Evolution of Anchor Polymer Systems Used in Arthroscopic Shoulder Surgery—A Comprehensive Review
Abstract
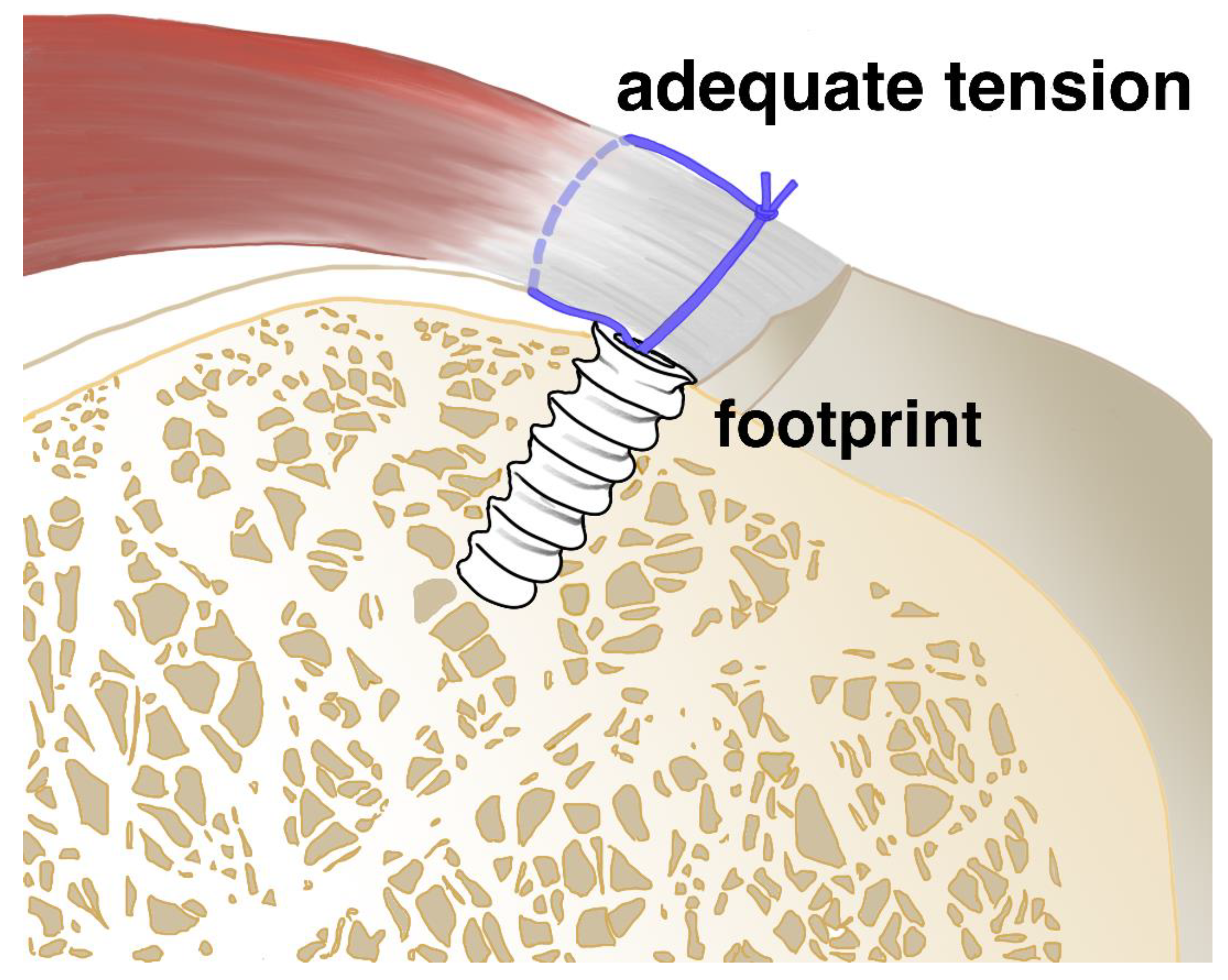
1. Introduction
2. Literature Search Strategy
3. Historical Evolution of Anchor Materials
3.1. From Metallic to Bioabsorbable Anchors
- Risk of migration into the joint space, potentially causing articular cartilage damage
- Interference with postoperative magnetic resonance imaging (MRI) due to metallic artifacts
- Permanent presence requiring removal during revision procedures
- Stress shielding effects leading to bone remodeling
3.2. Introduction of Bioabsorbable Polymers
- First Generation: Pure polyglycolic acid (PGA) anchors
- Second Generation: Poly-L-lactic acid (PLLA) anchors
- Third Generation: Copolymer systems (PLGA, PLDLA)
- Fourth Generation: Biocomposite anchors with ceramic fillers
- Fifth Generation: Advanced biocomposites with controlled degradation profiles
4. Bioabsorbable Polymer Systems
4.1. Polyglycolic Acid (PGA)
4.1.1. Chemical Properties and Degradation
- Initial degradation: Begins within the first week after implantation
- Complete resorption: Typically occurs within 6–12 weeks
- Degradation products: Glycolic acid, which is metabolized to carbon dioxide and water
4.1.2. Clinical Limitations
4.2. Poly-L-Lactic Acid (PLLA)
4.2.1. Chemical Properties and Degradation
- Degradation timeline: 2–5 years for complete resorption
- Degradation mechanism: Hydrolytic cleavage producing lactic acid
- Crystallinity: Higher crystalline content provides greater mechanical strength
4.2.2. Clinical Performance
- Maintained mechanical strength throughout the critical healing period
- Reduced inflammatory reactions compared to PGA
- Good biocompatibility profile
- Prolonged visibility on imaging studies (up to 7 years)
- Risk of foreign body reactions
- Potential for cyst formation around anchor sites
4.3. Poly-Lactic-co-Glycolic Acid (PLGA)
4.3.1. Chemical Composition and Tunable Properties
- PLGA 85:15 (85% lactide, 15% glycolide): Slower degradation (~24 months)
- PLGA 75:25: Intermediate degradation (~18 months)
- PLGA 50:50: Fastest degradation among PLGA formulations (~12 months)
4.3.2. Degradation Kinetics and Clinical Benefits
- Adequate mechanical support during critical healing periods
- Predictable resorption timeline
- Reduced risk of long-term foreign body reactions
- Compatibility with advanced imaging techniques
4.4. Transition Toward Metallic and Hybrid Systems
5. Biocomposite Anchor Systems
5.1. PLGA/β-Tricalcium Phosphate (β-TCP) Composites
5.1.1. Composition and Rationale
- 70–85% PLGA: Provides mechanical integrity and controlled degradation
- 15–30% β-TCP: Enhances osteoconductivity and bone ingrowth
5.1.2. Clinical Performance
- β-TCP provides osteoconductive scaffolding for bone ingrowth
- PLGA matrix maintains structural integrity during degradation
- Controlled release of calcium and phosphate ions promotes bone formation
5.2. Advanced Triple-Component Biocomposites
5.2.1. PLGA/β-TCP/Calcium Sulfate (CS) Systems
5.2.2. Degradation Timeline and Benefits
- Early Phase (0–12 weeks): CS degradation creates porosity for cellular infiltration
- Intermediate Phase (12–18 months): β-TCP provides osteoconductive framework
- Late Phase (18–24 months): PLGA matrix maintains structural support
5.2.3. Clinical Evidence
6. Biostable Polymer Systems: PEEK
6.1. Introduction to PEEK Anchors
6.1.1. Material Properties
- Chemical resistance: Excellent resistance to hydrolysis and chemical degradation
- Mechanical properties: High strength-to-weight ratio with optimal flexibility
- Biocompatibility: Excellent tissue tolerance with minimal inflammatory response
- Imaging compatibility: Radiolucent properties allowing clear postoperative imaging
6.1.2. Clinical Advantages
- Permanent mechanical fixation without degradation
- Superior imaging compatibility for postoperative monitoring
