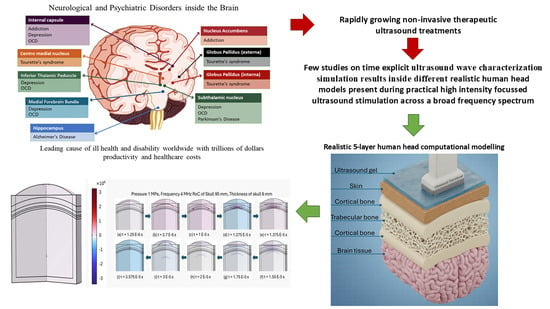
4.1. Pressure Acoustics—Frequency Domain Study
Simulations conducted in the frequency domain enable the identification of focal regions and provide insight into whether the system has reached a steady-state response, as illustrated in
Figure 4.
From the simulations in
Figure 4, it is clear that as the excitation frequency increases, the acoustic pressure amplitude drops significantly while the focal zone becomes narrower and more localized. At 0.5 MHz (
Figure 4A), the pressure field is strong, with a broad focal region and deeper penetration into the brain tissue. At 1–2 MHz (
Figure 4B,C), the focal spot sharpens, but attenuation through the skull reduces the transmitted pressure considerably. By 4–5 MHz (
Figure 4D,E), the transmitted pressure is dramatically diminished, and the focal region is much smaller, highlighting the strong frequency-dependent attenuation of bone. Another noticeable detail is the increased reflection and scattering patterns at higher frequencies, especially near skull boundaries, which further reduce the efficiency of acoustic energy transmission into the brain. This demonstrates the fundamental trade-off in transcranial ultrasound: lower frequencies penetrate better but yield less precise focusing, while higher frequencies provide tighter focal zones but suffer from strong attenuation and reduced intracranial pressure.
In order to figure out how the human anatomy would affect acoustic transmission, skull radius, skull thickness, and pressure change were studied.
In
Figure 5A, we can see that sound transmission loss increases both with frequency and with larger skull radius, indicating that thicker or more curved skulls act as stronger barriers to ultrasound propagation. At lower frequencies (below 1 MHz), transmission loss remains relatively moderate across skull radii, suggesting that low-frequency ultrasound penetrates more effectively regardless of skull thickness. However, as frequency increases beyond 2–3 MHz, transmission loss becomes highly sensitive to skull radius, with steeper rises observed in thicker skulls. This aligns with the physics of wave propagation, where higher frequencies are more prone to scattering, absorption, and reflection within dense structures.
Figure 5B complements this by showing that the focal depth of ultrasound is reduced with increasing frequency and skull radius, particularly evident at frequencies above 2 MHz. In these cases, the focus shifts closer to the skull, meaning that effective energy delivery into deeper brain regions becomes increasingly difficult. Together, the plots demonstrate that both skull geometry and operating frequency must be carefully balanced: smaller radii and lower frequencies favor transmission, while larger skulls and higher frequencies exacerbate losses and shift the focus away from the brain interior. This highlights why low-frequency ultrasound is generally favored for transcranial applications, despite its broader focal spot.
In
Figure 6A, the influence of skull thickness on sound transmission loss becomes evident when coupled with frequency. At low frequencies (<1 MHz), sound transmission loss remains relatively moderate across thickness values, suggesting that low-frequency ultrasound can penetrate even thicker skull regions with reasonable efficiency. However, as frequency increases, thicker skulls show a much greater attenuation, with losses exceeding 80–100 dB for skulls above 7–8 mm. This is consistent with the known physics of wave propagation: thicker bone leads to greater absorption, scattering, and phase aberrations, particularly at higher frequencies. Anatomically, this trend highlights why ultrasound transmission is more feasible through naturally thinner skull regions such as the temporal bone (~2–4 mm), compared to the occipital or parietal bones, which can exceed 6–8 mm in thickness. In
Figure 6B, the focus depth is also influenced: as both frequency and skull thickness increase, the focus shifts shallower, moving closer to the skull surface rather than penetrating deeper into the brain. For thin temporal regions, the focus can remain at depths of ~50 mm, making them favorable windows for transcranial applications. In contrast, thicker frontal or occipital regions reduce penetration depth, complicating targeted neuromodulation or therapy. These findings reinforce the anatomical dependency of ultrasound efficacy: thinner skull regions facilitate deeper, more efficient energy delivery, while thicker areas introduce strong transmission losses and degrade focal accuracy, making low-frequency sonication essential for accessing deeper brain structures in those regions.
