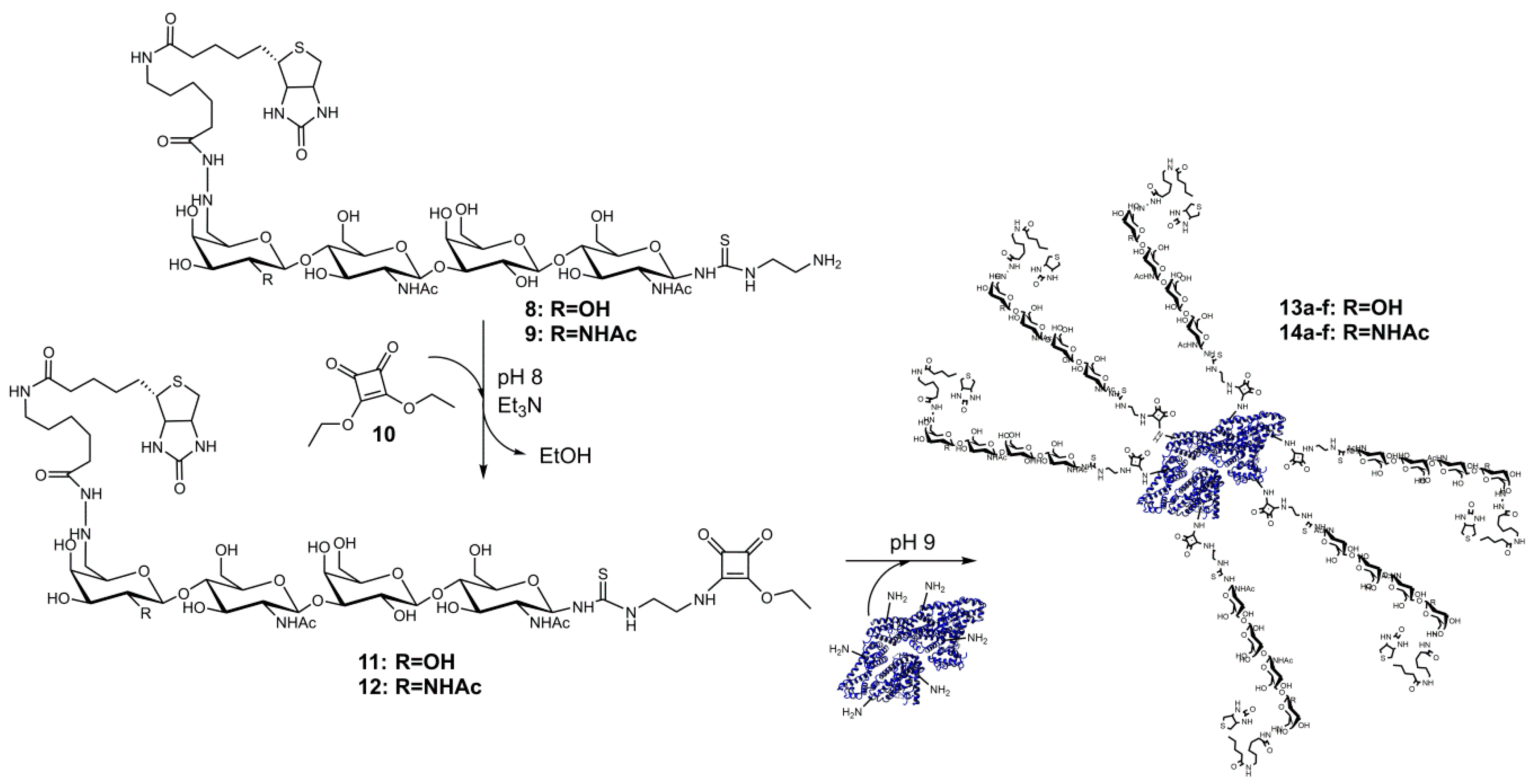
2.2. Synthesis of Compounds 11 and 12
2.3. Conjugation of Glycans to Bovine Serum Albumin
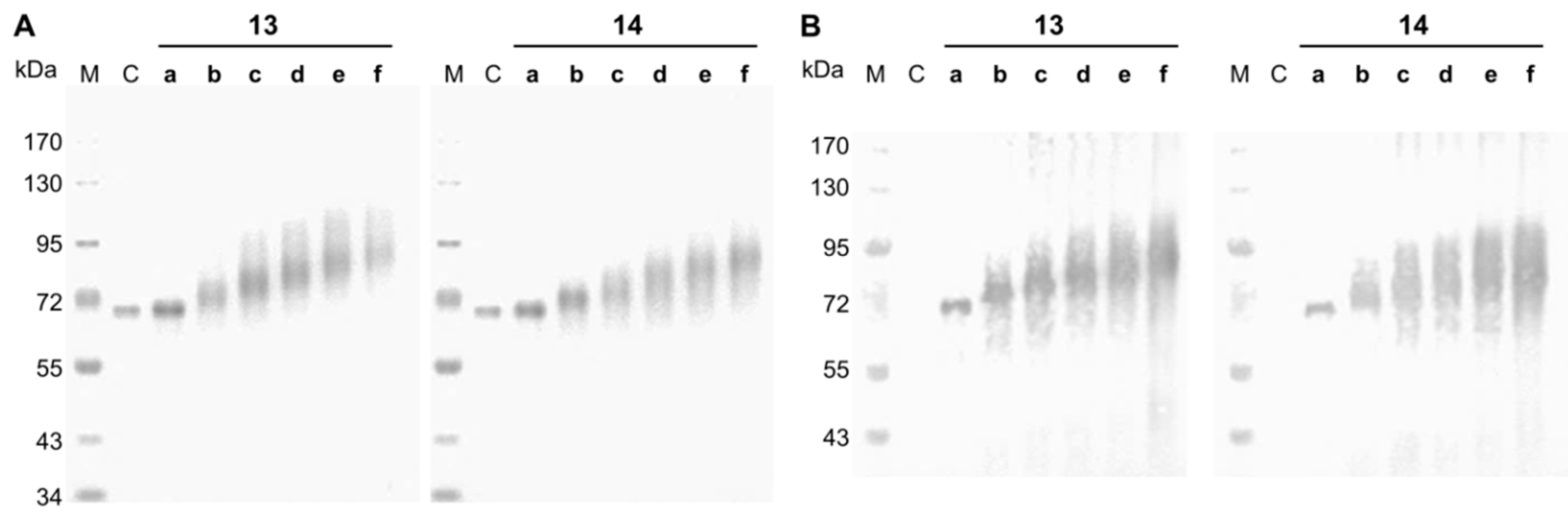
2.4. SDS-PAGE and Streptavidin Blot
2.5. Expression and Purification of Recombinant Galectins
2.6. Galectin Binding Assay with Immobilized Glycans and Neo-Glycoproteins
2.7. Inhibition of Galectin Binding with Neo-Glycoproteins
2.8. Surface Plasmon Resonance Spectroscopy
3. Results and Discussion
3.1. Biotinylation of LacNAc-LacNAc and LacdiNAc-LacNAc
3.2. Synthesis and Analysis of Neo-Glycoproteins Carrying Biotinylated Glycans
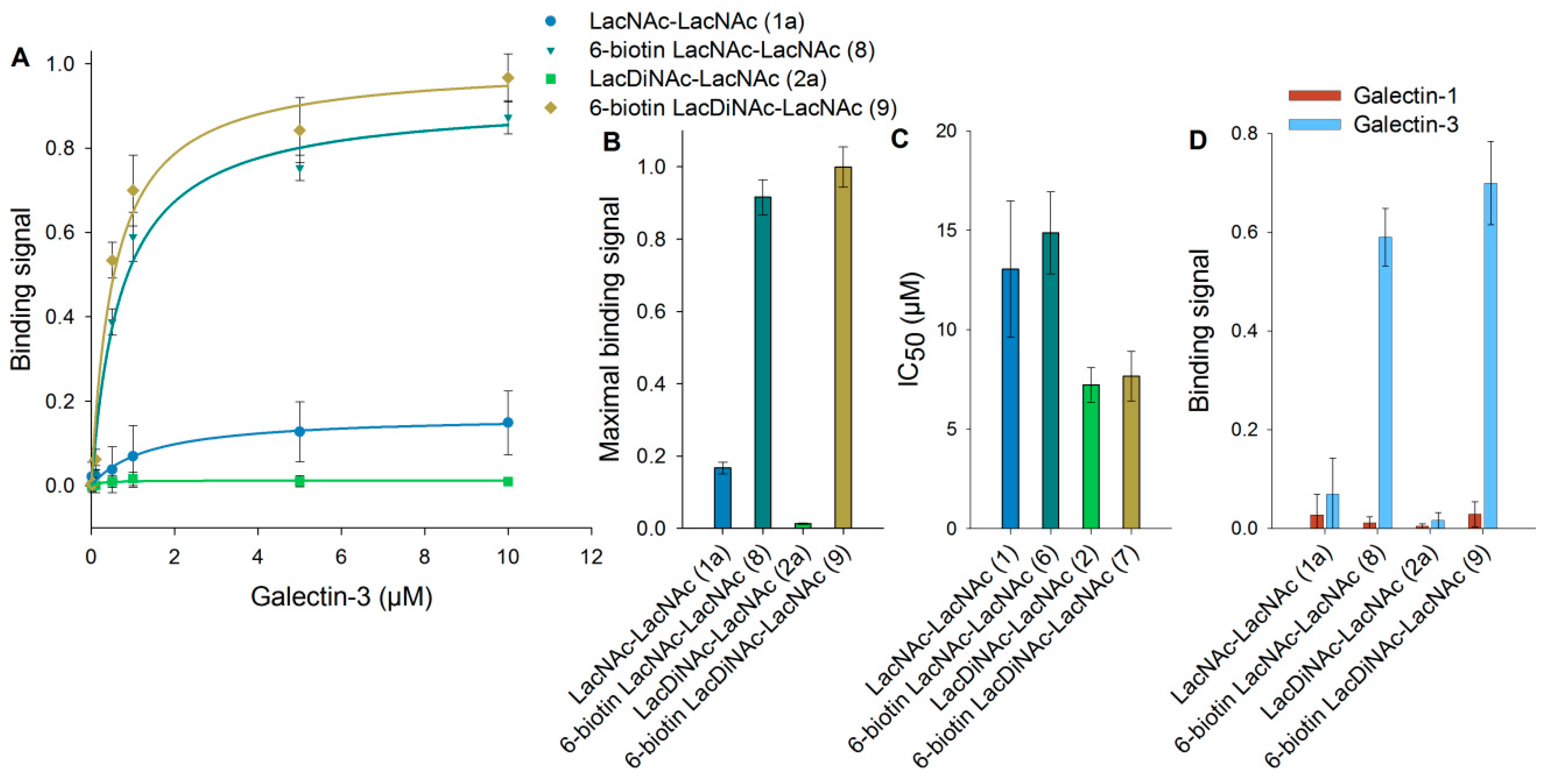
3.3. Binding of Galectin-3 to Immobilized 6-Biotinylated Tetrasaccharides
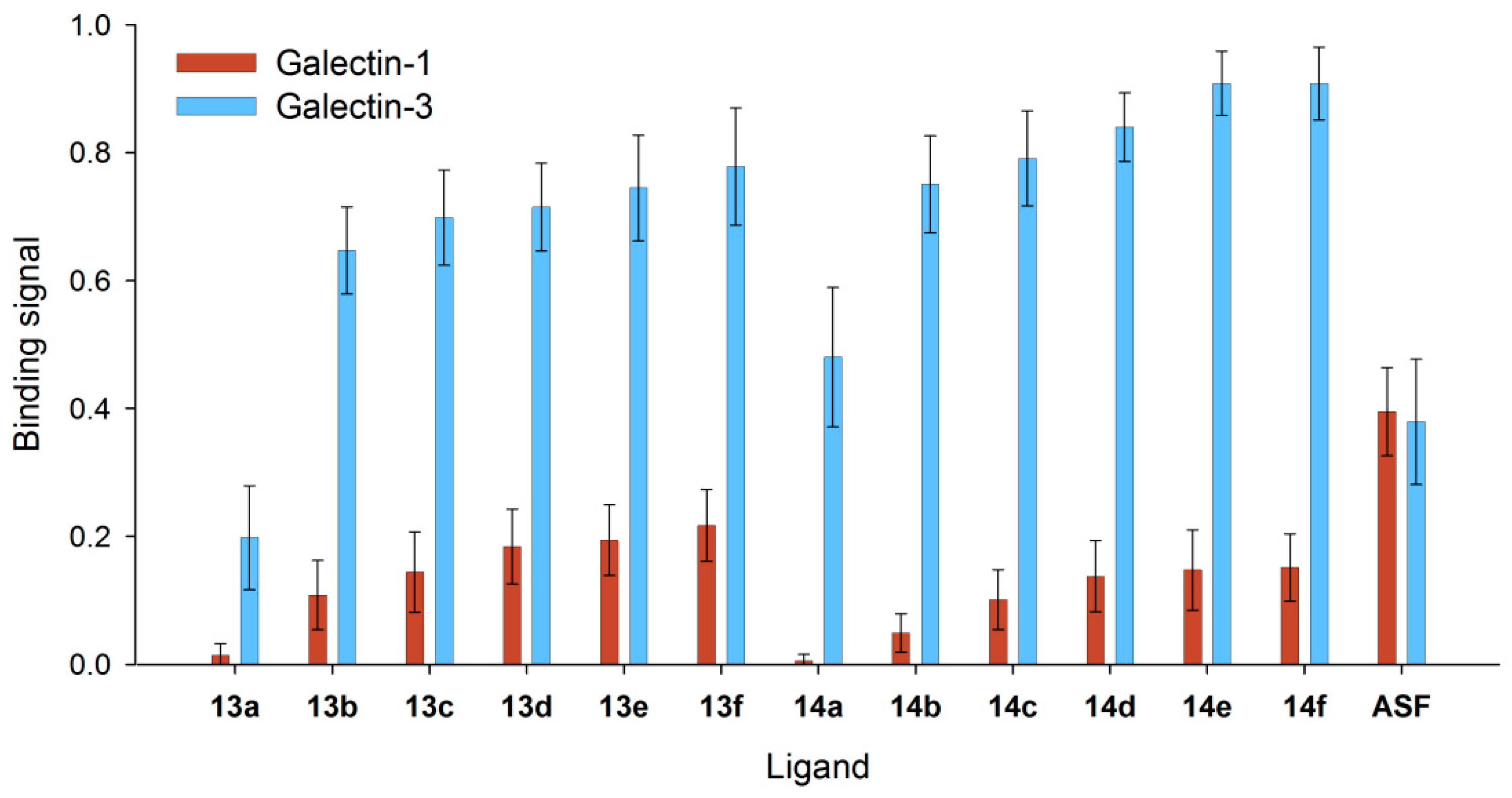
3.4. Binding of Galectin-3 and Galectin-1 to Neo-Glycoproteins
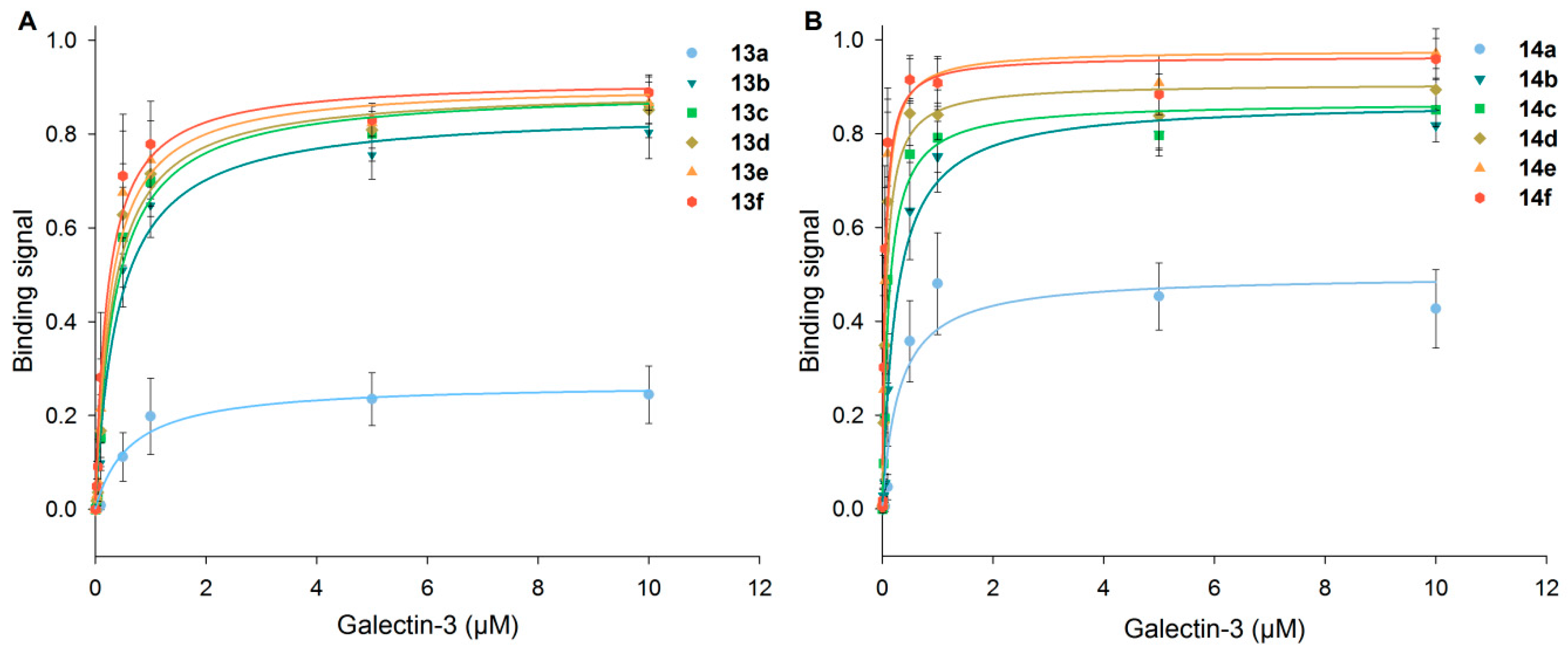
3.5. Detailed Characterization of Galectin-3 Binding to Neo-Glycoproteins
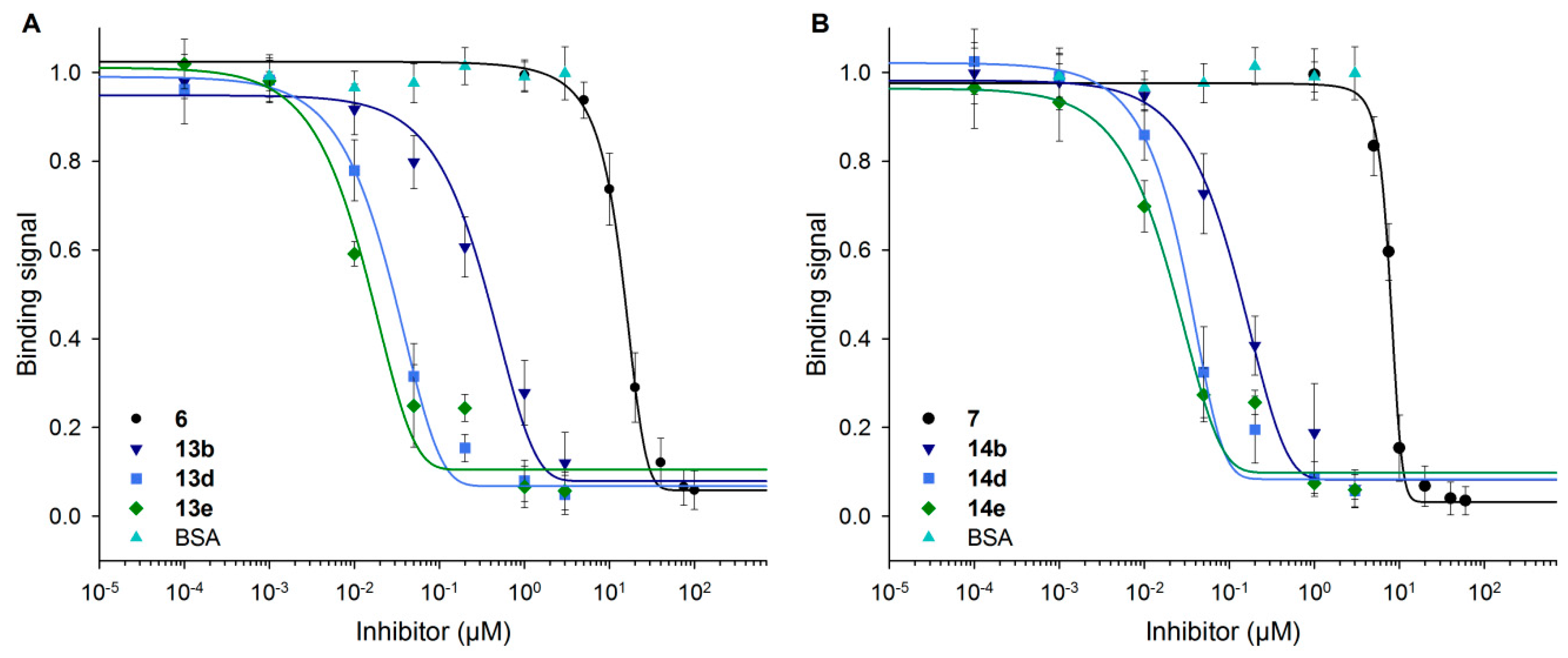
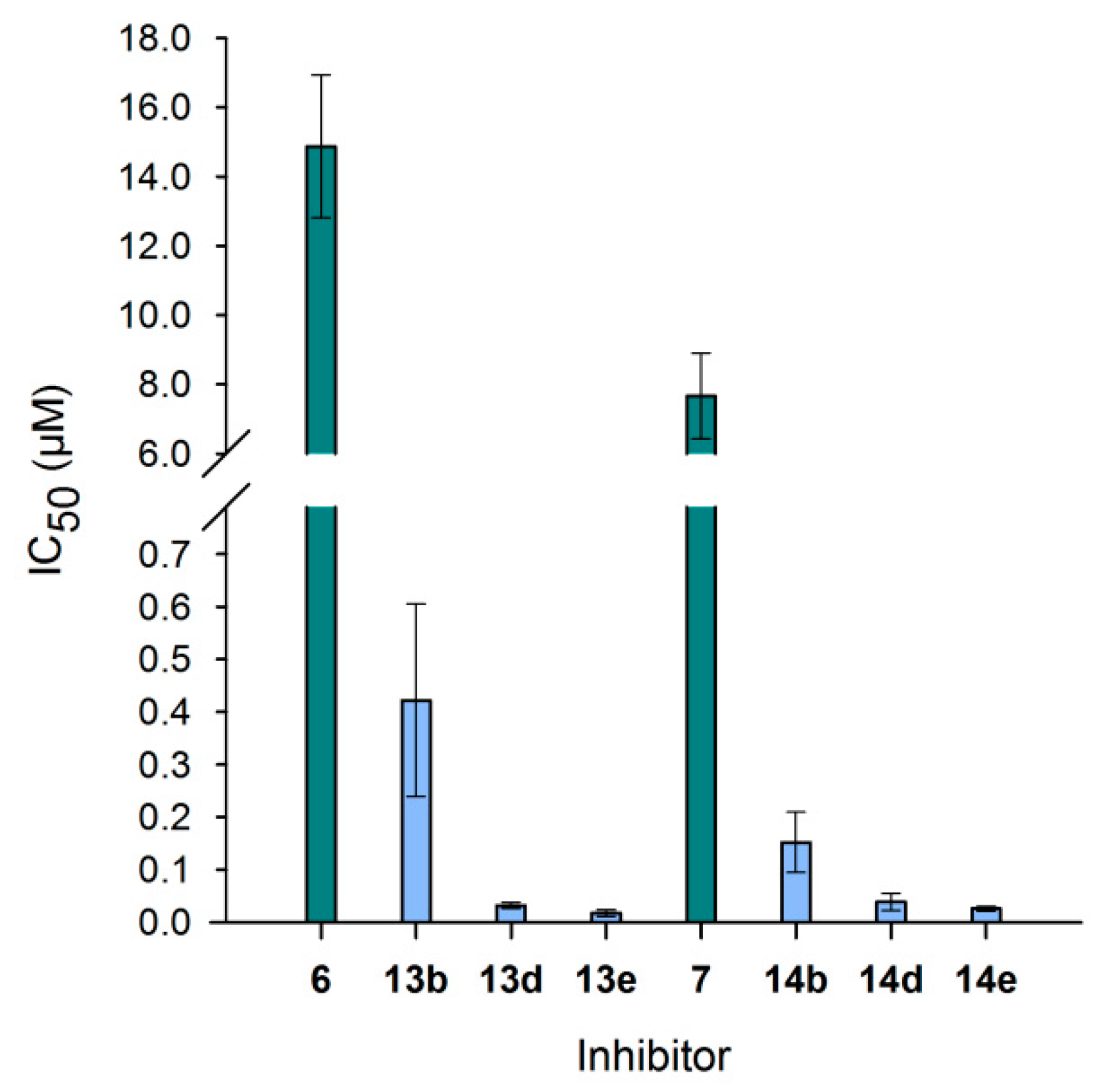
3.6. Neo-Glycoproteins as Inhibitors for Galectin-3
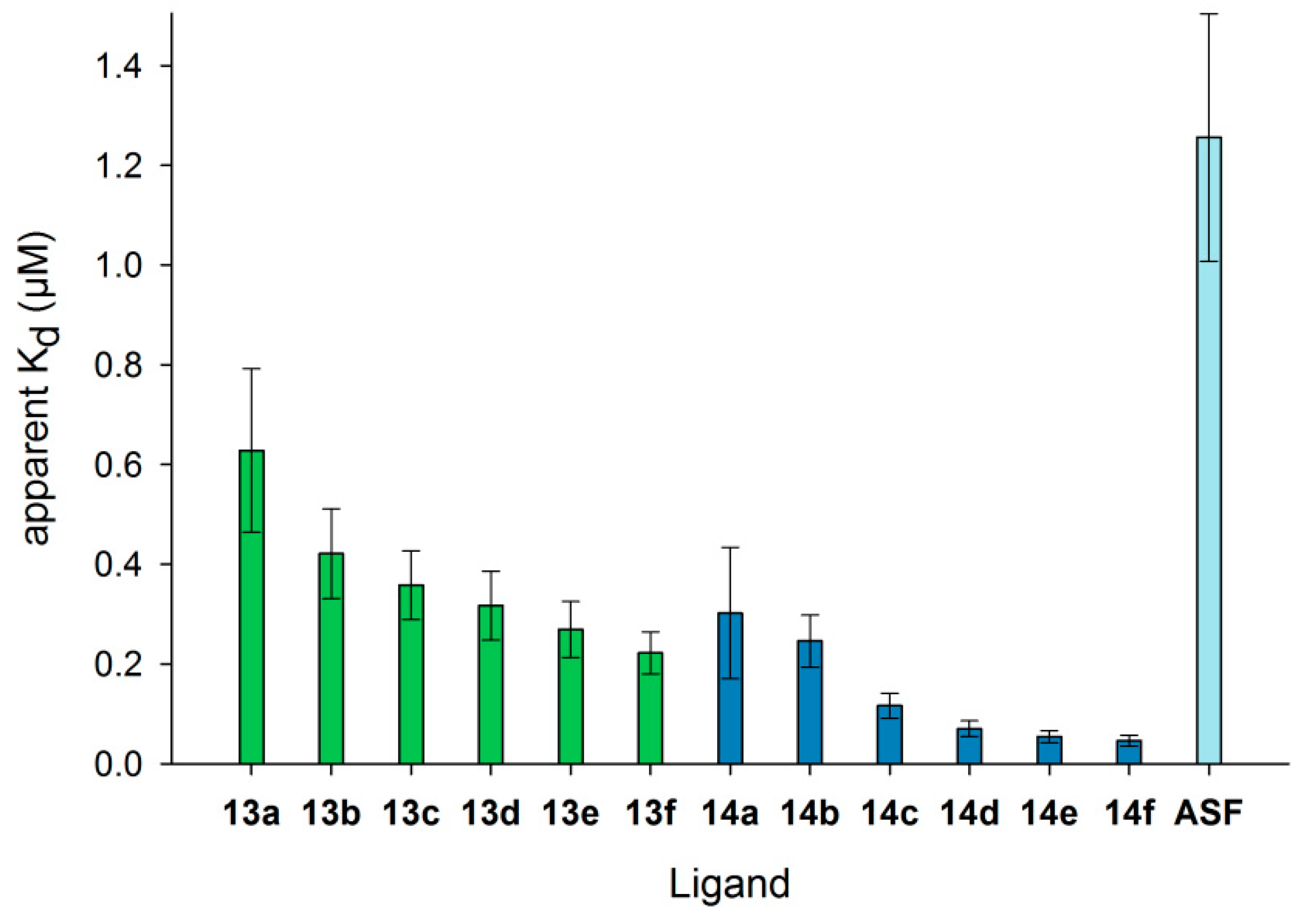
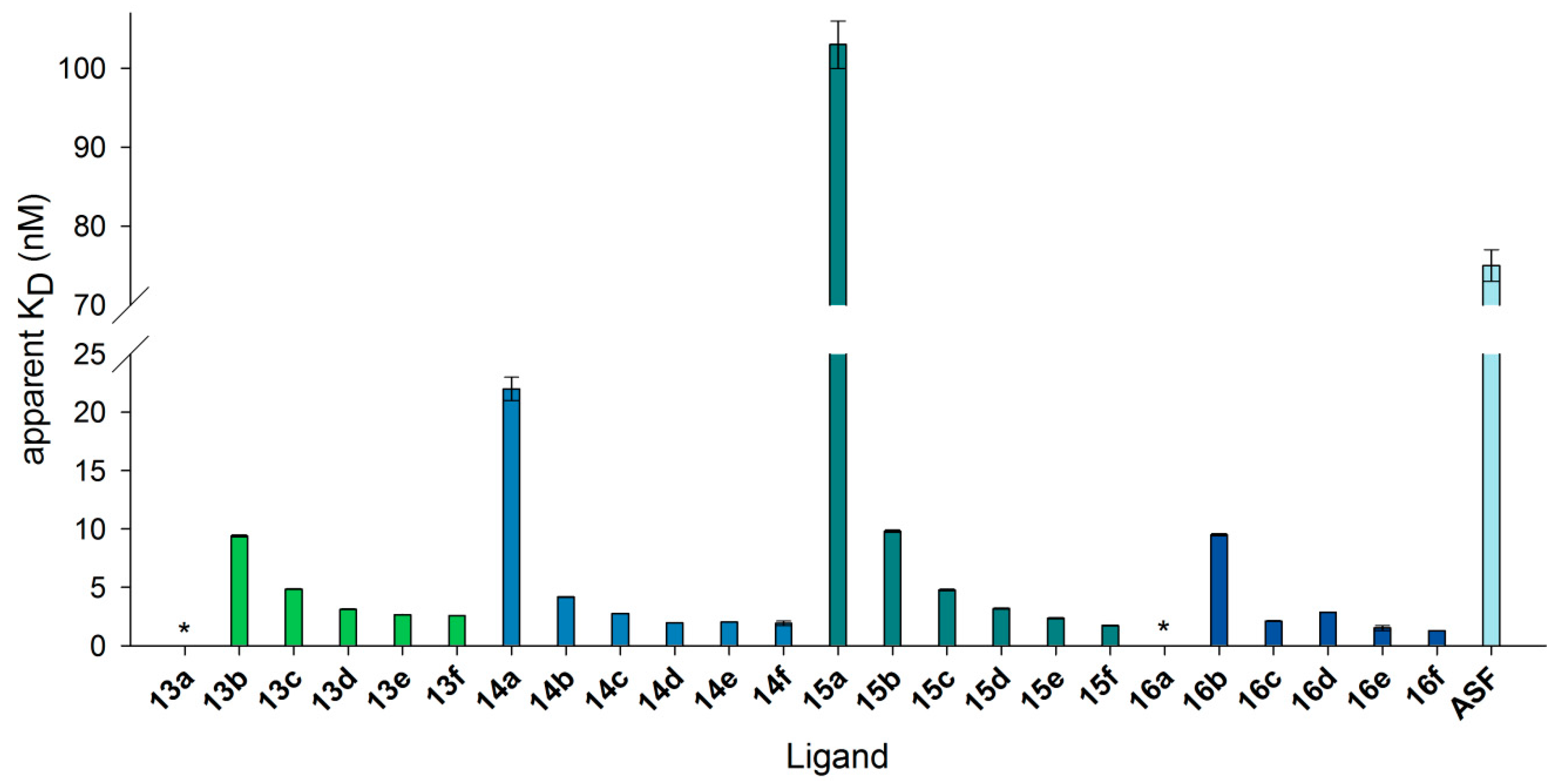
3.7. Neo-Glycoproteins as Galectin-3 Ligands in Surface Plasmon Resonance Spectroscopy
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Collins, B.E.; Paulson, J.C. Cell surface biology mediated by low affinity multivalent protein–glycan interactions. Curr. Opin. Chem. Biol. 2004, 8, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Gabius, H.-J. Cell surface glycans: The why and how of their functionality as biochemical signals in lectin-mediated information transfer. Crit. Rev. Immunol. 2006, 26, 43–79. [Google Scholar] [CrossRef] [PubMed]
- Gabius, H.-J. The magic of the sugar code. Trends Biochem. Sci. 2015, 40, 341. [Google Scholar] [CrossRef] [PubMed]
- Sasisekharan, R.; Myette, J.R. The sweet science of glycobiology: Complex carbohydrates, molecules that are particularly important for communication among cells, are coming under systematic study. Am. Sci. 2003, 91, 432–441. [Google Scholar] [CrossRef]
- Lis, H.; Sharon, N. Lectins: Carbohydrate-specific proteins that mediate cellular recognition. Chem. Rev. 1998, 98, 637–674. [Google Scholar] [CrossRef] [PubMed]
- Monsigny, M.; Mayer, R.; Roche, A.-C. Sugar-lectin interactions: Sugar clusters, lectin multivalency and avidity. Carbohydr. Lett. 2000, 4, 35–52. [Google Scholar] [PubMed]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Sharon, N. Bacterial lectins, cell-cell recognition and infectious disease. FEBS Lett. 1987, 217, 145–157. [Google Scholar] [CrossRef]
- Alvarez, C.P.; Lasala, F.; Carrillo, J.; Muñiz, O.; Corbí, A.L.; Delgado, R. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by ebola virus in cis and in trans. J. Virol. 2002, 76, 6841–6844. [Google Scholar] [CrossRef] [PubMed]