- Excellent biocompatibility with minimal tissue reaction
- Reliable mechanical properties throughout implant lifetime
6.2. Limitations and Challenges
6.2.1. Osseointegration Challenges
- Chemical inertness preventing cellular attachment
- Smooth surface characteristics limiting mechanical interlocking
- Lack of bioactive surface properties
6.2.2. Clinical Outcomes and Comparisons
7. Clinical Performance and Complications
7.1. Bioabsorbable Anchor Complications
7.1.1. Early Complications
7.1.2. Osteolysis and Cyst Formation
7.1.3. Loose Body Formation
7.2. PEEK Anchor Complications
7.2.1. Perianchor Cyst Formation
- Lower overall incidence compared to bioabsorbable anchors
- Different mechanism caused by mechanical micromotion rather than material degradation
- Generally smaller and less symptomatic cysts
7.2.2. Revision Surgery Challenges
8. Degradation Kinetics and Tissue Response
9. Future Directions and Emerging Technologies
10. Clinical Decision-Making Guidelines
10.1. Patient Factors
10.2. Surgical Factors
10.3. Material Properties
10.4. Evidence-Based Framework
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PGA | polyglycolic acid |
| PLLA | poly-L-lactic acid |
| PLGA | poly-lactic-co-glycolic acid |
| β-TCP | beta-tricalcium phosphate |
| CS | calcium sulfate |
| PEEK | polyetheretherketone |
References
- Lapner, P.; Henry, P.; Athwal, G.S.; Moktar, J.; McNeil, D.; MacDonald, P. Treatment of rotator cuff tears: A systematic review and meta-analysis. J. Shoulder Elb. Surg. 2022, 31, e120–e129. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, D.H.; Chung, S.W.; Yoon, J.P. Current concepts in arthroscopic rotator cuff repair. Clin. Shoulder Elb. 2025, 28, 103–112. [Google Scholar] [CrossRef]
- Dey Hazra, R.-O.; Ernat, J.J.; Rakowski, D.R.; Boykin, R.E.; Millett, P.J. The evolution of arthroscopic rotator cuff repair. Orthop. J. Sports Med. 2021, 9, 23259671211050899. [Google Scholar] [CrossRef]
- Gutman, M.J.; Kirsch, J.M.; Koa, J.; Fares, M.Y.; Abboud, J.A. Midterm outcomes of suture anchor fixation for displaced olecranon fractures. Clin. Shoulder Elb. 2024, 27, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, Y.S.; Park, I.; Lee, H.J.; Han, S.Y.; Jung, S.; Shin, S.J. A Comparison of Open-Construct PEEK Suture Anchor and Non-Vented Biocomposite Suture Anchor in Arthroscopic Rotator Cuff Repair: A Prospective Randomized Clinical Trial. Arthroscopy 2020, 36, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Visscher, L.E.; Jeffery, C.; Gilmour, T.; Anderson, L.; Couzens, G. The history of suture anchors in orthopaedic surgery. Clin. Biomech. 2019, 61, 70–78. [Google Scholar] [CrossRef]
- Jeong, H.J.; Lee, J.S.; Kim, Y.K.; Rhee, S.M.; Oh, J.H. Arthroscopic transosseous anchorless rotator cuff repair reduces bone defects related to peri-implant cyst formation: A comparison with conventional suture anchors using propensity score matching. Clin. Shoulder Elb. 2023, 26, 276–286. [Google Scholar] [CrossRef]
- Stenson, J.; Sanders, B.; Lazarus, M.; Austin, L. Arthroscopic Transosseous Rotator Cuff Repair. J. Am. Acad. Orthop. Surg. 2023, 31, e366–e375. [Google Scholar] [CrossRef] [PubMed]
- Denard, P.J.; Burkhart, S.S. The evolution of suture anchors in arthroscopic rotator cuff repair. Arthroscopy 2013, 29, 1589–1595. [Google Scholar] [CrossRef]
- Cho, C.H.; Bae, K.C.; Kim, D.H. Biomaterials Used for Suture Anchors in Orthopedic Surgery. Clin. Orthop. Surg. 2021, 13, 287–292. [Google Scholar] [CrossRef]
- Ock, J.; Seo, J.; Koh, K.H.; Kim, N. Comparing the biomechanical properties of conventional suture and all-suture anchors using patient-specific and realistic osteoporotic and non-osteoporotic phantom using 3D printing. Sci. Rep. 2023, 13, 20976. [Google Scholar] [CrossRef]
- Nouri, A.; Shirvan, A.R.; Li, Y.; Wen, C. Biodegradable metallic suture anchors: A review. Smart Mater. Manuf. 2023, 1, 100005. [Google Scholar] [CrossRef]
