Systematic Review of Artificial Intelligence and Electrocardiography for Cardiovascular Disease Diagnosis
Abstract
1. Introduction
- Conducting a systematic review based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [19], synthesizing over 100 studies published between 2019 and 2025 on the applications of AI in ECG analysis, covering a spectrum of cardiovascular conditions, including heart failure (HF), myocardial infarction (MI), atrial fibrillation (AF), and stress or anomaly detection;
- Classifying AI-ECG applications in detail by disease type, model architecture, and methodological approach, highlighting convergences and divergences across the literature;
- Comparing the performances of different AI-based methods through comprehensive tables that identify methodological regularities and shared diagnostic outcomes across studies;
- Critically evaluating persistent challenges such as data bias, signal noise, lack of model explainability, and demographic inequities, while offering actionable recommendations for future research and clinical translation; and
- Incorporating visual and tabular formats of data presentation to facilitate interpretation by clinical and technical audiences.
2. Methods
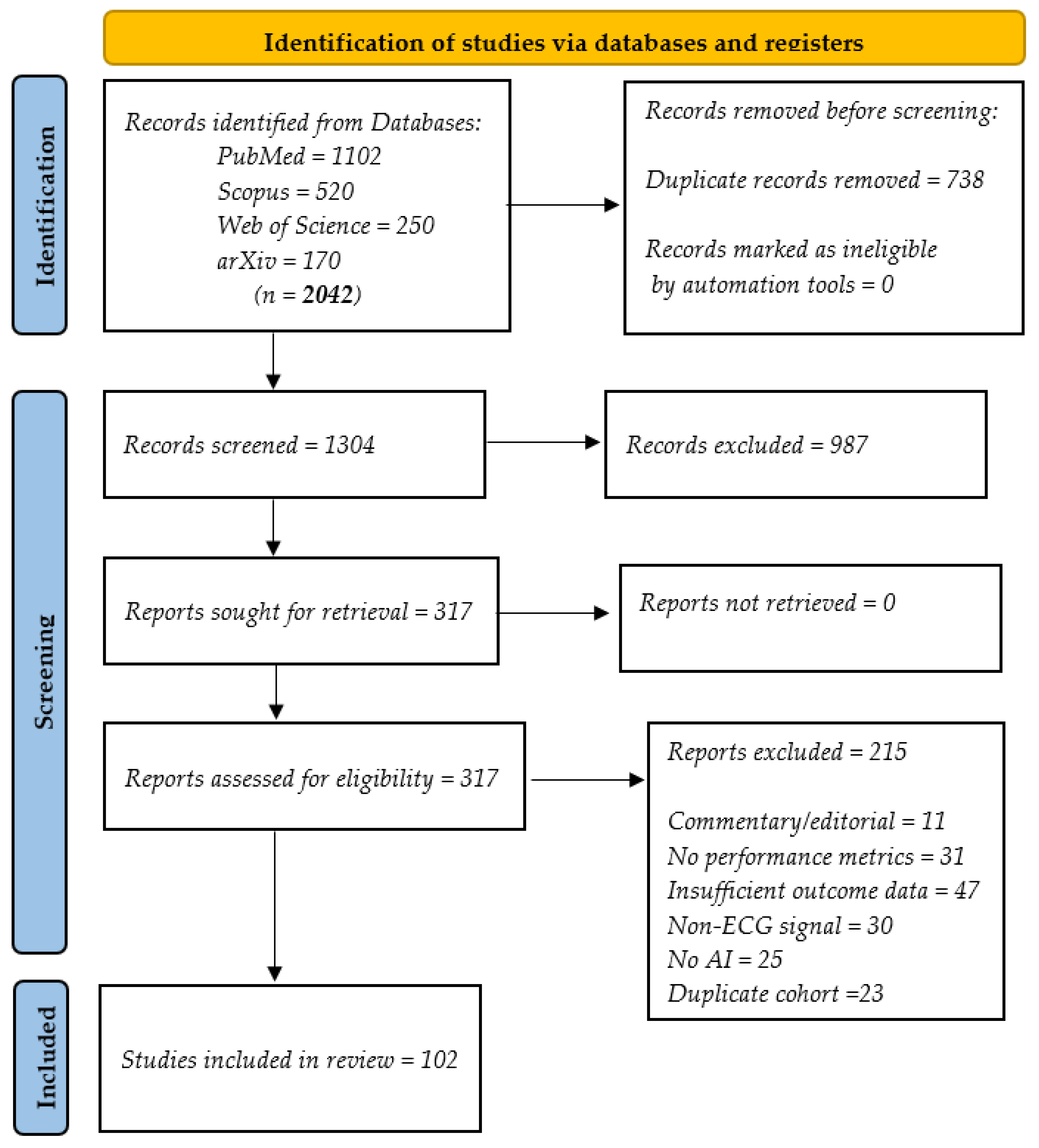
2.1. Selection of Resources
- Absence of ECG data
- Lack of AI or ML methods
- Non-original research (reviews, editorials, and commentaries)
- Animal/in vitro studies
- Insufficient performance or outcome data
- Non-English articles
2.2. Eligibility Criteria
- Original studies applying any AI, ML, DL, transformer-based, hybrid, or explainable AI (XAI) technique to 12-lead, single-lead, or wearable ECG data in human adults (≥18 years).
- Studies reporting diagnostic or prognostic performance (e.g., area under the curve [AUC], sensitivity, specificity, F-score, C-index, etc.).
- Articles that explicitly describe the datasets used for model training and/or testing.
- Publications available in English or Spanish with full text accessible.
- Conference abstracts, letters, editorials, reviews, or protocols without full primary data.
- In vivo, in silico, or phantom studies.
- Studies lacking clinical reference standards (including cardiologist adjudication, imaging, catheterization, or follow-up).
- Duplicate publications reporting the same cohort without new methodological or outcome data.
2.3. Sources of Data
3. Results
- Detection of occult structural heart disease,
- Diagnosis of arrhythmias,
- Risk stratification for HF and acute coronary syndromes, and
- Synthetic ECG generation for data augmentation and model training.
- AI-ECG applications across use cases
- Emerging applications: stress detection, real-life monitoring, and anomaly recognition
- Synthetic ECG generation using deep generative models (DGMs)
- Clinical validation studies and implementation challenges
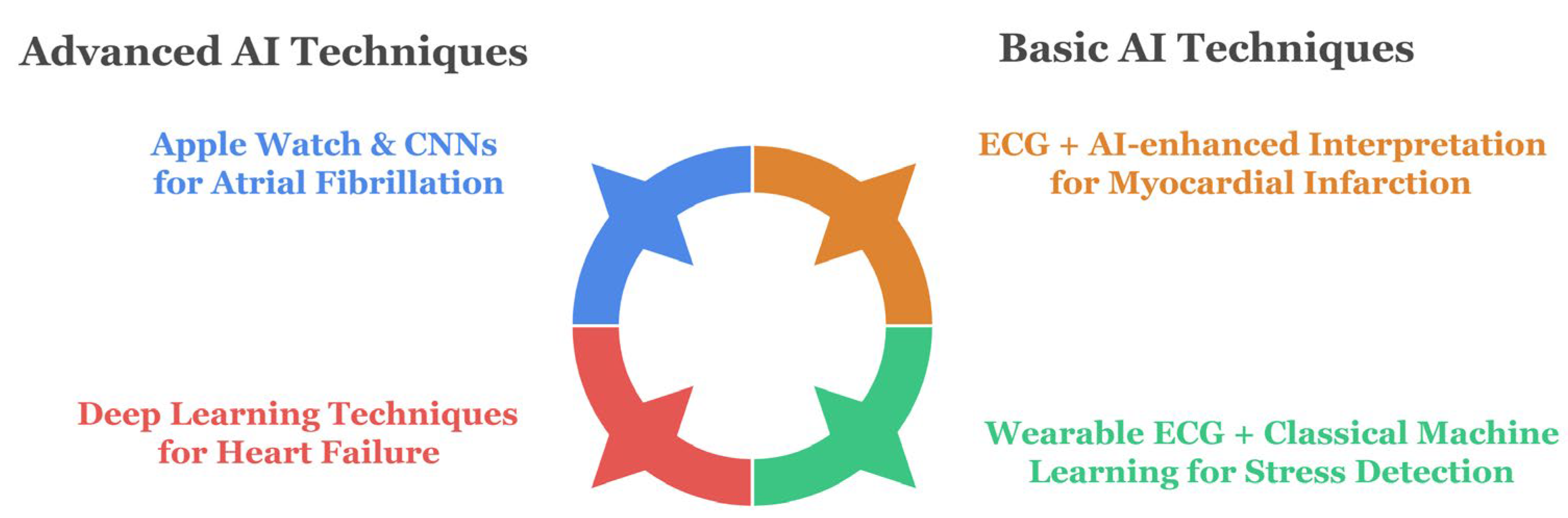
3.1. AI-ECG Applications Across Use Cases
3.1.1. Clinical Diagnostics by Disease Type
Atrial Fibrillation Detection
Myocardial Infarction Identification
- Huérfano-Maldonado et al. [18] identified CNN architectures in multimodal settings.