- Danguy, A.; Camby, I.; Kiss, R. Galectins and cancer. BBA Gen. Subj. 2002, 1572, 285–293. [Google Scholar] [CrossRef]
- Rabinovich, G.A. Galectins: An evolutionarily conserved family of animal lectins with multifunctional properties; a trip from the gene to clinical therapy. Cell Death Differ. 1999, 6, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Nangia-Makker, P.; Honjo, Y.; Sarvis, R.; Akahani, S.; Hogan, V.; Pienta, K.J.; Raz, A. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am. J. Pathol. 2000, 156, 899–909. [Google Scholar] [CrossRef]
- Dumic, J.; Dabelic, S.; Flögel, M. Galectin-3: An open-ended story. BBA Gen. Subj. 2006, 1760, 616–635. [Google Scholar] [CrossRef] [PubMed]
- Hittelet, A.; Legendre, H.; Nagy, N.; Bronckart, Y.; Pector, J.C.; Salmon, I.; Yeaton, P.; Gabius, H.J.; Kiss, R.; Camby, I. Upregulation of galectins-1 and-3 in human colon cancer and their role in regulating cell migration. Int. J. Cancer 2003, 103, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.K.; Dowling, C.A.; Jeng, K.C.G.; Chen, J.T.; Yang, R.Y.; Liu, F.T. Galectin-3 expression is induced in cirrhotic liver and hepatocellular carcinoma. Int. J. Cancer 1999, 81, 519–526. [Google Scholar] [CrossRef]
- Liu, F.-T.; Rabinovich, G.A. Galectins as modulators of tumour progression. Nat. Rev. Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Krześlak, A.; Lipińska, A. Galectin-3 as a multifunctional protein. Cell. Mol. Biol. Lett. 2004, 9, 305–328. [Google Scholar] [PubMed]
- Nakahara, S.; Oka, N.; Raz, A. On the role of galectin-3 in cancer apoptosis. Apoptosis 2005, 10, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, Y.; Fukumori, T.; Raz, A. Galectin-3 and metastasis. Glycoconj. J. 2002, 19, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Moon, B.-K.; Lee, Y.J.; Battle, P.; Jessup, J.M.; Raz, A.; Kim, H.-R.C. Galectin-3 protects human breast carcinoma cells against nitric oxide-induced apoptosis: Implication of galectin-3 function during metastasis. Am. J. Pathol. 2001, 159, 1055–1060. [Google Scholar] [CrossRef]
- Johnson, K.D.; Glinskii, O.V.; Mossine, V.V.; Turk, J.R.; Mawhinney, T.P.; Anthony, D.C.; Henry, C.J.; Huxley, V.H.; Glinsky, G.V.; Pienta, K.J. Galectin-3 as a potential therapeutic target in tumors arising from malignant endothelia. Neoplasia 2007, 9, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Nangia-Makker, P.; Nakahara, S.; Hogan, V.; Raz, A. Galectin-3 in apoptosis, a novel therapeutic target. J. Bioenerg. Biomembr. 2007, 39, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Mirandola, L.; Nguyen, D.D.; Rahman, R.L.; Grizzi, F.; Yuefei, Y.; Figueroa, J.A.; Jenkins, M.R.; Cobos, E.; Chiriva-Internati, M. Anti-galectin-3 therapy: A new chance for multiple myeloma and ovarian cancer? Int. Rev. Immunol. 2014, 33, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Salameh, B.A.; Leffler, H.; Nilsson, U.J. 3-(1, 2, 3-triazol-1-yl)-1-thio-galactosides as small, efficient, and hydrolytically stable inhibitors of galectin-3. Bioorg. Med. Chem. Lett. 2005, 15, 3344–3346. [Google Scholar] [CrossRef] [PubMed]