- Yang, Y.-S.; Shih, C.-A.; Fang, C.-J.; Huang, T.-T.; Hsu, K.-L.; Kuan, F.-C.; Su, W.-R.; Hong, C.-K. Biomechanical comparison of different suture anchors used in rotator cuff repair surgery–all-suture anchors are equivalent to other suture anchors: A systematic review and network meta-analysis. J. Exp. Orthop. 2023, 10, 45. [Google Scholar] [CrossRef]
- Keçeci, T.; Polat, Y.; Şahin, A.A.; Alparslan, M.; Sipahioğlu, S.; Çıraklı, A. Comparison of All-Suture Anchors and Metal Anchors in Arthroscopic Rotator Cuff Repair: Short-Term Clinical Outcomes and Anchor Pullout Risk. J. Clin. Med. 2025, 14, 2619. [Google Scholar] [CrossRef]
- Musa, A.A.; Bello, A.; Adams, S.M.; Onwualu, A.P.; Anye, V.C.; Bello, K.A.; Obianyo, I.I. Nano-Enhanced Polymer Composite Materials: A Review of Current Advancements and Challenges. Polymers 2025, 17, 893. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.K.; Thomas, B.; Thomas, S.B.; Tile, P.S.; Sakharwade, S.G. Carbon nanotubes as exceptional nanofillers in polymer and polymer/fiber nanocomposites: An extensive review. Mater. Today Commun. 2023, 37, 107358. [Google Scholar] [CrossRef]
- Damiri, F.; Fatimi, A.; Magdalena Musuc, A.; Paiva Santos, A.C.; Paszkiewicz, S.; Igwe Idumah, C.; Singh, S.; Varma, R.S.; Berrada, M. Nano-hydroxyapatite (nHAp) scaffolds for bone regeneration: Preparation, characterization and biological applications. J. Drug Deliv. Sci. Technol. 2024, 95, 105601. [Google Scholar] [CrossRef]
- Huang, D.; Li, Z.; Li, G.; Zhou, F.; Wang, G.; Ren, X.; Su, J. Biomimetic structural design in 3D-printed scaffolds for bone tissue engineering. Mater. Today Bio 2025, 32, 101664. [Google Scholar] [CrossRef] [PubMed]
- Milano, G.; Grasso, A.; Santagada, D.A.; Saccomanno, M.F.; Deriu, L.; Fabbriciani, C. Comparison between metal and biodegradable suture anchors in the arthroscopic treatment of traumatic anterior shoulder instability: A prospective randomized study. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 1785–1791. [Google Scholar] [CrossRef]
- Papalia, R.; Franceschi, F.; Diaz Balzani, L.; D’Adamio, S.; Denaro, V.; Maffulli, N. The arthroscopic treatment of shoulder instability: Bioabsorbable and standard metallic anchors produce equivalent clinical results. Arthroscopy 2014, 30, 1173–1183. [Google Scholar] [CrossRef]
- Godinho, G.G.; França, F.O.; Alves Freitas, J.M.; Aguiar, P.N.; de Carvalho Leite, M. Complications resulting from the use of metal anchors in shoulder arthroscopy. Rev. Bras. Ortop. 2009, 44, 143–147. [Google Scholar] [CrossRef]
- Nho, S.J.; Provencher, M.T.; Seroyer, S.T.; Romeo, A.A. Bioabsorbable anchors in glenohumeral shoulder surgery. Arthroscopy 2009, 25, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Piatti, M.; Gorla, M.; Alberio, F.; Omeljaniuk, R.J.; Rigamonti, L.; Gaddi, D.; Turati, M.; Bigoni, M. Comparison of all-suture anchors with metallic anchors in arthroscopic cuff repair: Structural and functional properties and clinical suitability. J. Orthop. 2023, 39, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Ozbaydar, M.; Elhassan, B.; Warner, J.J. The use of anchors in shoulder surgery: A shift from metallic to bioabsorbable anchors. Arthroscopy 2007, 23, 1124–1126. [Google Scholar] [CrossRef] [PubMed]
- Walton, R.A.; Liuzza, L.; Takawira, C.; Leonardi, C.; Lopez, M.J. Biocomposite Anchors Have Greater Yield Load and Energy Compared with All-Suture Anchors in an In Vitro Ovine Infraspinatus Tendon Repair Model. Arthrosc. Sports Med. Rehabil. 2024, 6, 100938. [Google Scholar] [CrossRef] [PubMed]
- Cummins, C.A.; Murrell, G.A. Mode of failure for rotator cuff repair with suture anchors identified at revision surgery. J. Shoulder Elb. Surg. 2003, 12, 128–133. [Google Scholar] [CrossRef]
- Oleksy, M.; Dynarowicz, K.; Aebisher, D. Advances in biodegradable polymers and biomaterials for medical applications—A review. Molecules 2023, 28, 6213. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Agrawal, C.M.; Barber, F.A.; Burkhart, S.S. Orthopaedic applications for PLA-PGA biodegradable polymers. Arthroscopy 1998, 14, 726–737. [Google Scholar] [CrossRef]