- Yu et al. [9] proposed a “subtraction ECG” approach for dynamic serial analysis, showing that this method improves early diagnosis compared with static ECG evaluation.
- Gautam et al. [29] explored context-independent ML models that can detect MI even in atypical presentations, highlighting their potential in generalized deployment, though their study focused broadly on HF and remote monitoring.
- Elvas et al. [33] demonstrated improved sensitivity for non-ST elevation infarctions by integrating 12-lead ECG with echocardiographic features.
- Sun et al. [34] applied ensemble learning techniques to prehospital ECGs, enabling earlier ischemia recognition in out-of-hospital settings.
- Tao et al. [35] used magnetocardiography and AI to detect ischemia and coronary stenosis in patients without classical ECG changes.
- Finally, Lefebvre and Hoekstra [36] emphasized the need for next-generation ECG techniques, such as body surface mapping, to improve sensitivity in the detection of early MI.
Heart Failure Monitoring
Stress and Anomaly Detection
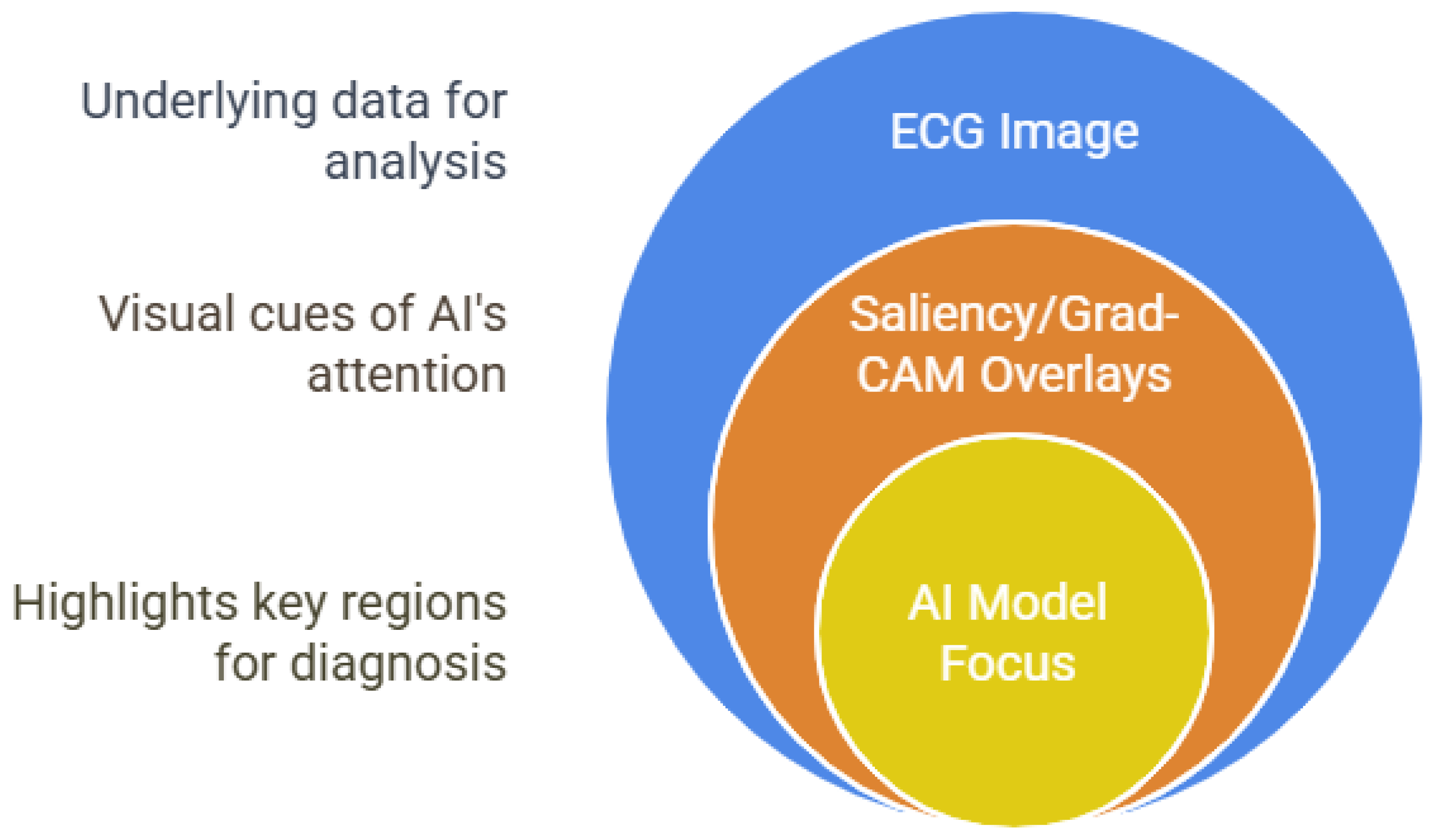
3.1.2. Explainable AI for ECG Diagnosis
3.2. Emerging Applications: Stress, Real-Life Monitoring, and Anomaly Detection
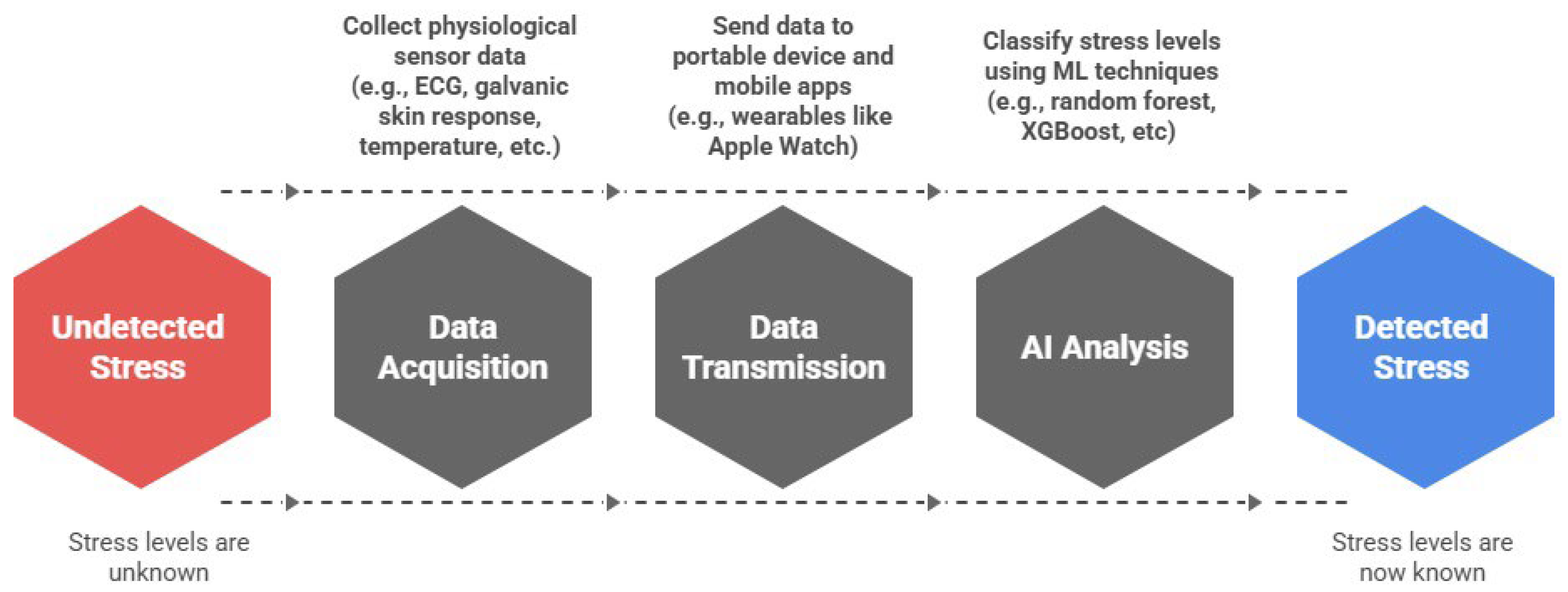
3.2.1. Stress Detection in Daily Life Using Wearable ECG
- Utilizing multimodal sensor fusion, combining ECG with galvanic skin response, temperature, and motion data for more robust detection;
- Validating models in ecologically valid, real-world contexts, rather than artificial experimental setups; and
- Adopting advanced ML models that outperform traditional statistical techniques in terms of sensitivity and generalizability.