- Sörme, P.; Qian, Y.; Nyholm, P.G.; Leffler, H.; Nilsson, U.J. Low micromolar inhibitors of galectin-3 based on 3′-derivatization of N-acetyllactosamine. ChemBioChem 2002, 3, 183–189. [Google Scholar] [CrossRef]
- Ahmad, N.; Gabius, H.J.; Andre, S.; Kaltner, H.; Sabesan, S.; Roy, R.; Liu, B.C.; Macaluso, F.; Brewer, C.F. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J. Biol. Chem. 2004, 279, 10841–10847. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, J.; Kuno, A.; Hirabayashi, J.; Sato, S. Visualization of galectin-3 oligomerization on the surface of neutrophils and endothelial cells using fluorescence resonance energy transfer. J. Biol. Chem. 2007, 282, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Ochieng, J.; Green, B.; Evans, S.; James, O.; Warfield, P. Modulation of the biological functions of galectin-3 by matrix metalloproteinases. BBA Gen. Subj. 1998, 1379, 97–106. [Google Scholar] [CrossRef]
- Šimonová, A.; Kupper, C.E.; Böcker, S.; Müller, A.; Hofbauerová, K.; Pelantová, H.; Elling, L.; Křen, V.; Bojarová, P. Chemo-enzymatic synthesis of LacdiNAc dimers of varying length as novel galectin ligands. J. Mol. Catal. B Enzym. 2014, 101, 47–55. [Google Scholar] [CrossRef]
- Breloy, I.; Söte, S.; Ottis, P.; Bonar, D.; Grahn, A.; Hanisch, F.-G. O-linked LacdiNAc-modified glycans in extracellular matrix glycoproteins are specifically phosphorylated at the subterminal GlcNAc. J. Biol. Chem. 2012, 287, 18275–18286. [Google Scholar] [CrossRef] [PubMed]
- Kenny, D.T.; Skoog, E.C.; Lindén, S.K.; Struwe, W.B.; Rudd, P.M.; Karlsson, N.G. Presence of terminal N-acetylgalactosamineβ1–4N-acetylglucosamine residues on O-linked oligosaccharides from gastric MUC5AC: Involvement in Helicobacter pylori colonization? Glycobiology 2012, 22, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Shinomi, M.; Hirano, K.; Ui-Tei, K.; Nishihara, S. LacdiNAc (GalNAcβ1–4GlcNAc) contributes to self-renewal of mouse embryonic stem cells by regulating leukemia inhibitory factor/STAT3 signaling. Stem Cells 2011, 29, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, T.K.; Honing, H.; Franke, N.; van Remoortere, A.; Schiphorst, W.; Liu, F.T.; Deelder, A.M.; Cummings, R.D.; Hokke, C.H.; van Die, I. LacdiNAc-glycans constitute a parasite pattern for galectin-3-mediated immune recognition. J. Immunol. 2004, 173, 1902–1907. [Google Scholar] [CrossRef] [PubMed]