- Nikolaidou, O.; Migkou, S.; Karampalis, C. Rehabilitation after rotator cuff repair. Open Orthop. J. 2017, 11, 154. [Google Scholar] [CrossRef]
- Schonebaum, D.I.; Garbaccio, N.; Escobar-Domingo, M.J.; Wood, S.; Smith, J.E.; Foster, L.; Mehdizadeh, M.; Cordero, J.J.; Foppiani, J.A.; Choudry, U. Comparing Biomechanical Properties of Bioabsorbable Suture Anchors: A Comprehensive Review. Biomimetics 2025, 10, 175. [Google Scholar] [CrossRef]
- Hussain, M.; Khan, S.M.; Shafiq, M.; Abbas, N. A review on PLA-based biodegradable materials for biomedical applications. Giant 2024, 18, 100261. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.I.; Lee, H.J.; Kim, D.J.; Sung, G.Y.; Kwak, D.H.; Kim, Y.S. Long-term Follow-up of Extensive Peri-anchor (Poly-L/D-lactic Acid) Cyst Formation after Arthroscopic Rotator Cuff Repair: A Case Report. Clin. Shoulder Elb. 2019, 22, 100–105. [Google Scholar] [CrossRef]
- Lu, Y.; Cheng, D.; Niu, B.; Wang, X.; Wu, X.; Wang, A. Properties of poly (lactic-co-glycolic acid) and progress of poly (lactic-co-glycolic acid)-based biodegradable materials in biomedical research. Pharmaceuticals 2023, 16, 454. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yin, G.; Sun, S.; Xu, P. Medical applications and prospects of polylactic acid materials. iScience 2024, 27, 111512. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, D.Y.; Kwon, J.E.; Park, J.S.; Oh, J.H. Perianchor Cyst Formation Around Biocomposite Biodegradable Suture Anchors After Rotator Cuff Repair. Am. J. Sports Med. 2015, 43, 2907–2912. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-C.; Wu, G.-L.; Yeh, M.-L. Fixation technique of biodegradable magnesium alloy suture anchor in the rotator cuff repair of the shoulder in a goat model: A technical note. BMC Musculoskelet. Disord. 2024, 25, 246. [Google Scholar] [CrossRef]
- Chung, S.W.; Oh, K.S.; Kang, S.J.; Yoon, J.P.; Kim, J.Y. Clinical Outcomes of Arthroscopic Rotator Cuff Repair Using Poly Lactic-co-glycolic Acid Plus β-tricalcium Phosphate Biocomposite Suture Anchors. Clin. Shoulder Elb. 2018, 21, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Barber, F.A.; Dockery, W.D.; Cowden, C.H., 3rd. The degradation outcome of biocomposite suture anchors made from poly L-lactide-co-glycolide and β-tricalcium phosphate. Arthroscopy 2013, 29, 1834–1839. [Google Scholar] [CrossRef]
- Barber, F.A.; Dockery, W.D.; Hrnack, S.A. Long-term degradation of a poly-lactide co-glycolide/β-tricalcium phosphate biocomposite interference screw. Arthroscopy 2011, 27, 637–643. [Google Scholar] [CrossRef]
- Scholes, C.; Fatima, M.; Moody, C.; Eng, K.; Page, R.S. Stage 2a IDEAL evaluation of a third-generation biocomposite suture anchor in arthroscopic rotator cuff repair: Subgroup cohort analysis of the PRULO registry with 12-month follow up. medRxiv 2024. [Google Scholar] [CrossRef]
- Vonhoegen, J.; John, D.; Hägermann, C. Osteoconductive resorption characteristics of a novel biocomposite suture anchor material in rotator cuff repair. J. Orthop. Surg. Res. 2019, 14, 12. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Tang, T. Current strategies to improve the bioactivity of PEEK. Int. J. Mol. Sci. 2014, 15, 5426–5445. [Google Scholar] [CrossRef]
- Pandey, V.; Madi, S. A Retrospective study on the efficacy, safety, and clinical and radiological outcomes of PEEK anchors (CEPTRE® knotted suture anchor and VIPLOK® knotless anchor) in the treatment of rotator cuff repairs. Cureus 2023, 15, e48632. [Google Scholar] [CrossRef]
- Baek, C.H.; Kim, J.G.; Kim, B.T.; Kim, S.J. Isolated latissimus dorsi transfer versus combined latissimus dorsi and teres major tendon transfer for irreparable anterosuperior rotator cuff tears. Clin. Orthop. Surg. 2024, 16, 761. [Google Scholar] [CrossRef]