The transducer pressure appeared to exert minimal influence on both STL and the focal depth. As illustrated in
Figure 7A, only minor fluctuations are observed in STL with varying transducer pressures; overall, an increase in applied pressure does not lead to a measurable decrease in STL. This observation is consistent with the underlying physics, since STL is defined as the ratio of the acoustic signal at the transducer to that measured at the focal zone, making it relatively insensitive to moderate variations in transducer contact pressure. Similarly,
Figure 7B shows that the focal depth exhibits only slight increases with increasing transducer pressure. This effect can be explained by the marginal improvement in acoustic coupling and wave propagation through the medium, which allows the ultrasound energy to reach slightly deeper regions. However, these changes are not significant within the examined pressure range. Overall, while the transducer pressure is critical for defining the absolute acoustic pressure at the focus, ensuring reproducibility and safety in experimental setups, it does not substantially alter STL or the focal depth, indicating that the system is robust to small variations in applied transducer force.
Based on the parametric analysis, the optimal experimental conditions were selected for subsequent simulations. Specifically, a low-frequency excitation in combination with a constant transducer pressure was chosen for modeling acoustic wave propagation in the time-explicit framework. This choice reflects the observed trends in STL and focal depth, where low frequencies provided favorable transmission characteristics, and variations in transducer pressure had a negligible impact. By fixing the pressure, the model ensures a reproducible and controlled absolute pressure at the focus, while low-frequency excitation maximizes wave penetration and minimizes attenuation through the cranial medium.
4.2. Pressure Acoustics—Time-Explicit Study
The time-explicit simulations provide a detailed view of the transient behavior of ultrasonic wave propagation through the medium. By applying a single pulse under a constant transducer pressure, these simulations capture the evolution of the acoustic pressure field over time, allowing for precise assessment of focal zone formation, wavefront propagation, and attenuation, as can be seen in
Figure 8. Unlike frequency-domain analyses, which represent steady-state responses, the time-explicit approach enables the visualization of dynamic phenomena, including reflections, interference, and the temporal buildup of pressure at the focus. At the time of maximum pressure ratio observed in the brain tissue, the region for which the signal pressure is more than 5% of the maximum pressure is considered the focus zone in this work.
Figure 8 illustrates the propagation of the ultrasonic wave through the medium as captured by the time-explicit simulation. The wavefront emanates from the transducer and travels through the biological tissues, gradually converging toward the focal region. A short frequency analysis of the obtained signal can be seen in
Supplemental Material Figure S2. As the wave propagates, constructive interference leads to an increase in pressure magnitude, clearly delineating the focal zone. The figure highlights the spatial localization of the maximum pressure, confirming that the pulse effectively concentrates acoustic energy at the intended focus. The Minor reflections and diffractions along the propagation path can also be observed, but they do not significantly affect the formation of the focal zone.