- Wuhrer, M.; Koeleman, C.A.M.; Deelder, A.M.; Hokke, C.H. Repeats of LacdiNAc and fucosylated LacdiNAc on N-glycans of the human parasite Schistosoma mansoni. FEBS J. 2006, 273, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Satoh, T.; Baba, S.; Yamashita, K. A1, 2-fucosylated and β-N-acetylgalactosaminylated prostate-specific antigen as an efficient marker of prostatic cancer. Glycobiology 2010, 20, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Kaya, T.; Kaneko, T.; Kojima, S.; Nakamura, Y.; Ide, Y.; Ishida, K.; Suda, Y.; Yamashita, K. High-sensitivity immunoassay with surface plasmon field-enhanced fluorescence spectroscopy using a plastic sensor chip: Application to quantitative analysis of total prostate-specific antigen and GalNAcβ1–4GlcNAc-linked prostate-specific antigen for prostate cancer diagnosis. Anal. Chem. 2015, 87, 1797–1803. [Google Scholar] [PubMed]
- Böcker, S.; Laaf, D.; Elling, L. Galectin binding to neo-glycoproteins: LacdiNAc conjugated BSA as ligand for human galectin-3. Biomolecules 2015, 5, 1671–1696. [Google Scholar] [CrossRef] [PubMed]
- Kupper, C.E.; Rosencrantz, R.R.; Henssen, B.; Pelantová, H.; Thönes, S.; Drozdova, A.; Křen, V.; Elling, L. Chemo-enzymatic modification of poly-N-acetyllactosamine (LacNAc) oligomers and N,N-diacetyllactosamine (LacdiNAc) based on galactose oxidase treatment. Beilstein J. Org. Chem. 2012, 8, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Sörme, P.; Kahl-Knutsson, B.; Wellmar, U.; Magnusson, B.-G.; Leffler, H.; Nilsson, U.J. Design and synthesis of galectin inhibitors. Methods Enzymol. 2003, 363, 157–169. [Google Scholar] [PubMed]
- Cumpstey, I.; Sundin, A.; Leffler, H.; Nilsson, U.J. C2-symmetrical thiodigalactoside bis-benzamido derivatives as high-affinity inhibitors of galectin-3: Efficient lectin inhibition through double arginine–arene interactions. Angew. Chem. 2005, 117, 5240–5242. [Google Scholar] [CrossRef]
- Kupper, C.E.; Böcker, S.; Liu, H.L.; Adamzyk, C.; van de Kamp, J.; Recker, T.; Lethaus, B.; Jahnen-Dechent, W.; Neuss, S.; Müller-Newen, G.; et al. Fluorescent SNAP-tag galectin fusion proteins as novel tools in glycobiology. Curr. Pharm. Des. 2013, 19, 5457–5467. [Google Scholar] [CrossRef] [PubMed]
- Witten, K.G.; Rech, C.; Eckert, T.; Charrak, S.; Richtering, W.; Elling, L.; Simon, U. Glyco-DNA–gold nanoparticles: Lectin-mediated assembly and dual-stimuli response. Small 2011, 7, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
- Wurm, F.R.; Klok, H.A. Be squared: Expanding the horizon of squaric acid-mediated conjugations. Chem. Soc. Rev. 2013, 42, 8220–8236. [Google Scholar] [CrossRef] [PubMed]
- Tietze, L.F.; Arlt, M.; Beller, M.; Glüsenkamp, K.-H.; Jähde, E.; Rajewsky, M.F. Anticancer agents, 15. Squaric acid diethyl ester: A new coupling reagent for the formation of drug biopolymer conjugates. Synthesis of squaric acid ester amides and diamides. Chem. Ber. 1991, 124, 1215–1221. [Google Scholar] [CrossRef]