- Promote standardized, multiethnic datasets to evaluate generalizability across global populations [74];
- Investigate adaptive learning models capable of updating in response to user feedback or contextual changes; and
- Develop consensus on validation protocols and clinical benchmarks, such as those established in cardiovascular AI research.
3.2.2. Real-Time Anomaly Detection with Tailored Deep Learning Models
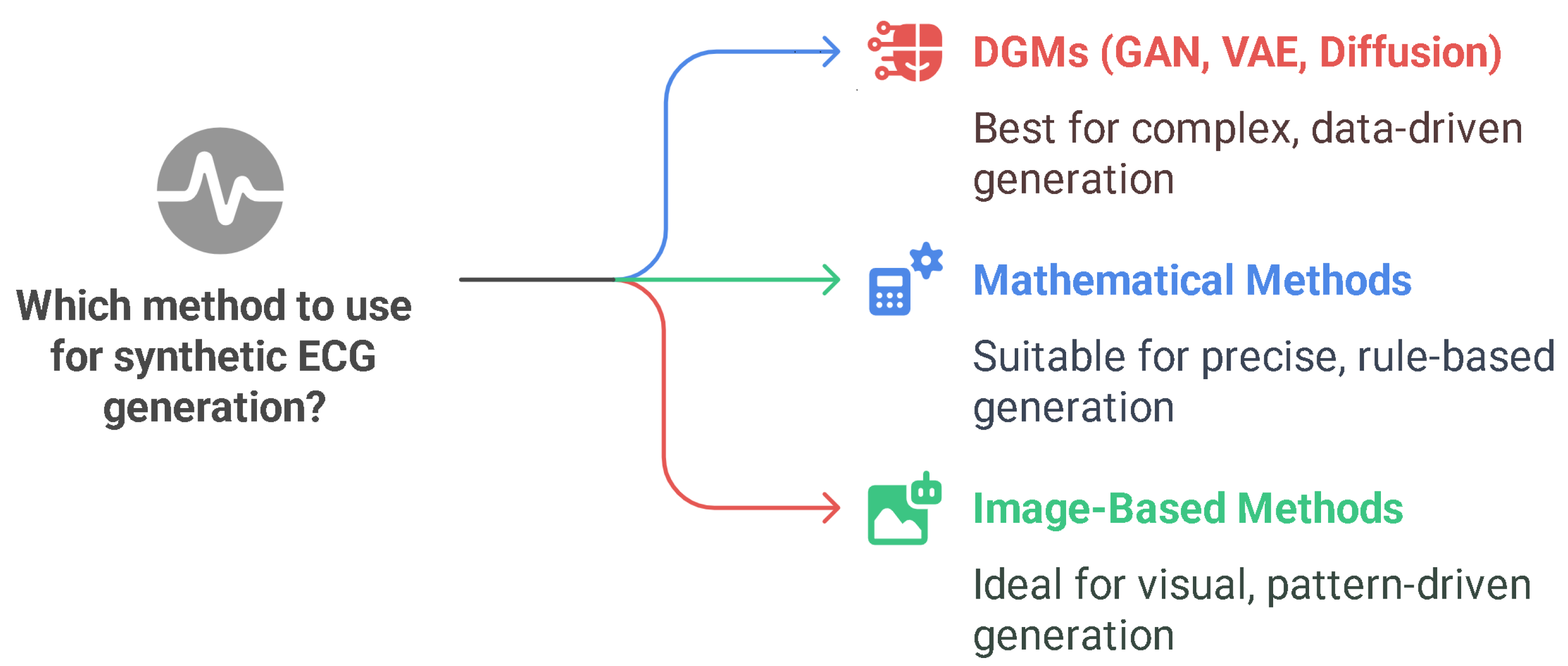
3.3. Synthetic ECG Generation with Deep Generative Models
- Deep Generative Models: Realism and Data-Driven Innovation
- Mathematical and Image-Based Approaches
- Comparative Evaluation and Validation Metrics
- Synthetic ECGs for Privacy Preservation and Regulatory Compliance
- Technical Advancements and Tailored Synthesis
- Current Gaps and Future Directions
3.4. Clinical Validation and Implementation Challenges
3.4.1. Generalizability and Bias
3.4.2. Infrastructure and Usability
3.4.3. Regulatory Frameworks
4. Discussion
- Systematic and Comprehensive Scope
- 2.
- Multi-Disease Coverage
- 3.
- Comparative Model Performance
- 4.
- XAI and Interpretability
- 5.
- Synthetic ECG Generation
- 6.
- Wearables and Real-World Signal Variability
- 7.
- Noise Reduction and Signal Preprocessing
- 8.
- Bias, Fairness, and Equity
- 9.
- Clinical Visualization and Recommendations
- 10.
- Integrative Synthesis Across Domains
5. Conclusions and Recommendations
- Broad Coverage of Applications: It offered a detailed classification of AI-ECG applications by disease, model architecture, and methodology, explicitly highlighting the convergences (e.g., the dominance of CNN-based models for arrhythmia and HF detection) and divergences (e.g., the impact of dataset diversity and model explainability) in the literature.
- Comparative Analyses: Through comparative tables and conceptual figures, this review highlighted regularities in performance metrics, methodological trends, and points of agreement among leading research groups, providing a practical resource for clinicians, engineers, and policymakers.
- Multidisciplinary Perspective: In contrast to previous reviews, which often focus narrowly on arrhythmia detection ELMs or synthetic data, this work delivered a broad, up-to-date, and critical synthesis encompassing clinical, technical, and implementation perspectives. This breadth is essential for understanding the real-world challenges and opportunities related to AI-ECG integration.
- Emerging Applications: The use of AI for stress detection and anomaly identification in real-life settings, as well as the generation of synthetic ECG data via GANs, VAEs, and diffusion models, is expanding the clinical and research scope of ECG analytics [48,110]. Continuous monitoring of stress through wearables and personalized anomaly detection systems represents a frontier that could transform preventive care [75,110].
- Explainability and Fairness: The integration of XAI tools (e.g., saliency maps, Grad-CAM, etc.) is improving transparency and clinical trust, but persistent dataset bias and lack of demographic diversity threaten generalizability and equity [7,61]. Without deliberate efforts to include diverse populations and to provide interpretability, the full benefits of AI-ECG may not be realized for all patient groups [66].
- Implementation Barriers: Real-world deployment is hindered by limited external validation, regulatory uncertainty, and challenges in EHR integration and clinician training. Many AI-ECG algorithms, though promising in research, have yet to be tested in prospective clinical trials or integrated into routine workflows [7,64].
- Standardize and Diversify Datasets
- 2.
- Advance Explainability
- 3.
- Promote Hybrid and Multimodal Models
- 4.
- Strengthen Regulatory and Validation frameworks
- 5.
- Facilitate Continuous Learning and Feedback
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, Y.; Fan, Z.; Li, J.; Zeng, Z.; Lu, G.; Lin, J.; Zhou, L.; Wu, T.; Cao, Q. Deep Learning-Based 12-Lead Electrocardiogram for Low Left Ventricular Ejection Fraction Detection in Patients. Can. J. Cardiol. 2024, 41, 278–290. [Google Scholar] [CrossRef]
- Lee, H.; Han, G.; Kim, K.; Kang, S.; Jang, J.; Jo, Y.; Son, J.; Lee, M.; Kwon, J.; Oh, B. Electrocardiographic-Driven Artificial Intelligence Model: A New Approach to Predicting One-Year Mortality in Heart Failure with Reduced Ejection Fraction Patients. Int. J. Med. Inform. 2025, 197, 105843. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cardiovascular Diseases (CVDs) [Internet]. WHO Fact Sheet. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 11 July 2025).
