Metabolic Engineering of the Phenylpropanoid and Its Primary, Precursor Pathway to Enhance the Flavor of Fruits and the Aroma of Flowers
Abstract
:1. Introduction
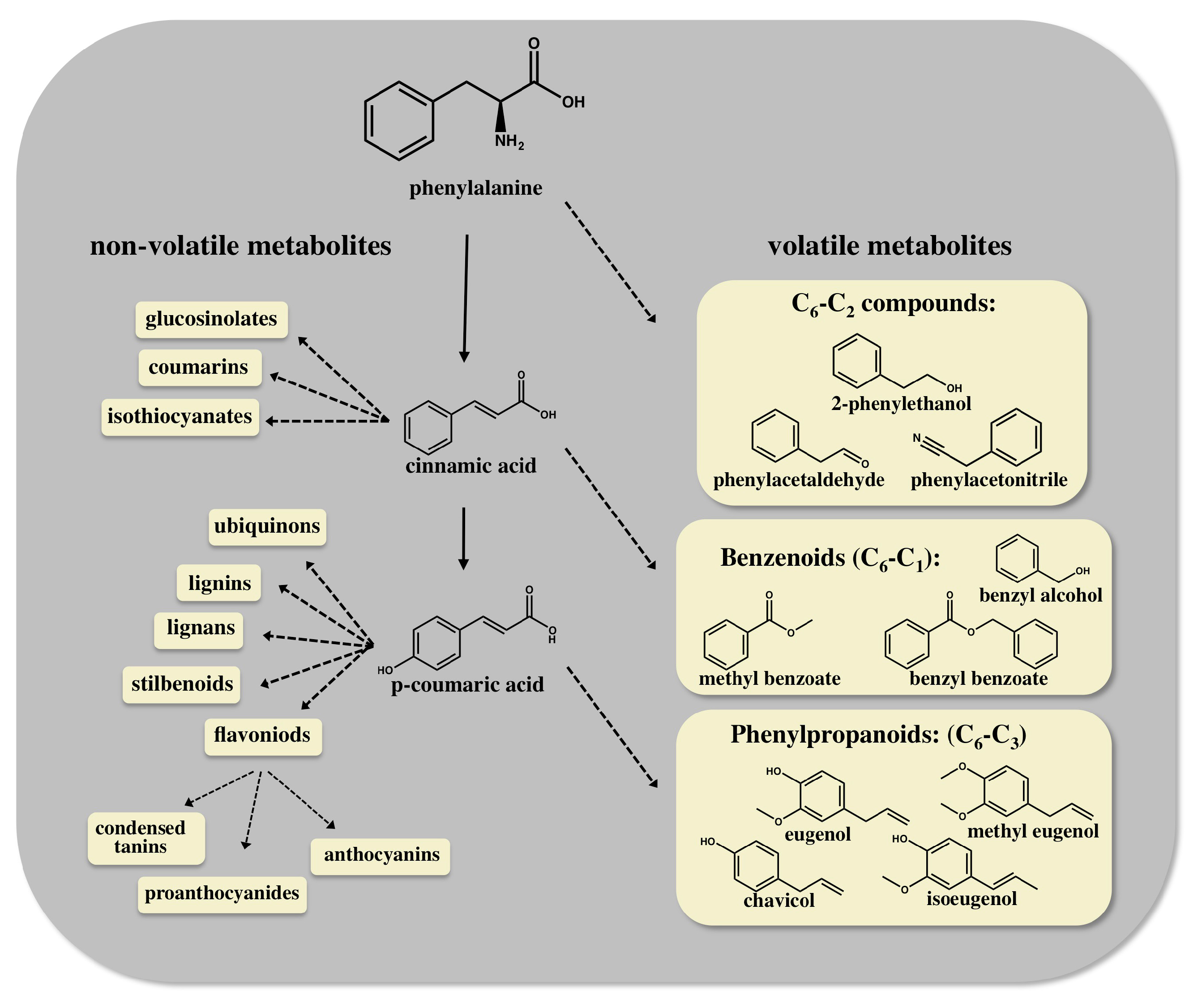
2. Enhancing Aroma and Flavor by Metabolic Engineering of the AAA Derived Pathways
3. Enhancing Aroma and Flavor by Metabolic Engineering of AAA Biosynthetic Pathways
3.1. Targeting the Shikimate Pathway (AroG*)
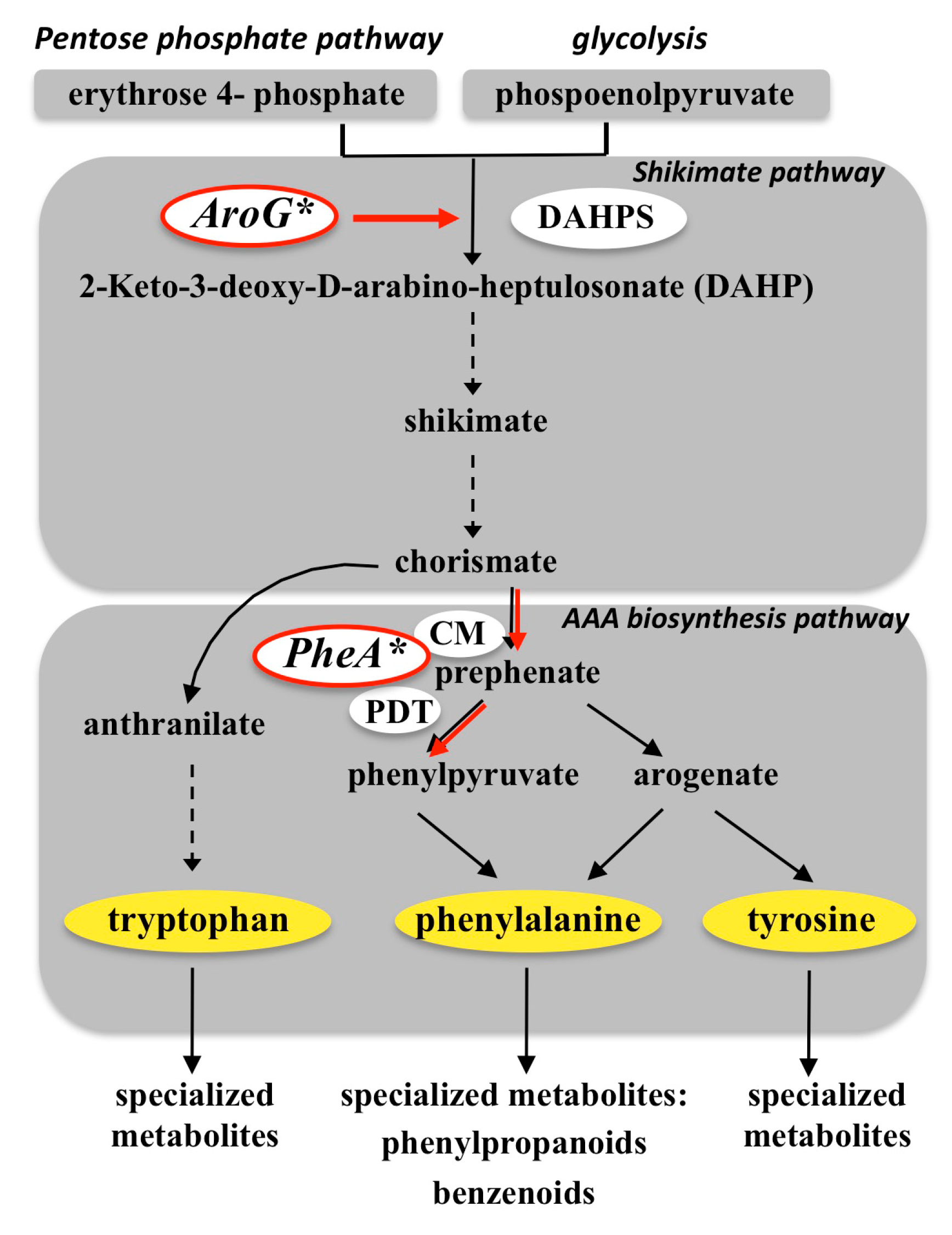
3.2. Targeting Phenylalanine Biosynthesis (PheA*)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pichersky, E.; Lewinsohn, E. Convergent Evolution in Plant Specialized Metabolism. Annu. Rev. Plant Biol. 2011, 62, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, I.T. Plant volatiles. Curr. Biol. 2010, 20, R392–R397. [Google Scholar] [CrossRef] [PubMed]
- Raguso, R.A. Wake up and smell the roses: The ecology and evolution of floral scent. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 549–569. [Google Scholar] [CrossRef]
- Pierik, R.; Ballaré, C.L.; Dicke, M. Ecology of plant volatiles: Taking a plant community perspective. Plant Cell Environ. 2014, 37, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Aragüez, I.; Valpuesta, V. Metabolic engineering of aroma components in fruits. Biotechnol. J. 2013, 8, 1144–1158. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, D.; Pichersky, E. Metabolic engineering of plant volatiles. Curr. Opin. Biotechnol. 2008, 19, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Klee, H.J. Improving the flavor of fresh fruits: Genomics, biochemistry, and biotechnology. New Phytol. 2010, 187, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Tzin, V.; Galili, G. New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Mol. Plant 2010, 3, 956–972. [Google Scholar] [CrossRef] [PubMed]
- Muhlemann, J.K.; Klempien, A.; Dudareva, N. Floral volatiles: From biosynthesis to function. Plant Cell Environ. 2014, 37, 1936–1949. [Google Scholar] [CrossRef] [PubMed]