- Noh, K.-C.; Morya, V.K. Double pulley-triple row technique for arthroscopic rotator cuff repair: A technical note. Clin. Orthop. Surg. 2024, 17, 181. [Google Scholar] [CrossRef]
- Abdullah, M.R.; Goharian, A.; Abdul Kadir, M.R.; Wahit, M.U. Biomechanical and bioactivity concepts of polyetheretherketone composites for use in orthopedic implants-a review. J. Biomed. Mater. Res. Part A 2015, 103, 3689–3702. [Google Scholar] [CrossRef] [PubMed]
- Bradford, J.P.; Hernandez-Moreno, G.; Pillai, R.R.; Hernandez-Nichols, A.L.; Thomas, V. Low-Temperature Plasmas Improving Chemical and Cellular Properties of Poly (Ether Ether Ketone) Biomaterial for Biomineralization. Materials 2023, 17, 171. [Google Scholar] [CrossRef] [PubMed]
- Durham, J.W., 3rd; Montelongo, S.A.; Ong, J.L.; Guda, T.; Allen, M.J.; Rabiei, A. Hydroxyapatite coating on PEEK implants: Biomechanical and histological study in a rabbit model. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 723–731. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, P.; Zhang, X.; Jingguo, X.; Yongjie, W.; Zou, X.; Mei, X.; Zhang, S.; Zhang, S. Strategies to improve bioactive and antibacterial properties of polyetheretherketone (PEEK) for use as orthopedic implants. Mater. Today Bio 2022, 16, 100402. [Google Scholar] [CrossRef]
- Salentiny, Y.; Lassandro, N.; Karanassos, M.; Boudard, G.; Bataille, J.F.; Guignand, D.; Le Rue, O.; Moreel, P.; Navez, G.; George, T. Clinical and radiological outcome after arthroscopic rotator cuff repair using PEEK-CF anchors. Orthop. Traumatol. Surg. Res. 2024, 110, 103714. [Google Scholar] [CrossRef]
- Chahla, J.; Liu, J.N.; Manderle, B.; Beletsky, A.; Cabarcas, B.; Gowd, A.K.; Inoue, N.; Chubinskaya, S.; Trenhaile, S.; Forsythe, B.; et al. Bony Ingrowth of Coil-Type Open-Architecture Anchors Compared With Screw-Type PEEK Anchors for the Medial Row in Rotator Cuff Repair: A Randomized Controlled Trial. Arthroscopy 2020, 36, 952–961. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, Y.-B. Clinical and radiologic outcomes of combined use of biocomposite and peek suture anchors during arthroscopic rotator cuff repair: A prospective observational study. J. Clin. Med. 2020, 9, 2545. [Google Scholar] [CrossRef]
- Suroto, H.; Anindita Satmoko, B.; Prajasari, T.; De Vega, B.; Wardhana, T.H.; Samijo, S.K. Biodegradable vs nonbiodegradable suture anchors for rotator cuff repair: A systematic review and meta-analysis. EFORT Open Rev. 2023, 8, 731–747. [Google Scholar] [CrossRef] [PubMed]
- Glueck, D.; Wilson, T.C.; Johnson, D.L. Extensive osteolysis after rotator cuff repair with a bioabsorbable suture anchor: A case report. Am. J. Sports Med. 2005, 33, 742–744. [Google Scholar] [CrossRef] [PubMed]
- Cole, E.W.; Werner, B.C.; Denard, P.J. Perianchor cyst formation is similar between all-suture and conventional suture anchors used for arthroscopic rotator cuff repair in the same shoulder. Arthrosc. Sports Med. Rehabil. 2022, 4, e949–e955. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kang, J.-S.; Park, I.; Shin, S.-J. Serial changes in perianchor cysts following arthroscopic labral repair using all-suture anchors. Clin. Orthop. Surg. 2020, 13, 229. [Google Scholar] [CrossRef]
- Ranson, J.; Hoggett, L.; Mulgrew, E.; Jain, N. Biochemical and biomechanical influence on peri anchor cyst formation in rotator cuff repair. Acta Orthop. Belg. 2022, 88, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Jing, L.; He, P.; Wu, W.; Wang, Z.; Zhao, F.; Zhang, J.; Li, D.; Xu, P.; Yan, H. Period of formation of and factors associated with perianchor cysts around Healicoil and Footprint PEEK anchors following arthroscopic rotator cuff repair. J. Shoulder Elb. Surg. 2025. [Google Scholar] [CrossRef]
- Barber, F.A. Biodegradable shoulder anchors have unique modes of failure. Arthroscopy 2007, 23, 316–320. [Google Scholar] [CrossRef]