Figure 9 depicts how transcranial ultrasound transmission properties change with increasing skull thickness at constant frequency (2 MHz) and source pressure (1 MPa) for two radii of curvature (65 mm and 95 mm). The maximum pressure ratio exhibits a modest decreasing trend as thickness increases for both radii, with higher values consistently observed for the 95 mm curvature. In contrast, the focus zone demonstrates nonmonotonic behavior, with increasing skull thickness, particularly for thinner skulls. For a given frequency (2 MHz) and pressure (1 MPa), the 95 mm curvature generally yields a wider focus zone than the 65 mm, except at intermediate thicknesses where both curves converge. The observed non-monotonic behaviors arise from complex wave interactions at skull-tissue interfaces. Mode conversion at skull boundaries transforms longitudinal waves into shear waves, which subsequently reconvert to longitudinal waves upon transmission, creating multiple wave groups with different arrival times and amplitudes [
82,
83]. Constructive and destructive interference between direct transmitted waves and skull-reverberated waves produces oscillatory pressure patterns, influencing focus quality [
84]. The skull’s heterogeneous microstructure and varying porosity generate frequency-dependent scattering effects that interact nonlinearly with thickness changes [
43,
47]. These mechanisms do not scale linearly with anatomical parameters, explaining why optimal transmission occurs at intermediate rather than extreme values. Understanding these interactions is crucial for patient-specific treatment planning in transcranial ultrasound applications.
The findings highlight that thinner skulls and larger curvatures favor higher pressure transmission and more controlled focal regions. Notably, extreme values in the focus zone occur at both thin and thick skulls, highlighting the complex interplay between anatomical and acoustic parameters when targeting precise focal regions in transcranial ultrasound applications.
The maximum pressure ratio (
Figure 10) shows that the maximum pressure ratio is highest at lower frequencies (0.5–1 MHz) for all skull thicknesses and radii of curvature but drops dramatically at 2 MHz before partially recovering at 4–5 MHz, especially for thinner skulls. The effect is more pronounced with thicker skulls, but for both thicknesses, the 65 mm radius configuration yields slightly higher transmission values at lower frequencies than the 95 mm, though this reverses near 4 MHz. The 6 mm skull with 65 mm curvature demonstrates a pronounced peak at 1 MHz, suggesting a strong dependency of transcranial ultrasound transmission on both bone thickness and the radius of curvature. For all cases, thicker skulls (6 mm) generally yield higher pressure ratios at 1 MHz, but the advantage diminishes at higher frequencies, where transmission efficiency becomes more similar across geometries.
The focus zone figure shows that zone width tends to decrease as frequency increases, especially from 0.5 MHz to 5 MHz, regardless of skull thickness or curvature. Interestingly, lower frequencies yield larger and less tightly focused zones, which might be less desirable for focal targeting but can increase the effective treatment volume. Conversely, higher frequencies produce smaller focus zones but suffer from poorer pressure transmission.
The results emphasize the importance of patient-specific parameter optimization (frequency, skull thickness, transducer geometry) for maximizing both energy delivery and focal control in therapeutic or research-oriented transcranial ultrasound applications. The findings highlight that: 1. Lower frequencies enhance both pressure transmission and result in broader focal zones, making them suitable for situations where deep penetration and energy delivery are prioritized over tight localization. 2. Skull thickness significantly diminishes both the maximum pressure ratio and the focus zone at higher frequencies, underlining the challenge of individualized parameter selection for patient-specific applications. The anomaly in the results trend is insignificant in front of the non-monotonic behavior observed due to skull thickness variation. Thus, the complex interplay of reflection, refraction, and mode conversion is not highly nonlinear, as observed in skull thickness variation, and also, as further presented, in radius of curvature variation.
Figure 11 shows a slight decrease in pressure transmission as the radius of curvature increases for both 4 mm and 6 mm skull thicknesses. The 4 mm skull consistently yields a higher maximum pressure ratio compared to the 6 mm skull, emphasizing the beneficial effect of thinner bone on acoustic energy delivery. The rate of decrease is gradual after 65 mm RoC value, with the lowest values observed at the largest radii. The focus zone chart reveals that focus zone width tends to increase as radius increases, particularly beyond 75 mm, and this effect is most pronounced for the 6 mm skull. For smaller radii (<75 mm), the focus zone remains nearly constant, but it widens sharply with further increases in radius of curvature. Thinner skulls enable higher pressure transmission across all radii of curvature. Thus, larger radii of curvature lead to broader focus zones but reduced maximum pressure ratios, especially in thicker skulls. For applications requiring high energy transmission and a tightly focused beam, smaller radii and thinner skulls offer clear advantages. The observed behaviors result from intricate acoustic interactions at the interfaces between cranium and tissue. As skull curvature varies, multiple physical mechanisms contribute simultaneously: acoustic impedance mismatch between skull bone (Z ≈ 4.7 MRayls) and surrounding tissues (Z ≈ 1.5 MRayls), which creates substantial reflection coefficients that fluctuate with geometric changes [
43]. Additionally, refraction effects due to varying skull curvature alter wave propagation paths, particularly affecting focus zone geometry.