- Schuurink, R.C.; Haring, M.A.; Clark, D.G. Regulation of volatile benzenoid biosynthesis in petunia flowers. Trends Plant Sci. 2006, 11, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Tieman, D.; Taylor, M.; Schauer, N.; Fernie, A.R.; Hanson, A.D.; Klee, H.J. Tomato aromatic amino acid decarboxylases participate in synthesis of the flavor volatiles 2-phenylethanol and 2-phenylacetaldehyde. Proc. Natl. Acad. Sci. USA 2006, 103, 8287–8292. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.; Kurtzer, R.; Skowranek, K.; Kiessling, P.; Fridman, E.; Pichersky, E.; Schwab, W. Metabolic engineering in strawberry fruit uncovers a dormant biosynthetic pathway. Metab. Eng. 2011, 13, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Lunkenbein, S.; Coiner, H.; de Vos, C.H.R.; Schaart, J.G.; Boone, M.J.; Krens, F.A.; Schwab, W.; Salentijn, E.M. Molecular characterization of a stable antisense chalcone synthase phenotype in strawberry (Fragaria × ananassa). J. Agric. Food Chem. 2006, 54, 2145–2153. [Google Scholar] [CrossRef] [PubMed]
- Zvi, M.M.; Shklarman, E.; Masci, T.; Kalev, H.; Debener, T.; Shafir, S.; Ovadis, M.; Vainstein, A. PAP1 transcription factor enhances production of phenylpropanoid and terpenoid scent compounds in rose flowers. New Phytol. 2012, 195, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Aranovich, D.; Lewinsohn, E.; Zaccai, M. Post-harvest enhancement of aroma in transgenic lisianthus (Eustoma grandiflorum) using the Clarkia breweri benzyl alcohol acetyltransferase (BEAT) gene. Postharvest Biol. Technol. 2007, 43, 255–260. [Google Scholar] [CrossRef]
- Beekwilder, J.; Alvarez-Huerta, M.; Neef, E.; Verstappen, F.W.A.; Bouwmeester, H.J.; Aharoni, A. Functional characterization of enzymes forming volatile esters from strawberry and banana. Plant Physiol. 2004, 135, 1865–1878. [Google Scholar] [CrossRef] [PubMed]
- Guterman, I.; Masci, T.; Chen, X.L.; Negre, F.; Pichersky, E.; Dudareva, N.; Weiss, D.; Vainstein, A. Generation of phenylpropanoid pathway derived volatiles in transgenic plants: Rose alcohol acetyltransferase produces phenylethyl acetate and benzyl acetate in petunia flowers. Plant Mol. Biol. 2006, 60, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Lücker, J.; Bouwmeester, H.J.; Schwab, W.; Blaas, J.; van der Plas, L.H.W.; Verhoeven, H.A. Expression of Clarkia S-linalool synthase