- Hou, S.J.; Saksena, R.; Kovac, P. Preparation of glycoconjugates by dialkyl squarate chemistry revisited. Carbohydr. Res. 2008, 343, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Roth, Z.; Yehezkel, G.; Khalaila, I. Identification and quantification of protein glycosylation. Int. J. Carbohydr. Chem. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Jahouh, F.; Xu, P.; Vann, W.F.; Kovac, P.; Banoub, J.H. Mapping the glycation sites in the neoglycoconjugate from hexasaccharide antigen of Vibrio cholerae, serotype ogawa and the recombinant tetanus toxin C-fragment carrier. J. Mass Spectrom. 2013, 48, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, W.; Orwenyo, J.; Banerjee, A.; Vasta, G.R.; Wang, L.X. Design and synthesis of glycoprotein-based multivalent glyco-ligands for influenza hemagglutinin and human galectin-3. Bioorg. Med. Chem. 2013, 21, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Glinsky, V.V.; Landon, L.A.; Matthews, L.; Deutscher, S.L. Peptides specific to the galectin-3 carbohydrate recognition domain inhibit metastasis-associated cancer cell adhesion. Carcinogenesis 2005, 26, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Z.; Xia, B.Y.; Stowell, S.R.; Lasanajak, Y.; Smith, D.F.; Cummings, R.D. Novel fluorescent glycan microarray strategy reveals ligands for galectins. Chem. Biol. 2009, 16, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Stowell, S.R.; Arthur, C.M.; Mehta, P.; Slanina, K.A.; Blixt, O.; Leffler, H.; Smith, D.F.; Cummings, R.D. Galectin-1,-2, and-3 exhibit differential recognition of sialylated glycans and blood group antigens. J. Biol. Chem. 2008, 283, 10109–10123. [Google Scholar] [CrossRef] [PubMed]
- Stowell, S.R.; Dias-Baruffi, M.; Penttila, L.; Renkonen, O.; Nyame, A.K.; Cummings, R.D. Human galectin-1 recognition of poly-N-acetyllactosamine and chimeric polysaccharides. Glycobiology 2004, 14, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, A.; Stowell, S.; Blixt, O.; Cummings, R.D. Dimeric galectin-1 binds with high affinity to alpha 2,3-sialylated and non-sialylated terminal N-acetyllactosamine units on surface-bound extended glycans. J. Biol. Chem. 2005, 280, 5549–5562. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, E.M.; Andre, S.; Kurmyshkina, O.V.; Pochechueva, T.V.; Severov, V.V.; Pazynina, G.V.; Gabius, H.J.; Bovin, N.V. Galectin-loaded cells as a platform for the profiling of lectin specificity by fluorescent neoglycoconjugates: A case study on galectins-1 and-3 and the impact of assay setting. Glycobiology 2008, 18, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lucendo, M.F.; Solis, D.; Andre, S.; Hirabayashi, J.; Kasai, K.; Kaltner, H.; Gabius, H.J.; Romero, A. Growth-regulatory