- Nusselt, T.; Freche, S.; Klinger, H.M.; Baums, M.H. Intraosseous foreign body granuloma in rotator cuff repair with bioabsorbable suture anchor. Arch. Orthop. Trauma Surg. 2010, 130, 1037–1040. [Google Scholar] [CrossRef][Green Version]
- Kim, S.H.; Yang, S.H.; Rhee, S.M.; Lee, K.J.; Kim, H.S.; Oh, J.H. The formation of perianchor fluid associated with various suture anchors used in rotator cuff repair: All-suture, polyetheretherketone, and biocomposite anchors. Bone Jt. J. 2019, 101-b, 1506–1511. [Google Scholar] [CrossRef]
- Ro, K.; Pancholi, S.; Son, H.S.; Rhee, Y.G. Perianchor Cyst Formation After Arthroscopic Rotator Cuff Repair Using All-Suture-Type, Bioabsorbable-Type, and PEEK-Type Anchors. Arthroscopy 2019, 35, 2284–2292. [Google Scholar] [CrossRef] [PubMed]
- Güleçyüz, M.F.; Pietschmann, M.F.; Scharpf, A.; Eid, A.S.; Michalski, S.; Simon, J.M.; Niethammer, T.R.; Müller, P.E. Revisability of polyetheretherketone suture anchors utilised in primary and revision Bankart repair. J. Orthop. Sci. 2020, 25, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Vert, M. Degradable and bioresorbable polymers in surgery and in pharmacology: Beliefs and facts. J. Mater. Sci. Mater. Med. 2009, 20, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, C.G.; Clanton, T.O. Bioabsorbable implants: Review of clinical experience in orthopedic surgery. Ann. Biomed. Eng. 2004, 32, 171–177. [Google Scholar] [CrossRef]
- Umrath, F.; Schmitt, L.-F.; Kliesch, S.-M.; Schille, C.; Geis-Gerstorfer, J.; Gurewitsch, E.; Bahrini, K.; Peters, F.; Reinert, S.; Alexander, D. Mechanical and functional improvement of β-TCP scaffolds for use in bone tissue engineering. J. Funct. Biomater. 2023, 14, 427. [Google Scholar] [CrossRef]
- Jakimiuk, A.; Maintz, M.; Müller-Gerbl, M.; Thieringer, F.M.; Keller, M.; Guebeli, A.; Honigmann, P. 3D-printed patient-specific implants made of polylactide (PLDLLA) and β-tricalcium phosphate (β-TCP) for corrective osteotomies of the distal radius. 3D Print. Med. 2024, 10, 42. [Google Scholar] [CrossRef]
- Gunatillake, P.A.; Adhikari, R. Biodegradable synthetic polymers for tissue engineering. Eur. Cells Mater. 2003, 5, 1–16; discussion 16. [Google Scholar] [CrossRef]
- Ruan, C.; Hu, N.; Ma, Y.; Li, Y.; Liu, J.; Zhang, X.; Pan, H. The interfacial pH of acidic degradable polymeric biomaterials and its effects on osteoblast behavior. Sci. Rep. 2017, 7, 6794. [Google Scholar] [CrossRef]
- Smith, A.N.; Ulsh, J.B.; Gupta, R.; Tang, M.M.; Peredo, A.P.; Teinturier, T.D.; Mauck, R.L.; Gullbrand, S.; Hast, M.W. Characterization of degradation kinetics of additively manufactured PLGA under variable mechanical loading paradigms. J. Mech. Behav. Biomed. Mater. 2024, 153, 106457. [Google Scholar] [CrossRef]
- Lalitha Sridhar, S.; Vernerey, F. Localized enzymatic degradation of polymers: Physics and scaling laws. Phys. Rev. Appl. 2018, 9, 031001. [Google Scholar] [CrossRef]
- Rhee, S.M.; Kim, D.H.; Kim, M.S. Magnetic resonance imaging for relationship between the severity of perianchor fluid collection and rotator cuff integrity after arthroscopic double-row suture-bridge rotator cuff repair. Orthop. Traumatol. Surg. Res. 2024, 110, 103897. [Google Scholar] [CrossRef]
- Barber, F.A.; Dockery, W.D. Long-term absorption of poly-L-lactic Acid interference screws. Arthroscopy 2006, 22, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Randelli, P.; Compagnoni, R.; Aliprandi, A.; Cannaò, P.M.; Ragone, V.; Tassi, A.; Cabitza, P. Long-term degradation of poly-lactic co-glycolide/β-tricalcium phosphate biocomposite anchors in arthroscopic bankart repair: A prospective study. Arthroscopy 2014, 30, 165–171. [Google Scholar] [CrossRef]
- Matsuki, K.; Sugaya, H.; Takahashi, N.; Kawasaki, T.; Yoshimura, H.; Kenmoku, T. Degradation of Cylindrical Poly-Lactic Co-Glycolide/Beta-Tricalcium Phosphate Biocomposite Anchors After Arthroscopic Bankart Repair: A Prospective Study. Orthopedics 2018, 41, e348–e353. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Zhang, Z.; Zhu, W.; Weng, X. Polyetheretherketone development in bone tissue engineering and orthopedic surgery. Front. Bioeng. Biotechnol. 2023, 11, 1207277. [Google Scholar] [CrossRef]