- McLaren, J.; de Alencar, J.; Aslanger, E.; Meyers, H.; Smith, S. From ST-Segment Elevation MI to Occlusion MI: The New Paradigm Shift in Acute Myocardial Infarction. JACC Adv. 2024, 3, 101314. [Google Scholar] [CrossRef] [PubMed]
- Dorraki, M.; Liao, Z.; Abbott, D.; Psaltis, P.; Baker, E.; Bidargaddi, N.; Wardill, H.; van den Hengel, A.; Narula, J.; Verjans, J. Improving Cardiovascular Disease Prediction with Machine Learning Using Mental Health Data: A Prospective UK Biobank Study. JACC Adv. 2024, 3 Pt 2, 101180. [Google Scholar] [CrossRef] [PubMed]
- Monfredi, O.; Moore, C.; Sullivan, B.; Keim-Malpass, J.; Fairchild, K.; Loftus, T.; Bihorac, A.; Krahn, K.; Dubrawski, A.; Lake, D.; et al. Continuous ECG Monitoring Should Be the Heart of Bedside AI-Based Predictive Analytics Monitoring for Early Detection of Clinical Deterioration. J. Electrocardiol. 2023, 76, 35–38. [Google Scholar] [CrossRef]
- Shahid, S.; Iqbal, M.; Saeed, H.; Hira, S.; Batool, A.; Khalid, S.; Tahirkheli, N. Diagnostic Accuracy of Apple Watch Electrocardiogram for Atrial Fibrillation: A Systematic Review and Meta-Analysis. JACC Adv. 2025, 4, 101538. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, M.; Li, Y.; Gao, J.; Sun, Y.; Liu, F.; Wei, S. A Dual-Path Interactive ECG Denoising Algorithm. In Proceedings of the 2024 17th International Congress on Image and Signal Processing, BioMedical Engineering and Informatics (CISP-BMEI), Shanghai, China, 26–27 October 2024; pp. 1–6. [Google Scholar] [CrossRef]
- Yu, K.; Feng, L.; Chen, Y.; Wu, M.; Zhang, Y.; Zhu, P.; Chen, W.; Wu, Q.; Hao, J. Accurate Wavelet Thresholding Method for ECG Signals. Comput. Biol. Med. 2024, 169, 107835. [Google Scholar] [CrossRef]
- Madan, P.; Singh, V.; Singh, D.; Diwakar, M.; Pant, B.; Kishor, A. A Hybrid Deep Learning Approach for ECG-Based Arrhythmia Classification. Bioengineering 2022, 9, 152. [Google Scholar] [CrossRef]
- Abd Al-Alim, M.; Mubarak, R.; Salem, N.; Sadek, I. A Machine-Learning Approach for Stress Detection Using Wearable Sensors in Free-Living Environments. Comput. Biol. Med. 2024, 179, 108918. [Google Scholar] [CrossRef]
- Zanchi, B.; Monachino, G.; Fiorillo, L.; Conte, G.; Auricchio, A.; Tzovara, A.; Faraci, F. Synthetic ECG Signals Generation: A Scoping Review. Comput. Biol. Med. 2025, 184, 109453. [Google Scholar] [CrossRef]
- Kapsecker, M.; Möller, M.; Jonas, S. Disentangled Representational Learning for Anomaly Detection in Single-Lead Electrocardiogram Signals Using Variational Autoencoder. Comput. Biol. Med. 2025, 184, 109422. [Google Scholar] [CrossRef]
- Amann, J.; Blasimme, A.; Vayena, E.; Frey, D.; Madai, V.I.; Precise4Q consortium. Explainability for Artificial Intelligence in Healthcare: A Multidisciplinary Perspective. BMC Med. Inform. Decis. Mak. 2020, 20, 310. [Google Scholar] [CrossRef] [PubMed]
- Ayano, Y.; Schwenker, F.; Dufera, B.; Debelee, T. Interpretable Machine Learning Techniques in ECG-Based Heart Disease Classification: A Systematic Review. Diagnostics 2023, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Agarwal, C.; Kaur, I.; Gupta, P. Machine Learning for Cardiac Arrhythmia Detection: A Systematic Survey. J. Phys. Conf. Ser. 2023, 2570, 012028. [Google Scholar] [CrossRef]
- Wang, J.; Lu, S.; Wang, S.-H.; Zhang, Y.-D. A Review on Extreme Learning Machine. Multimed. Tools Appl. 2022, 81, 41611–41660. [Google Scholar] [CrossRef]
- Huérfano-Maldonado, Y.; Mora, M.; Vilches, K.; Hernández-García, R.; Gutiérrez, R.; Vera, M. A Comprehensive Review of Extreme Learning Machine in Medical Imaging. Neurocomputing 2023, 556, 126618. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.; Group, P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Zhou, S.; Sapp, J.; AbdelWahab, A.; Trayanova, N. Deep Learning Applied to Electrocardiogram Interpretation. Can. J. Cardiol. 2021, 37, 17–18. [Google Scholar] [CrossRef]
- Xie, C.; Wang, Z.; Yang, C.; Liu, J.; Liang, H. Machine Learning for Detecting Atrial Fibrillation from ECGs: Systematic Review and Meta-Analysis. Rev. Cardiovasc. Med. 2024, 25, 8. [Google Scholar] [CrossRef]
- Martínez-Sellés, M.; Marina-Breysse, M. Current and Future Use of Artificial Intelligence in Electrocardiography. J. Cardiovasc. Dev. Dis. 2023, 10, 175. [Google Scholar] [CrossRef]
- Fussek, Ł.; Niewiadomska, J.; Bondos, B.; Stępień, A.; Paluch, A.; Skrzypek, J.; Niekra, A.; Kochan, R.; Wieczorek, E.; Lee, K.; et al. Artificial Intelligence in ECG Interpretation: Review Article. J. Educ. Health Sport 2025, 80, 57853. [Google Scholar] [CrossRef]
- Kher, R.; Patel, D. A Comprehensive Review on Wearable Health Monitoring Systems. Open Biomed. Eng. J. 2021, 15, 213–225. [Google Scholar] [CrossRef]
- Ansari, Y.; Mourad, O.; Qaraqe, K.; Serpedin, E. Deep Learning for ECG Arrhythmia Detection and Classification: An Overview of Progress for Period 2017–2023. Front. Physiol. 2023, 14, 1246746. [Google Scholar] [CrossRef]
- Weipeng, C.; Xi-Zhao, W.; Zhong, M.; Jinzhu, G. A Review on Neural Networks with Random Weights. Neurocomputing 2017, 275, 278–288. [Google Scholar] [CrossRef]
- Neri, L.; Oberdier, M.T.; van Abeelen, K.C.J.; Menghini, L.; Tumarkin, E.; Tripathi, H.; Jaipalli, S.; Orro, A.; Paolocci, N.; Gallelli, I.; et al. Electrocardiogram Monitoring Wearable Devices and Artificial-Intelligence-Enabled Diagnostic Capabilities: A Review. Sensors 2023, 23, 4805. [Google Scholar] [CrossRef]
- Alimbayeva, Z.; Alimbayev, C.; Ozhikenov, K.; Bayanbay, N.; Ozhikenova, A. Wearable ECG Device and Machine Learning for Heart Monitoring. Sensors 2024, 24, 4201. [Google Scholar] [CrossRef]
- Gautam, N.; Ghanta, S.N.; Mueller, J.; Mansour, M.; Chen, Z.; Puente, C.; Ha, Y.M.; Tarun, T.; Dhar, G.; Sivakumar, K.; et al. Artificial Intelligence, Wearables and Remote Monitoring for Heart Failure: Current and Future Applications. Diagnostics 2022, 12, 2964. [Google Scholar] [CrossRef]
- Xiao, R.; Ding, C.; Hu, X.; Clifford, G.; Wright, D.; Shah, A.; Al-Zaiti, S.; Zègre-Hemsey, J. Integrating Multimodal In-formation in Machine Learning for Classifying Acute Myocardial Infarction. Physiol. Meas. 2023, 44, 044002. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, H.; Liu, X. Cardiovascular Disease Detection Based on Multi-Modal Data Fusion and Multi-Branch Residual Network. In Proceedings of the 2023 International Conference on Frontiers of Artificial Intelligence and Machine Learning (FAIML ‘23), Beijing, China, 14–16 April 2023; ACM: New York, NY, USA, 2024; pp. 25–28. [Google Scholar] [CrossRef]