human galectin-1: Crystallographic characterisation of the structural changes induced by single-site mutations and their impact on the thermodynamics of ligand binding. J. Mol. Biol. 2004, 343, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, S.; Glushka, J.; Moremen, K.; Pierce, M. Enzymatic synthesis of natural and C-13 enriched linear poly-N-acetyllactosamines as ligands for galectin-1. Glycobiology 1999, 9, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Qun, Z.; Cummings, R.D. L-14 lectin recognition of laminin and its promotion of in vitro cell adhesion. Arch. Biochem. Biophys. 1993, 300, 6–17. [Google Scholar] [CrossRef]
- Diehl, C.; Engstrom, O.; Delaine, T.; Hakansson, M.; Genheden, S.; Modig, K.; Leffler, H.; Ryde, U.; Nilsson, U.J.; Akke, M. Protein flexibility and conformational entropy in ligand design targeting the carbohydrate recognition domain of galectin-3. J. Am. Chem. Soc. 2010, 132, 14577–14589. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, J.; Kanigsberg, A.; Slaaby, R.; Leffler, H.; Barondes, S.H.; Rini, J.M. X-ray crystal structure of the human galectin-3 carbohydrate recognition domain at 2.1-angstrom resolution. J. Biol. Chem. 1998, 273, 13047–13052. [Google Scholar] [CrossRef] [PubMed]
- Lobsanov, Y.D.; Gitt, M.A.; Leffler, H.; Barondes, S.H.; Rini, J.M. X-ray crystal structure of the human dimeric S-Lac lectin, L-14-II, in complex with lactose at 2.9-A resolution. J. Biol. Chem. 1993, 268, 27034–27038. [Google Scholar] [PubMed]
- Collins, P.M.; Bum-Erdene, K.; Yu, X.; Blanchard, H. Galectin-3 Interactions with Glycosphingolipids. J. Mol. Biol. 2014, 426, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Öberg, C.T.; Leffler, H.; Nilsson, U.J. Inhibition of galectins with small molecules. Chimia 2011, 65, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Pieters, R.J. Inhibition and detection of galectins. ChemBioChem 2006, 7, 721–728. [Google Scholar] [CrossRef] [PubMed]
- van Hattum, H.; Branderhorst, H.M.; Moret, E.E.; Nilsson, U.J.; Leffler, H.; Pieters, R.J. Tuning the preference of thiodigalactoside- and lactosamine-based ligands to galectin-3 over galectin-1. J. Med. Chem. 2013, 56, 1350–1354. [Google Scholar] [CrossRef] [PubMed]
- Marchiori, M.F.; Souto, D.E.; Bortot, L.O.; Pereira, J.F.; Kubota, L.T.; Cummings, R.D.; Dias-Baruffi, M.; Carvalho, I.; Campo, V.L. Synthetic 1,2,3-triazole-linked glycoconjugates bind with high affinity to human galectin-3. Bioorg. Med. Chem. 2015, 23, 3414–3425. [Google Scholar] [CrossRef] [PubMed]
- Dam, T.K.; Gabius, H.-J.; André, S.; Kaltner, H.; Lensch, M.; Brewer, C.F. Galectins bind to the multivalent glycoprotein asialofetuin with enhanced affinities and a gradient of decreasing binding constants. Biochemistry 2005, 44, 12564–12571. [Google Scholar] [CrossRef] [PubMed]