- Tang, S.; Shen, Y.; Jiang, L.; Zhang, Y. Surface Modification of nano-hydroxyapatite/polymer composite for bone tissue repair applications: A Review. Polymers 2024, 16, 1263. [Google Scholar] [CrossRef]
- Zhou, L.; Qian, Y.; Zhu, Y.; Liu, H.; Gan, K.; Guo, J. The effect of different surface treatments on the bond strength of PEEK composite materials. Dent. Mater. 2014, 30, e209–e215. [Google Scholar] [CrossRef]
- Sheikh, Z.; Javaid, M.A.; Hamdan, N.; Hashmi, R. Bone Regeneration Using Bone Morphogenetic Proteins and Various Biomaterial Carriers. Materials 2015, 8, 1778–1816. [Google Scholar] [CrossRef]
- De Taillac, L.B.; Porté-Durrieu, M.C.; Labrugère, C.; Bareille, R.; Amédée, J.; Baquey, C. Grafting of RGD peptides to cellulose to enhance human osteoprogenitor cells adhesion and proliferation. Compos. Sci. Technol. 2004, 64, 827–837. [Google Scholar] [CrossRef]
- Rambhia, K.J.; Ma, P.X. Controlled drug release for tissue engineering. J. Control. Release 2015, 219, 119–128. [Google Scholar] [CrossRef]
- Ni, X.; Xing, X.; Deng, Y.; Li, Z. Applications of stimuli-responsive hydrogels in bone and cartilage regeneration. Pharmaceutics 2023, 15, 982. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xue, W.; Yun, Z.; Liu, Q.; Sun, X. Biomedical applications of stimuli-responsive “smart” interpenetrating polymer network hydrogels. Mater. Today Bio 2024, 25, 100998. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wu, Z.; Zhang, K.; Weir, M.D.; Xu, H.H.; Cheng, L.; Huang, X.; Zhou, W. Unlocking the potential of stimuli-responsive biomaterials for bone regeneration. Front. Pharmacol. 2024, 15, 1437457. [Google Scholar] [CrossRef]
- Peng, X.; Peng, Q.; Wu, M.; Wang, W.; Gao, Y.; Liu, X.; Sun, Y.; Yang, D.; Peng, Q.; Wang, T.; et al. A pH and Temperature Dual-Responsive Microgel-Embedded, Adhesive, and Tough Hydrogel for Drug Delivery and Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 19560–19573. [Google Scholar] [CrossRef]
- Ansari, M.A.A.; Dash, M.; Camci-Unal, G.; Jain, P.K.; Nukavarapu, S.; Ramakrishna, S.; Falcone, N.; Dokmeci, M.R.; Najafabadi, A.H.; Khademhosseini, A. Engineered stimuli-responsive smart grafts for bone regeneration. Curr. Opin. Biomed. Eng. 2023, 28, 100493. [Google Scholar] [CrossRef]
- Creton, C.; Ciccotti, M. Fracture and adhesion of soft materials: A review. Rep. Prog. Phys. 2016, 79, 046601. [Google Scholar] [CrossRef]
- Song, P.; Zhou, D.; Wang, F.; Li, G.; Bai, L.; Su, J. Programmable biomaterials for bone regeneration. Mater. Today Bio 2024, 29, 101296. [Google Scholar] [CrossRef]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Zhou, C.; Li, Q.; Liu, L.; Jiang, C.; Dai, H.; Zhang, H.; Zhao, J.; Liang, W. Nanotechnology in Orthopedic Care: Advances in Drug Delivery, Implants, and Biocompatibility Considerations. Int. J. Nanomed. 2025, 20, 9251–9274. [Google Scholar] [CrossRef]
- Omidian, H.; Wilson, R.L. PLGA Implants for Controlled Drug Delivery and Regenerative Medicine: Advances, Challenges, and Clinical Potential. Pharmaceuticals 2025, 18, 631. [Google Scholar] [CrossRef]
- Lee, W.-Y.; Jeon, Y.-S.; Kim, K.-C.; Shin, H.-D.; Joo, Y.-B.; Chung, H.-J. Comparative Analysis of Bone Mineral Density of the Lumbar Spine, Hip, and Proximal Humerus in Patients with Unilateral Rotator Cuff Tears. Clin. Orthop. Surg. 2024, 16, 751. [Google Scholar] [CrossRef]
- Costăchescu, B.; Niculescu, A.G.; Grumezescu, A.M.; Teleanu, D.M. Screw Osteointegration-Increasing Biomechanical Resistance to Pull-Out Effect. Materials 2023, 16, 5582. [Google Scholar] [CrossRef]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef]