- Singh, N.; Kaur, K.; Chaudhary, A.; Goyal, S. Preprocessing of Medical Images Using Deep Learning: A Comprehensive Review. In Proceedings of the 2023 Second International Conference on Augmented Intelligence and Sustainable Systems (ICAISS), Trichy, India, 23–25 August 2023; IEEE: Piscataway, NJ, USA, 2023; pp. 521–527. [Google Scholar] [CrossRef]
- Elvas, L.; Nunes, M.; Ferreira, J.; Dias, M.; Rosário, L. AI-Driven Decision Support for Early Detection of Cardiac Events: Unveiling Patterns and Predicting Myocardial Ischemia. J. Pers. Med. 2023, 13, 1421. [Google Scholar] [CrossRef]
- Sun, Q.; Liang, C.; Chen, T.; Ji, B.; Liu, R.; Wang, L.; Tang, M.; Chen, Y.; Wang, C. Early Detection of Myocardial Ischemia in 12-Lead ECG Using Deterministic Learning and Ensemble Learning. Comput. Methods Programs Biomed. 2022, 226, 107124. [Google Scholar] [CrossRef]
- Tao, R.; Zhang, S.; Zhang, R.; Shen, C.; Ma, J.; Cui, J.; Chen, Y.; Wang, B.; Li, H.; Xie, X.; et al. AI-Enabled Diagnosis and Localization of Myocardial Ischemia and Coronary Artery Stenosis from Magnetocardiographic Recordings. Sci. Rep. 2025, 15, 6094. [Google Scholar] [CrossRef]
- Lefebvre, C.; Hoekstra, J. Early Detection and Diagnosis of Acute Myocardial Infarction: The Potential for Improved Care with Next-Generation, User-Friendly Electrocardiographic Body Surface Mapping. Am. J. Emerg. Med. 2007, 25, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Seoni, S.; Molinari, F.; Acharya, U.R.; Lih, O.S.; Barua, P.D.; García, S.; Salvi, M. Application of Spatial Uncertainty Predictor in CNN-BiLSTM Model Using Coronary Artery Disease ECG Signals. Inf. Sci. 2024, 665, 120383. [Google Scholar] [CrossRef]
- Cheng, J.; Zou, Q.; Zhao, Y. ECG Signal Classification Based on Deep CNN and BiLSTM. BMC Med. Inform. Decis. Mak. 2021, 21, 133. [Google Scholar] [CrossRef] [PubMed]
- Attia, Z.; Friedman, P.; Noseworthy, P.; Lopez-Jimenez, F.; Ladewig, D.; Satam, G.; Pellikka, P.; Munger, T.; Asirvatham, S.; Scott, C.; et al. Age and Sex Estimation Using Artificial Intelligence from Standard 12-Lead ECGs. Circ. Arrhythmia Electrophysiol. 2019, 12, e007284. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, L.; Sun, W.; Zhu, Y.; Zhang, Z.; Chen, L.; Xie, M.; Zhang, L. Artificial Intelligence in Diagnosis of Heart Failure. J. Am. Heart Assoc. 2025, 14, e039511. [Google Scholar] [CrossRef]
- Zainab, H.; Khan, A.; Khan, R.; Hussain, H. Integration of AI and Wearable Devices for Continuous Cardiac Health Monitoring. Int. J. Multidiscip. Sci. Arts 2024, 3, 123–128. [Google Scholar] [CrossRef]
- Ribeiro, A.; Ribeiro, M.; Paixão, G.; Oliveira, D.; Gomes, P.; Canazart, J.; Ferreira, M.; Andersson, C.; Macfarlane, P.; Meira, W.; et al. Automatic Diagnosis of the 12-Lead ECG Using a Deep Neural Network. Nat. Commun. 2020, 11, 1760. [Google Scholar] [CrossRef]
- Murat, F.; Sadak, F.; Yildirim, O.; Talo, M.; Murat, E.; Karabatak, M.; Demir, Y.; Tan, R.; Acharya, U. Review of Deep Learning-Based Atrial Fibrillation Detection Studies. Int. J. Environ. Res. Public Health 2021, 18, 11302. [Google Scholar] [CrossRef]
- Ojha, J.; Haugerud, H.; Yazidi, A.; Lind, P.G. Exploring Interpretable AI Methods for ECG Data Classification. In Proceedings of the Fifth Workshop on Intelligent Cross-Data Analysis and Retrieval (ICDAR ‘24), Phuket, Thailand, 10–14 June 2024; ACM: New York, NY, USA, 2024; pp. 1–8. [Google Scholar] [CrossRef]
- Petmezas, G.; Stefanopoulos, L.; Kilintzis, V.; Tzavelis, A.; Rogers, J.A.; Katsaggelos, A.K.; Maglaveras, N. State-of-the-Art Deep Learning Methods on Electrocardiogram Data: Systematic Review. JMIR Med. Inform. 2022, 10, e38454. [Google Scholar] [CrossRef]
- Mosslah, A.; Hazim, R.; Alwan, M. Performance Analysis of ECG Signal Noise Removal Techniques: A Review. IOSR J. Comput. Eng. 2021, 20, 85–88. [Google Scholar]
- Hamad, A.; Jasim, A. ECG Signal De-Noising Based on Deep Learning Autoencoder and Discrete Wavelet Transform. Int. J. Eng. Technol. 2020, 9, 415. [Google Scholar] [CrossRef]
- Keshan, N.; Parimi, P.V.; Bichindaritz, I. Machine Learning for Stress Detection from ECG Signals in Automobile Drivers. In Proceedings of the 2015 IEEE International Conference on Big Data (Big Data), Santa Clara, CA, USA, 29 October–1 November 2015; pp. 2661–2669. [Google Scholar] [CrossRef]
- Venton, J.; Aston, P.J. Investigating the Robustness of Deep Learning to Electrocardiogram Noise. In Proceedings of the 2021 Computing in Cardiology (CinC), Brno, Czech Republic, 12–15 September 2021; pp. 1–4. [Google Scholar] [CrossRef]
- Rahman, S.; Pal, S.; Yearwood, J.; Karmakar, C. Robustness of Deep Learning Models in Electrocardiogram Noise Detection and Classification. Comput. Methods Programs Biomed. 2024, 253, 108249. [Google Scholar] [CrossRef]
- Arsene, C.T.C.; Hankins, R.; Yin, H. Deep Learning Models for Denoising ECG Signals. In Proceedings of the 27th European Signal Processing Conference (EUSIPCO), A Coruña, Spain, 2–6 September 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Peng, H.; Chang, X.; Yao, Z.; Shi, D.; Chen, Y. A Deep Learning Framework for ECG Denoising and Classification. Biomed. Signal Process. Control 2024, 94, 106441. [Google Scholar] [CrossRef]
- Reza, D.; Mandala, S.; Zaki, S.M.; Ming, E. Deep Learning Autoencoder Study on ECG Signals. J. Nas. Tek. Elektro 2023, 12, 82–88. [Google Scholar] [CrossRef]
- Yoon, D.; Lim, H.S.; Jung, K.; Kim, T.Y.; Lee, S. Deep Learning-Based Electrocardiogram Signal Noise Detection and Screening Model. Healthc. Inform. Res. 2019, 25, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Pravin, C.; Ojha, V. A Novel ECG Signal Denoising Filter Selection Algorithm Based on Conventional Neural Networks. In Proceedings of the 19th IEEE International Conference on Machine Learning and Applications (ICMLA 2020), Miami, FL, USA, 14–17 December 2020; pp. 998–1003. [Google Scholar] [CrossRef]
- Kovalchuk, O.; Barmak, O.; Radiuk, P.; Klymenko, L.; Krak, I. Towards Transparent AI in Medicine: ECG-Based Arrhythmia Detection with Explainable Deep Learning. Technologies 2025, 13, 34. [Google Scholar] [CrossRef]