- Lundquist, J.J.; Toone, E.J. The cluster glycoside effect. Chem. Rev. 2002, 102, 555–578. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, L.L.; Young, T.; Gruber, T.D.; Mortell, K.H. Multivalency in protein–carbohydrate recognition. In Glycoscience; Fraser-Reid, B., Tatsuta, K., Thiem, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 2483–2523. [Google Scholar]
- Pieters, R.J. Maximising multivalency effects in protein-carbohydrate interactions. Org. Biomol. Chem. 2009, 7, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Pieters, R.J.; Arnusch, C.J.; Breukink, E. Membrane permeabilization by multivalent anti-microbial peptides. Protein Pept. Lett. 2009, 16, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Lepur, A.; Salomonsson, E.; Nilsson, U.J.; Leffler, H. Ligand induced galectin-3 protein self-association. J. Biol. Chem. 2012, 287, 21751–21756. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.K.; Wolfenden, M.L.; Nangia-Makker, P.; Michel, A.K.; Raz, A.; Cloninger, M.J. Multivalent scaffolds induce galectin-3 aggregation into nanoparticles. Beilstein J. Org. Chem. 2014, 10, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xue, X.C.; Jin, X.F.; Wang, L.J.; Sha, Y.L.; Li, Z.J. Synthesis of multivalent N-acetyl lactosamine modified quantum dots for the study of carbohydrate and galectin-3 interactions. Tetrahedron 2012, 68, 7148–7154. [Google Scholar] [CrossRef]
- Wolfenden, M.; Cousin, J.; Nangia-Makker, P.; Raz, A.; Cloninger, M. Glycodendrimers and modified ELISAs: Tools to elucidate multivalent interactions of galectins 1 and 3. Molecules 2015, 20, 7059–7096. [Google Scholar] [CrossRef] [PubMed]
- Téllez-Sanz, R.; Garcia-Fuentes, L.; Vargas-Berenguel, A. Human galectin-3 selective and high affinity inhibitors. Present state and future perspectives. Curr. Med. Chem. 2013, 20, 2979–2990. [Google Scholar] [CrossRef] [PubMed]
- Hakon, L.; Ulf, J.N. Low-molecular weight inhibitors of galectins. In Galectins and Disease Implications for Targeted Therapeutics; ACS: Washington, DC, USA, 2012; Volume 1115, pp. 47–59. [Google Scholar]
- Vrasidas, I.; Andre, S.; Valentini, P.; Bock, C.; Lensch, M.; Kaltner, H.; Liskamp, R.M.; Gabius, H.J.; Pieters, R.J. Rigidified multivalent lactose molecules and their interactions with mammalian galectins: A route to selective inhibitors. Org. Biomol. Chem. 2003, 1, 803–810. [Google Scholar] [CrossRef] [PubMed]
- André, S.; Sansone, F.; Kaltner, H.; Casnati, A.; Kopitz, J.; Gabius, H.J.; Ungaro, R. Calix[n]arene-based glycoclusters: Bioactivity of thiourea-linked galactose/lactose moieties as inhibitors of binding of medically relevant lectins to a glycoprotein and cell-surface glycoconjugates and selectivity among human adhesion/growth-regulatory galectins. ChemBioChem 2008, 9, 1649–1661. [Google Scholar] [PubMed]