in transgenic petunia plants results in the accumulation of S-linalyl-beta-D-glucopyranosid. Plant J. 2001, 27, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Lavy, M.; Zuker, A.; Lewinsohn, E.; Larkov, O.; Ravid, U.; Vainstein, A.; Weiss, D. Linalool and linalool oxide production in transgenic carnation flowers expressing the Clarkia breweri linalool synthase gene. Mol. Breed. 2002, 9, 103–111. [Google Scholar] [CrossRef]
- Tzin, V.; Malitsky, S.; Aharoni, A.; Galili, G. Expression of a bacterial bi-functional chorismate mutase/prephenate dehydratase modulates primary and secondary metabolism associated with aromatic amino acids in Arabidopsis. Plant J. 2009, 60, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Tzin, V.; Malitsky, S.; Ben Zvi, M.M.; Bedair, M.; Sumner, L.; Aharoni, A.; Galili, G. Expression of a bacterial feedback-insensitive 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase of the shikimate pathway in Arabidopsis elucidates potential metabolic bottlenecks between primary and secondary metabolism. New Phytol. 2012, 194, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.; Ovadia, R.; Perl, A.; Bar, E.; Lewinsohn, E.; Galili, G.; Oren-Shamir, M. Enhanced formation of aromatic amino acids increases fragrance without affecting flower longevity or pigmentation in Petunia × hybrida. Plant Biotechnol. J. 2015, 13, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Tzin, V.; Rogachev, I.; Meir, S.; Moyal Ben Zvi, M.; Masci, T.; Vainstein, A.; Aharoni, A.; Galili, G. Tomato fruits expressing a bacterial feedback-insensitive 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase of the shikimate pathway possess enhanced levels of multiple specialized metabolites and upgraded aroma. J. Exp. Bot. 2013, 64, 4441–4452. [Google Scholar] [CrossRef] [PubMed]
- Tzin, V.; Rogachev, I.; Meir, S.; Moyal Ben Zvi, M.; Masci, T.; Vainstein, A.; Aharoni, A.; Galili, G. Altered levels of aroma and volatiles by metabolic engineering of shikimate pathway genes in tomato fruits. AIMS Bioengin. 2015, 2, 75–92. [Google Scholar] [CrossRef]