- Citro, V.; Clerici, M.; Boccaccini, A.R.; Della Porta, G.; Maffulli, N.; Forsyth, N.R. Tendon tissue engineering: An overview of biologics to promote tendon healing and repair. J. Tissue Eng. 2023, 14, 20417314231196275. [Google Scholar] [CrossRef] [PubMed]
- Morya, V.K.; Lang, J.; Lee, Y.-b.; Kim, J.W.; Lee, K.U.; Noh, K.-C. A Narrative Review on the Double Pulley-Triple Row Technique for Large to Massive Rotator Cuff Repair. Clin. Orthop. Surg. 2025, 17, 359. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-R.; Chun, Y.-M. Robot-assisted orthopedic surgeries around shoulder joint: Where we are? Biomed. Eng. Lett. 2023, 13, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Kunze, K.N.; Ferguson, D.; Pareek, A.; Colyvas, N. Robotic-Assisted Arthroscopy Promises Enhanced Procedural Efficiency, Visualization, and Control but Must Overcome Barriers to Adoption. HSS J.® 2025, 21, 283–288. [Google Scholar] [CrossRef]
- De Marinis, R.; Marigi, E.M.; Atwan, Y.; Yang, L.; Oeding, J.F.; Gupta, P.; Pareek, A.; Sanchez-Sotelo, J.; Sperling, J.W. Current clinical applications of artificial intelligence in shoulder surgery: What the busy shoulder surgeon needs to know and what’s coming next. JSES Rev. Rep. Tech. 2023, 3, 447–453. [Google Scholar] [CrossRef]
- Han, F.; Huang, X.; Wang, X.; Chen, Y.-f.; Lu, C.; Li, S.; Lu, L.; Zhang, D.W. Artificial Intelligence in Orthopedic Surgery: Current Applications, Challenges, and Future Directions. MedComm 2025, 6, e70260. [Google Scholar] [CrossRef] [PubMed]

| Anchor Type | Cyst Formation Rate | Severe Cyst Rate | Other Reported Complications | Timeline | Reference |
|---|---|---|---|---|---|
| PLLA | 15–30% | 5–10% | Inflammatory reaction, osteolysis, foreign body response | 12–24 months | [32,35] |
| PLGA/β-TCP | 40–50% | 10–15% | Mild osteolytic changes | 6–18 months | [41] |
| PLGA/β-TCP/CS | <5% | <2% | Minimal osteolysis; no anchor pull-out or migration | 12–21 months | [54] |
| Material | Initial Strength Loss | 50% Mass Loss | Complete Resorption | Reference |
|---|---|---|---|---|
| PGA | 2–4 weeks | 6–8 weeks | 12–16 weeks | [27,28] |
| PLLA | 6–12 months | 18–36 months | 36–60 months | [27,28,31,74] |
| PLGA (85:15) | 6–12 months | 12–18 months | 24–30 months | [27,38,39] |
| PLGA/β-TCP | 8–12 months | 18–24 months | 30–36 months | [38,39,75,76] |
| PLGA/β-TCP/CS | 6–9 months | 15–21 months | 21–24 months | [41] |
| Clinical Scenario | First Choice | Second Choice | Rationale |
|---|---|---|---|
| Primary rotator cuff repair (young patient) | PLGA/β-TCP/CS | PLGA/β-TCP | Optimal degradation profile and osteoconductivity |
| Primary rotator cuff repair (elderly patient) | PEEK | PLGA/β-TCP | Stable fixation in low-density bone; avoids degradation issues |
| Revision surgery | PEEK | PLGA/β-TCP | Preserves bone stock and ensures radiolucent fixation |
| Large/massive tears | PEEK | PLGA/β-TCP | High mechanical strength and fatigue resistance |
| Bankart repair | PLGA/β-TCP | PEEK | Balanced mechanical and biological performance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, E.-J.; Kwon, K.-E.; Kim, J.-H. Evolution of Anchor Polymer Systems Used in Arthroscopic Shoulder Surgery—A Comprehensive Review. Bioengineering 2025, 12, 1146. https://doi.org/10.3390/bioengineering12111146
Yoon E-J, Kwon K-E, Kim J-H. Evolution of Anchor Polymer Systems Used in Arthroscopic Shoulder Surgery—A Comprehensive Review. Bioengineering. 2025; 12(11):1146. https://doi.org/10.3390/bioengineering12111146
Chicago/Turabian StyleYoon, Eun-Ji, Kyeong-Eon Kwon, and Jong-Ho Kim. 2025. "Evolution of Anchor Polymer Systems Used in Arthroscopic Shoulder Surgery—A Comprehensive Review" Bioengineering 12, no. 11: 1146. https://doi.org/10.3390/bioengineering12111146
APA StyleYoon, E.-J., Kwon, K.-E., & Kim, J.-H. (2025). Evolution of Anchor Polymer Systems Used in Arthroscopic Shoulder Surgery—A Comprehensive Review. Bioengineering, 12(11), 1146. https://doi.org/10.3390/bioengineering12111146