- André, S.; Grandjean, C.; Gautier, F.M.; Bernardi, S.; Sansone, F.; Gabius, H.J.; Ungaro, R. Combining carbohydrate substitutions at bioinspired positions with multivalent presentation towards optimising lectin inhibitors: Case study with calixarenes. Chem. Commun. 2011, 47, 6126–6128. [Google Scholar] [CrossRef] [PubMed]
| Compound | MW (kDa) | ΔMW (kDa) | Modified Lysine Residues (mol/mol BSA) |
|---|---|---|---|
| BSA | 66.4 | 0.0 | 0.0 |
| 13a | 67.0 | 0.6 | 0.5 |
| 13b | 70.6 | 4.2 | 3.3 |
| 13c | 74.6 | 8.2 | 6.4 |
| 13d | 78.4 | 12.0 | 9.4 |
| 13e | 83.0 | 16.6 | 13.0 |
| 13f | 84.6 | 18.2 | 14.2 |
| 14a | 66.8 | 0.4 | 0.3 |
| 14b | 69.3 | 2.9 | 2.3 |
| 14c | 71.9 | 5.5 | 4.3 |
| 14d | 74.3 | 7.9 | 6.2 |
| 14e | 78.1 | 11.7 | 9.2 |
| 14f | 80.6 | 14.2 | 11.1 |
| Compound | IC50 (µM) | Relative Potency | Relative Potency per Glycan |
|---|---|---|---|
| 6 | 14.8 ± 2.06 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| 13b | 0.42 ± 0.18 | 35.2 ± 15.3 | 10.7 ± 4.6 |
| 13d | 0.03 ± 0.01 | 464.1 ± 83.7 | 49.4 ± 8.9 |
| 13e | 0.02 ± 0.01 | 856.0 ± 287.5 | 65.8 ± 22.1 |
| 7 | 7.66 ± 1.24 | 1.0 ± 0.2 | 1.0 ± 0.2 |
| 14b | 0.15 ± 0.06 | 50.3 ± 19.1 | 21.9 ± 8.3 |
| 14d | 0.04 ± 0.02 | 197.6 ± 84.8 | 31.9 ± 13.7 |
| 14e | 0.03 ± 0.00 | 297.9 ± 52.4 | 32.4 ± 5.7 |
| 6-Biotin LacNAc-LacNAc | 6-Biotin LacdiNAc-LacNAc | LacNAc-LacNAc | LacdiNAc-LacNAc | ||||
|---|---|---|---|---|---|---|---|
| 13a | 0.5 | 14a | 0.3 | 15a | 1.6 | 16a | 1.7 |
| 13b | 3.3 | 14b | 2.3 | 15b | 7.5 | 16b | 7.5 |
| 13c | 6.4 | 14c | 4.3 | 15c | 14.4 | 16c | 14.1 |
| 13d | 9.4 | 14d | 6.2 | 15d | 17.8 | 16d | 18.0 |
| 13e | 13.0 | 14e | 9.2 | 15e | 24.2 | 16e | 24.4 |
| 13f | 14.2 | 14f | 11.1 | 15f | 29.0 | 16f | 27.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Böcker, S.; Elling, L. Biotinylated N-Acetyllactosamine- and N,N-Diacetyllactosamine-Based Oligosaccharides as Novel Ligands for Human Galectin-3. Bioengineering 2017, 4, 31. https://doi.org/10.3390/bioengineering4020031
Böcker S, Elling L. Biotinylated N-Acetyllactosamine- and N,N-Diacetyllactosamine-Based Oligosaccharides as Novel Ligands for Human Galectin-3. Bioengineering. 2017; 4(2):31. https://doi.org/10.3390/bioengineering4020031
Chicago/Turabian StyleBöcker, Sophia, and Lothar Elling. 2017. "Biotinylated N-Acetyllactosamine- and N,N-Diacetyllactosamine-Based Oligosaccharides as Novel Ligands for Human Galectin-3" Bioengineering 4, no. 2: 31. https://doi.org/10.3390/bioengineering4020031
APA StyleBöcker, S., & Elling, L. (2017). Biotinylated N-Acetyllactosamine- and N,N-Diacetyllactosamine-Based Oligosaccharides as Novel Ligands for Human Galectin-3. Bioengineering, 4(2), 31. https://doi.org/10.3390/bioengineering4020031