- Manela, N.; Oliva, M.; Ovadia, R.; Sikron-Persi, N.; Ayenew, B.; Fait, A.; Galili, G.; Perl, A.; Weiss, D.; Oren-Shanir, M. Phenylalanine and tyrosine levels are rate-limiting factors in production of health promoting metabolites in Vitis vinifera cv. Gamay Red cell suspension. Front. Plant Sci. 2015, 6, 538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Butelli, E.; Alseekh, S.; Tohge, T.; Rallapalli, G.; Luo, J.; Kawar, P.G.; Hill, L.; Santino, A.; Fernie, A.R.; et al. Multi-level engineering facilitates the production of phenylpropanoid compounds in tomato. Nat. Commun. 2015, 26, 8635. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Butelli, E.; Hill, L.; Parr, A.; Niggeweg, R.; Bailey, P.; Weisshaar, B.; Martin, C. AtMYB12 regulates caffeoyl quinic acid and flavonol synthesis in tomato: Expression in fruit results in very high levels of both types of polyphenol. Plant J. 2008, 56, 316–326. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peled-Zehavi, H.; Oliva, M.; Xie, Q.; Tzin, V.; Oren-Shamir, M.; Aharoni, A.; Galili, G. Metabolic Engineering of the Phenylpropanoid and Its Primary, Precursor Pathway to Enhance the Flavor of Fruits and the Aroma of Flowers. Bioengineering 2015, 2, 204-212. https://doi.org/10.3390/bioengineering2040204
Peled-Zehavi H, Oliva M, Xie Q, Tzin V, Oren-Shamir M, Aharoni A, Galili G. Metabolic Engineering of the Phenylpropanoid and Its Primary, Precursor Pathway to Enhance the Flavor of Fruits and the Aroma of Flowers. Bioengineering. 2015; 2(4):204-212. https://doi.org/10.3390/bioengineering2040204
Chicago/Turabian StylePeled-Zehavi, Hadas, Moran Oliva, Qingjun Xie, Vered Tzin, Michal Oren-Shamir, Asaph Aharoni, and Gad Galili. 2015. "Metabolic Engineering of the Phenylpropanoid and Its Primary, Precursor Pathway to Enhance the Flavor of Fruits and the Aroma of Flowers" Bioengineering 2, no. 4: 204-212. https://doi.org/10.3390/bioengineering2040204
APA StylePeled-Zehavi, H., Oliva, M., Xie, Q., Tzin, V., Oren-Shamir, M., Aharoni, A., & Galili, G. (2015). Metabolic Engineering of the Phenylpropanoid and Its Primary, Precursor Pathway to Enhance the Flavor of Fruits and the Aroma of Flowers. Bioengineering, 2(4), 204-212. https://doi.org/10.3390/bioengineering2040204