- Abdullah, T.; Zahid, M.; Tang, T.B.; Ali, W.; Nasser, M. Explainable Deep Learning Model for Cardiac Arrhythmia Classification. In Proceedings of the 2022 International Conference on Future Technologies in Security and Communication (ICFTSC 2022), Sarawak, Malaysia, 1–2 December 2022; pp. 87–92. [Google Scholar] [CrossRef]
- Gliner, V.; Levy, I.; Tsutsui, K.; Acha, M.R.; Schliamser, J.; Schuster, A.; Yaniv, Y. Clinically Meaningful Interpretability of an AI Model for ECG Classification. NPJ Digit. Med. 2025, 8, 109. [Google Scholar] [CrossRef]
- Amann, J.; Vetter, D.; Blomberg, S.N.; Christensen, H.C.; Coffee, M.; Gerke, S.; Gilbert, T.K.; Hagendorff, T.; Holm, S.; Livne, M.; et al. To Explain or Not to Explain?—Artificial Intelligence Explainability in Clinical Decision Support Systems. PLoS Digit. Health 2022, 1, e0000016. [Google Scholar] [CrossRef]
- She, W.J.; Siriaraya, P.; Iwakoshi, H.; Kuwahara, N.; Senoo, K. An Explainable AI Application (AF’fective) to Support Monitoring of Patients with Atrial Fibrillation after Catheter Ablation: Qualitative Focus Group, Design Session, and Interview Study. JMIR Hum. Factors 2025, 12, e65923. [Google Scholar] [CrossRef]
- Hammer, A.; Goettling, M.; Malberg, H.; Linke, A.; Schmidt, M. An Explainable AI for Trustworthy Detection of Atrial Fibrillation on Reduced Lead ECGs in Mobile Applications. Eur. Heart J. 2024, 45 (Suppl. S1), ehae666.3497. [Google Scholar] [CrossRef]
- Wang, J.; Lv, J.; Li, R.; Gong, Y.; Chen, Y.; Parameshachari, B.D.; Slowik, A.; Wei, W. XDTEncoder: A Deep Explainable Arrhythmia Classification Framework for Smart Healthcare. ACM Trans. Multimed. Comput. Commun. Appl. 2024. just accepted. [Google Scholar] [CrossRef]
- Hughes, J.W.; Olgin, J.E.; Avram, R.; Abreau, S.A.; Sittler, T.; Radia, K.; Hsia, H.; Walters, T.; Lee, B.; Gonzalez, J.E.; et al. Performance of a Convolutional Neural Network and Explainability Technique for 12-Lead Electrocardiogram Interpretation. JAMA Cardiol. 2021, 6, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Kalmady, S.V.; Hindle, A.; Sandhu, R.; Sun, W.; Sepehrvand, N.; Greiner, R.; Kaul, P. Diagnostic and Prognostic Electrocardiogram-Based Models for Rapid Clinical Applications. Can. J. Cardiol. 2024, 40, 1788–1803. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Kadian, T.; Shetty, M.K.; Gupta, A. Explainable AI Decision Model for ECG Data of Cardiac Disorders. Biomed. Signal Process. Control 2022, 75, 103584. [Google Scholar] [CrossRef]
- Salih, A.M.; Galazzo, I.B.; Gkontra, P.; Rauseo, E.; Lee, A.M.; Lekadir, K.; Radeva, P.; Petersen, S.E.; Menegaz, G. A Review of Evaluation Approaches for Explainable AI with Applications in Cardiology. Artif. Intell. Rev. 2024, 57, 240. [Google Scholar] [CrossRef]
- Bayona, A.V.; Wang, J.; Xiang, Y. Artificial Intelligence in Cardiovascular Prognosis and Diagnosis: A Review. Explor. Med. 2025, 6, 1001347. [Google Scholar] [CrossRef]
- Meyers, H.P.; Bracey, A.; Lee, D.; Lichtenheld, A.; Li, W.J.; Singer, D.D.; Rollins, Z.; Kane, J.A.; Dodd, K.W.; Meyers, K.E.; et al. Accuracy of OMI ECG Findings versus STEMI Criteria for Diagnosis of Acute Coronary Occlusion Myocardial Infarction. IJC Heart Vasc. 2021, 33, 100767. [Google Scholar] [CrossRef]
- Skouteli, C.; Prenzas, N.; Kakas, A.; Pattichis, C.S. Explainable AI Modeling in the Prediction of Cardiovascular Disease Risk. Stud. Health Technol. Inform. 2024, 316, 978–982. [Google Scholar] [CrossRef]
- Hempel, P.; Ribeiro, A.H.; Vollmer, M.; Bender, T.; Dörr, M.; Krefting, D.; Spicher, N. Explainable AI Associates ECG Aging Effects with Increased Cardiovascular Risk in a Longitudinal Population Study. NPJ Digit. Med. 2025, 8, 25. [Google Scholar] [CrossRef]
- Svennberg, E.; Han, J.K.; Caiani, E.G.; Engelhardt, S.; Ernst, S.; Friedman, P.; Garcia, R.; Ghanbari, H.; Hindricks, G.; Man, S.H.; et al. State of the Art of Artificial Intelligence in Clinical Electrophysiology in 2025: A Scientific Statement of the European Heart Rhythm Association (EHRA) of the ESC, the Heart Rhythm Society (HRS), and the ESC Working Group on E-Cardiology. EP Eur. 2025, 27, euaf071. [Google Scholar] [CrossRef]
- Hota, A.; Park, S.-W. Stress Detection Using Physiological Signals Based on Machine Learning. In Proceedings of the 2022 International Conference on Computational Science and Computational Intelligence (CSCI), Las Vegas, NV, USA, 14–16 December 2022; pp. 379–384. [Google Scholar] [CrossRef]
- Pinge, A.; Gad, V.; Jaisighani, D.; Ghosh, S.; Sen, S. Detection and Monitoring of Stress Using Wearables: A Systematic Review. Front. Comput. Sci. 2024, 6, 1478851. [Google Scholar] [CrossRef]
- Canali, S.; Schiaffonati, V.; Aliverti, A. Challenges and Recommendations for Wearable Devices in Digital Health: Data Quality, Interoperability, Health Equity, Fairness. PLoS Digit. Health 2022, 1, e0000104. [Google Scholar] [CrossRef] [PubMed]
- Smets, E.; Rios Velazquez, E.; Schiavone, G.; Chakroun, I.; D’Hondt, E.; De Raedt, W.; Cornelis, J.; Janssens, O.; Van Hoecke, S.; Claes, S.; et al. Large-Scale Wearable Data Reveal Digital Phenotypes for Daily-Life Stress Detection. NPJ Digit. Med. 2018, 1, 67. [Google Scholar] [CrossRef]
- Ebrahimi, Z.; Loni, M.; Daneshtalab, M.; Gharehbaghi, A. A Review on Deep Learning Methods for ECG Arrhythmia Classification. Expert Syst. Appl. X 2020, 7, 100033. [Google Scholar] [CrossRef]
- Dhyani, S.; Butola, R. Autoencoders for ECG Anomaly Detection: A Survey. J. Comput. Educ. Sports Health 2025, 2, 47–58. [Google Scholar]
- Mirza, M.U.; Dalmaz, O.; Çukur, T. Skip Connections for Medical Image Synthesis with Generative Adversarial Networks. In Proceedings of the 2022 30th Signal Processing and Communications Applications Conference (SIU), Safranbolu, Turkey, 18–21 May 2022; pp. 1–4. [Google Scholar] [CrossRef]
- Nowroozilarki, Z.; Mortazavi, B.J.; Jafari, R. Variational Autoencoders for Biomedical Signal Morphology Clustering and Noise Detection. IEEE J. Biomed. Health Inform. 2023, 28, 169–180. [Google Scholar] [CrossRef]
- Raje, V.V.; Goel, S.; Patil, S.V.; Kokate, M.D.; Mane, D.A.; Lavate, S. Realtime Anomaly Detection in Healthcare IoT: A Machine Learning-Driven Security Framework. J. Electr. Syst. 2023, 19, 192–202. [Google Scholar] [CrossRef]
- Hong, S.; Zhou, Y.; Shang, J.; Xiao, C.; Sun, J. Opportunities and Challenges of Deep Learning Methods for Electrocardiogram Data: A Systematic Review. Comput. Biol. Med. 2020, 122, 103801. [Google Scholar] [CrossRef]