The clinical translation of these findings is particularly relevant for the individualized planning of transcranial ultrasound neuromodulation and ablation therapies. Since the results demonstrate strong dependence of transmission and focal parameters on skull thickness, curvature, and acoustic diffusivity, clinical implementation should incorporate patient-specific skull parameter extraction. This can be achieved through preoperative CT or MRI-based acoustic mapping, where skull density ratio, cortical/trabecular segmentation, and local curvature are quantified to derive the acoustic impedance and attenuation coefficients used in numerical models. Recent studies show that CT Hounsfield units can be linearly correlated with ultrasound speed and density, allowing for conversion models that automatically generate acoustic property maps for use in FEM or k-space simulations [
85,
86,
87,
88]. Such individualized modeling enables pre-treatment planning, optimizing sonication frequency, focal depth, and transducer curvature for each patient while minimizing off-target exposure. Integration of this framework into clinical workflows, potentially through automated segmentation and parameter extraction pipelines, could substantially enhance treatment precision, safety, and reproducibility in both tFUS neuromodulation and HIFU ablation settings.
There are potential limitations to the model setup and the numerical model used in this work, anticipated by the authors. The 2D-axisymmetric geometry limits the actual asymmetric skull geometry representation and the transducer misalignments during clinical trials. As with any numerical method, there exists a potential for systematic errors to arise. The possible origins of these inaccuracies include the application of a direct solver in the study and the discretization technique employed in the finite element analysis. A direct solver simultaneously resolves all equations, which may lead to the accumulation of errors due to computational approximations of the solution. Moreover, second-order discretization, specifically quadratic Lagrangian elements, may influence the mesh quality, as the discretization depends on the approximation accuracy between adjacent components.
Another key limitation of the present work is that the proposed five-layer human head model has not yet been experimentally validated through in vitro or in vivo measurements, although an initial validation can be found in
Supplemental Material Figure S1. Future studies should aim to perform quantitative experimental verification of the simulated pressure transmission and focal characteristics using human skull phantoms or ex vivo skull specimens under comparable sonication conditions. Such validation is essential to confirm the predictive accuracy of the model across different skull geometries, densities, and frequencies. Nevertheless, the results presented here show strong concordance with previously reported experimental and hybrid numerical–experimental studies. For example, Pichardo et al. (2011) demonstrated an exponential dependence of transmission loss on skull thickness and density across frequencies of 0.2–1 MHz using excised human skulls [
43], while Clement & Hynynen (2002) reported similar focal distortion and pressure attenuation trends in experimental measurements through human cranial bone [
89]. More recent validation efforts by Marquet et al. (2009) confirmed that finite-element and full-wave simulation frameworks using CT-derived skull maps accurately predict focal shift, transmission loss, and harmonic generation within 10–15% of measured values [
90]. These agreements reinforce the reliability of the present model as a realistic computational platform for future patient-specific treatment planning and parameter optimization in tFUS and HIFU applications.
The findings highlight the following outcomes: 1. Lower frequencies enhance both pressure transmission and result in broader focal zones, making them suitable for situations where deep penetration and energy delivery are prioritized over tight localization. 2. Skull thickness significantly diminishes both the maximum pressure ratio and the focus zone at higher frequencies, underlining the challenge of individualized parameter selection for patient-specific applications. 3. Optimizing the balance between frequency, skull anatomy, and transducer curvature is essential for maximizing the efficacy and safety of transcranial ultrasound therapy or stimulation in neurological and psychiatric disorder treatments.