- Chukwu, E.C.; Moreno-Sánchez, P.A. Enhancing Arrhythmia Diagnosis with Data-Driven Methods: A 12-Lead ECG-Based Explainable AI Model. In Digital Health and Wireless Solutions, Proceedings of the Nordic Conference on Digital Health and Wireless Solutions, NCDHWS, Oulu, Finland, 7–8 May 2024; Särestöniemi, M., Keikhosrokiani, P., Singh, D., Harjula, E., Tiulpin, A., Jansson, M., Isomursu, M., van Gils, M., Saarakkala, S., Reponen, J., Eds.; Springer: Cham, Switzerland, 2024; Volume 2084. [Google Scholar] [CrossRef]
- Adib, E.; Afghah, F.; Prevost, J.J. Synthetic ECG Signal Generation Using Generative Neural Networks. PLoS ONE 2025, 20, e0271270. [Google Scholar] [CrossRef]
- Núñez, J.F.; Arjona, J.; Béjar, J. Synthetic ECG Generation for Data Augmentation and Transfer Learning in Arrhythmia Classification. arXiv 2024, arXiv:2411.18456. [Google Scholar]
- Goodfellow, I.; Pouget-Abadie, J.; Mirza, M.; Xu, B.; Warde-Farley, D.; Ozair, S.; Courville, A.; Bengio, Y. Generative Adversarial Networks. Adv. Neural Inf. Process. Syst. 2014, 3, 139–144. [Google Scholar] [CrossRef]
- Rayavarapu, S.M.; ShanmukhaPrasanthi, T.; Lavanya, Y.L.; Kumar, G.S.; SaibhushanaRao, G.; Singham, A. Synthesis of ECG Signals Using Generative Adversarial Networks. In Proceedings of the 2023 Second International Conference on Electrical, Electronics, Information and Communication Technologies (ICEEICT), Trichirappalli, India, 24–26 July 2023; pp. 1–4. [Google Scholar] [CrossRef]
- Akpinar, M.H.; Sengur, A.; Salvi, M.; Seoni, S.; Faust, O.; Mir, H.; Molinari, F.; Acharya, U.R. Synthetic Data Generation via Generative Adversarial Networks in Healthcare: A Systematic Review of Image and Signal-Based Studies. IEEE Open J. Eng. Med. Biol. 2024, 6, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Rivolta, M.W.; Badilini, F.; Sassi, R. A Systematic Survey of Data Augmentation of ECG Signals for AI Applications. Sensors 2023, 23, 5237. [Google Scholar] [CrossRef]
- Thambawita, V.; Isaksen, J.L.; Hicks, S.A.; Ghouse, J.; Ahlberg, G.; Linneberg, A.; Grarup, N.; Ellervik, C.; Olesen, M.S.; Hansen, T.; et al. DeepFake Electrocardiograms Using Generative Adversarial Networks Are the Beginning of the End for Privacy Issues in Medicine. Sci. Rep. 2021, 11, 21896. [Google Scholar] [CrossRef] [PubMed]
- Prakash, N.K.; Subramanian, K. Machine Learning-Based Cardiac Arrhythmia Detection from ECG Signal. In Proceedings of the 2020 International Conference on Smart Systems and Innovative Technologies (ICSSIT), Tirunelveli, India, 25–26 September 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–6. [Google Scholar] [CrossRef]
- Berger, L.; Haberbusch, M.; Moscato, F. Generative Adversarial Networks in Electrocardiogram Synthesis: Recent Developments and Challenges. Artif. Intell. Med. 2023, 143, 102632. [Google Scholar] [CrossRef] [PubMed]
- Heng, Y.; Yinghua, M.; Khan, F.G.; Zhang, Y.; Xie, C.; Li, Y. Survey: Application and Analysis of Generative Adversarial Networks in Medical Images. Artif. Intell. Rev. 2025, 58, 39. [Google Scholar] [CrossRef]
- Ibrahim, M.; Al Khalil, Y.; Amirrajab, S.; Sun, C.; Breeuwer, M.; Pluim, J.; Elen, B.; Ertaylan, G.; Dumontier, M. Generative AI for Synthetic Data across Multiple Medical Modalities: A Systematic Review of Recent Developments and Challenges. Comput. Biol. Med. 2025, 189, 109834. [Google Scholar] [CrossRef]
- Upreti, R. Conditional Deep Generative Models for Generating Synthetic Electrocardiograms. Master’s Thesis, Oslo Metropolitan University, Oslo, Norway, 2023. [Google Scholar]
- Musa, N.; Gital, A.Y.; Aljojo, N.; Chiroma, H.; Adewole, K.S.; Mojeed, H.A.; Faruk, N.; Abdulkarim, A.; Emmanuel, I.; Folawiyo, Y.Y.; et al. A Systematic Review and Meta-Data Analysis on the Applications of Deep Learning in Electrocardiogram. J. Ambient Intell. Humaniz. Comput. 2023, 14, 9677–9750. [Google Scholar] [CrossRef]
- Raghu, A.; Shanmugam, D.; Pomerantsev, E.; Guttag, J.; Stultz, C.M. Data Augmentation for Electrocardiograms. In Proceedings of the CHIL 2022 Call for Papers—Conference on Health, Inference and Learning, Virtual, 7–8 April 2022; Volume 174, pp. 282–310. [Google Scholar]
- Khamparia, A.; Gupta, D. Generative Artificial Intelligence for Biomedical and Smart Health Informatics; IEEE Press: Piscataway, NJ, USA; Wiley: Amethi, India, 2024. [Google Scholar]
- Ma, S.; Cui, J.; Xiao, W.; Liu, L. Deep Learning-Based Data Augmentation and Model Fusion for Automatic Arrhythmia Identification and Classification Algorithms. Comput. Intell. Neurosci. 2022, 2022, 1577778. [Google Scholar] [CrossRef]
- Escrivaes, I.; Barbosa, L.; Torres, H.; Oliveira, B.; Vilaça, J.; Morais, P. ECG Classification Using Artificial Intelligence: Model Optimization and Robustness Assessment. In Proceedings of the 2022 IEEE 10th International Conference on Serious Games and Applications for Health (SeGAH), Rio de Janeiro, Brazil, 16–18 November 2022; IEEE: Piscataway, NJ, USA, 2022; pp. 1–8. [Google Scholar] [CrossRef]
- Kelly, C.J.; Karthikesalingam, A.; Suleyman, M.; Corrado, G.; King, D. Key Challenges for Delivering Clinical Impact with Artificial Intelligence. BMC Med. 2019, 17, 195. [Google Scholar] [CrossRef]
- Palermi, S.; Vecchiato, M.; Ng, F.S.; Attia, Z.; Cho, Y.; Anselmino, M.; De Ferrari, G.M.; Saglietto, A.; Sau, A.; Chiu, I.M.; et al. Artificial Intelligence and the Electrocardiogram: A Modern Renaissance. Eur. J. Intern. Med. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Tan, B. Electrocardiogram Soft Computing Using Hybrid Deep Learning CNN-ELM. Appl. Soft Comput. 2020, 86, 105778. [Google Scholar] [CrossRef]
- Ghosh, S.; Mukherjee, H.; Obaidullah, S.M.; Santosh, K.C.; Das, N.; Roy, K. A Survey on Extreme Learning Machine and Evolution of Its Variants. In Recent Trends in Image Processing and Pattern Recognition, Proceedings of the International Conference on Recent Trends in Image Processing and Pattern Recognition, RTIP2R 2018, Solapur, India, 21–22 December 2025; Santosh, K., Hegadi, R., Eds.; Springer: Singapore, 2019; Volume 1035. [Google Scholar] [CrossRef]
- Traunmueller, P.; JahaniJoo, A.; Khooyooz, S.; Aminifar, A.; TaheriNejad, N. Wearable Healthcare Devices for Monitoring Stress and Attention Level in Workplace Environments. arXiv 2024, arXiv:2406.05813. [Google Scholar]
- Hygrell, T.; Viberg, F.; Dahlberg, E.; Charlton, P.H.; Gudmundsdottir, K.K.; Mant, J.; Lindman Hörnlund, J.; Svennberg, E. An Artificial Intelligence–Based Model for Prediction of Atrial Fibrillation from Single-Lead Sinus Rhythm Electrocar-diograms Facilitating Screening. EP Eur. 2023, 25, 1332–1338. [Google Scholar] [CrossRef]
- Linh, V.T.N.; Han, S.; Koh, E.; Kim, S.; Jung, H.S.; Koo, J. Advances in wearable electronics for monitoring human organs: Bridging external and internal health assessments. Biomaterials 2025, 314, 122865. [Google Scholar] [CrossRef]
- Ayyub, A.; Politis, C.; Usman, M.A. A Comprehensive Review of AI-Based Detection of Arrhythmia Using Electrocardiogram (ECG). Comput. Biol. Med. 2025, 196, 110594. [Google Scholar] [CrossRef]
- Wu, Z.; Guo, C. Deep Learning and Electrocardiography: Systematic Review of Current Techniques in Cardiovascular Disease Diagnosis and Management. Biomed. Eng. Online 2025, 24, 23. [Google Scholar] [CrossRef]
- Sahoo, S.; Dash, M.; Behera, S.; Sabut, S. Machine Learning Approach to Detect Cardiac Arrhythmias in ECG Signals: A Survey. IRBM 2020, 41, 261–274. [Google Scholar] [CrossRef]
- Can, Y.S.; Chalabianloo, N.; Ekiz, D.; Ersoy, C. Continuous Stress Detection Using Wearable Sensors in Real Life: Algorithmic Programming Contest Case Study. Sensors 2019, 19, 1849. [Google Scholar] [CrossRef]



| Ref. | Model/Method | Sensitivity | Notable Features | Consensus Points |
|---|---|---|---|---|
| [4] | Rule-based + AI | 43.6% (STEMI) | Misses > 50% occlusions | Need for OMI paradigm |
| [18] | CNN + Imaging | >70% | Multimodal, prehospital ECG | Multimodal fusion improves outcome |
| [9] | Subtraction ECG | – | Serial comparison, early Dx | Dynamic ECG analysis is superior |
| [29] | ML, context-free | – | Context-independent detection | AI outperforms classic rules |
| Ref. | Model | Dataset/ Size | Internal AUC | External AUC | Key Notes |
|---|---|---|---|---|---|
| [1] | DenseNet-121 | 136,775/ ECG–echo | 0.965 | 0.848 | Large single-center dataset |
| [37] | CNN-BiLSTM | 67,332/ ECG | 0.943 | 0.867 | Uncertainty estimation integrated |
| [38] | CNN-BiLSTM | 145,220/ ECG | 0.958 | 0.823 | Large-scale dataset withuncertainty |
| Methodology | Dataset | SNR Improvement | Key Authors | Consensus/Notes |
|---|---|---|---|---|
| Wavelet (WT) + ACF | MIT-BIH | +6.5 dB | [9] | Best for muscle noise, wearable data |
| DP-IDAE | MIT-BIH, PTB-XL | +7.06 dB | [8] | Outperforms FIR, WT; preserves shape |
| Classic FIR/WT | MIT-BIH | <1 dB | (Traditional) | Inferior to AI-based methods |
| Issue | Consensus in Literature | Recommendations from Review |
|---|---|---|
| Lack of transparency | XAI improves trust (e.g., saliency maps) [15,44] | Integrate XAI in all clinical, AI-ECG deployments |
| Dataset bias | Major bias noted [4], underreporting of diversity [13] | Mandate diversity reporting, open data access |
| Limited accessibility | <30% public datasets available [12] | Standardize metadata, build public ECG repositories |
| Ref | SYS | N | DIS | CMP | INT | SYN | WRB | BIA | VIS | REC | INT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [6] | ❌ | NS | CVD | ❌ | ❌ | ❌ | ❌ | ❌ | ❌ | ❌ | ❌ |
| [7] | ✅ | 9 | AF | ✅ | ❌ | ❌ | ✅ | ❌ | ✅ | ✅ | ❌ |
| [12] | ✅ | 70 | ⚠️️ | ⚠️ | ⚠️ | ✅ | ❌ | ⚠️ | ❌ | ⚠️ | ❌ |
| [15] | ✅ | 50+ | CVD | ✅ | ✅ | ❌ | ❌ | ✅ | ✅ | ⚠️ | ⚠️ |
| [16] | ✅ | 80 | NS | ✅ | ⚠️ | ❌ | ❌ | ❌ | ❌ | ❌ | ❌ |
| [21] | ✅ | 27 | AF | ✅ | ❌ | ❌ | ✅ | ❌ | ✅ | ⚠️ | ❌ |
| [22] | ❌ | NS | CVD | ❌ | ⚠️ | ❌ | ⚠️ | ❌ | ❌ | ⚠️ | ❌ |
| [23] | ❌ | NS | CVD | ❌ | ⚠️ | ❌ | ❌ | ❌ | ❌ | ⚠️ | ❌ |
| [26] | ❌ | NS | ⚠️ | ❌ | ❌ | ❌ | ❌ | ❌ | ❌ | ❌ | ⚠️ |
| [43] | ✅ | 51 | AF | ⚠️ | ❌ | ❌ | ⚠️ | ❌ | ✅ | ⚠️ | ⚠️ |
| [40] | ✅ | 95 | HF | ✅ | ⚠️ | ❌ | ❌ | ⚠️ | ✅ | ✅ | ✅ |
| [29] | ❌ | NS | HF | ❌ | ❌ | ❌ | ✅ | ⚠️ | ⚠️ | ✅ | ⚠️ |
| [24] | ❌ | NS | CVD | ❌ | ❌ | ❌ | ✅ | ❌ | ⚠️ | ⚠️ | ⚠️ |
| [25] | ✅ | >100 | ARR | ✅ | ⚠️ | ❌ | ⚠️ | ✅ | ✅ | ✅ | ✅ |
| [27] | ✅ | >100 | CVD | ⚠️ | ⚠️ | ❌ | ✅ | ✅ | ✅ | ✅ | ✅ |
| [45] | ✅ | 94 | CVD | ✅ | ✅ | ❌ | ⚠️ | ✅ | ✅ | ✅ | ✅ |
| [64] | ✅ | 76 | MUL | ✅ | ✅ | ❌ | ✅ | ✅ | ✅ | ✅ | ✅ |
| [66] | ✅ | >100 | CVD | ✅ | ✅ | ✅ | ⚠️ | ✅ | ✅ | ✅ | ✅ |
| [67] | ✅ | >100 | MUL | ✅ | ✅ | ❌ | ✅ | ✅ | ✅ | ✅ | ✅ |
| [71] | ✅ | NS | MUL, VT | ✅ | ✅ | ❌ | ✅ | ✅ | ✅ | ✅ | ✅ |
| [73] | ✅ | >100 | ST | ✅ | ✅ | ❌ | ✅ | ⚠️ | ✅ | ✅ | ⚠️ |
| [74] | ❌ | NS | NS | ❌ | ⚠️ | ❌ | ✅ | ✅ | ❌ | ✅ | ⚠️ |
| [77] | ❌ | – | AD | ⚠️ | ✅ | ✅ | ❌ | ❌ | ⚠️ | ⚠️ | ❌ |
| [81] | ✅ | 60 | MUL | ✅ | ⚠️ | ❌ | ⚠️ | ✅ | ⚠️ | ✅ | ⚠️ |
| [87] | ✅ | 65 | NS | ✅ | ⚠️ | ✅ | ⚠️ | ⚠️ | ✅ | ✅ | ❌ |
| [88] | ✅ | 82 | NS | ✅ | ❌ | ✅ | ⚠️ | ⚠️ | ✅ | ✅ | ❌ |
| [95] | ✅ | 88 | MUL | ✅ | ✅ | ❌ | ⚠️ | ✅ | ✅ | ✅ | ⚠️ |
| [91] | ⚠️ | NS | NS | ⚠️ | ❌ | ✅ | ⚠️ | ✅ | ⚠️ | ✅ | ❌ |
| [92] | ✅ | NS | NS | ⚠️ | ❌ | ✅ | ❌ | ⚠️ | ⚠️ | ✅ | ❌ |
| [93] | ✅ | NS | NS | ⚠️ | ❌ | ✅ | ❌ | ✅ | ⚠️ | ✅ | ⚠️ |
| [101] | ❌ | NS | MUL | ⚠️ | ✅ | ⚠️ | ✅ | ✅ | ⚠️ | ✅ | ⚠️ |
| [76] | ❌ | NS | ARR | ❌ | ✅ | ❌ | ❌ | ⚠️ | ❌ | ❌ | ❌ |
| [106] | ❌ | NS | MULT | ❌ | ⚠️ | ❌ | ✅ | ⚠️ | ⚠️ | ❌ | ❌ |
| [107] | ✅ | 67 | ARR | ✅ | ⚠️ | ❌ | ❌ | ❌ | ✅ | ❌ | ❌ |
| [108] | ✅ | 39 | MULT | ✅ | ⚠️ | ❌ | ⚠️ | ❌ | ⚠️ | ✅ | ⚠️ |
| [109] | ✅ | 27 | ARR | ✅ | ❌ | ❌ | ❌ | ❌ | ❌ | ❌ | ❌ |
| Our | ✅ | >100 | MULT | ✅ | ✅ | ✅ | ✅ | ✅ | ✅ | ✅ | ✅ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velandia, H.; Pardo, A.; Vera, M.I.; Vera, M. Systematic Review of Artificial Intelligence and Electrocardiography for Cardiovascular Disease Diagnosis. Bioengineering 2025, 12, 1248. https://doi.org/10.3390/bioengineering12111248
Velandia H, Pardo A, Vera MI, Vera M. Systematic Review of Artificial Intelligence and Electrocardiography for Cardiovascular Disease Diagnosis. Bioengineering. 2025; 12(11):1248. https://doi.org/10.3390/bioengineering12111248
Chicago/Turabian StyleVelandia, Hernando, Aldo Pardo, María Isabel Vera, and Miguel Vera. 2025. "Systematic Review of Artificial Intelligence and Electrocardiography for Cardiovascular Disease Diagnosis" Bioengineering 12, no. 11: 1248. https://doi.org/10.3390/bioengineering12111248
APA StyleVelandia, H., Pardo, A., Vera, M. I., & Vera, M. (2025). Systematic Review of Artificial Intelligence and Electrocardiography for Cardiovascular Disease Diagnosis. Bioengineering, 12(11), 1248. https://doi.org/10.3390/bioengineering12111248








