Heart Rate Variability Patterns Reflect Yoga Intervention in Chronically Stressed Pregnant Women: A Quasi-Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Participants
2.2. Questionnaires: Maternal Perceived Stress, Sociodemographic Questionnaire, Physical Activity Questionnaire
2.3. Hatha Yoga and Yoga Nidra
2.4. ECG Acquisition and SampEn and Entropy Rate Computation
2.5. Heart Rate Variability (HRV) Computation Approach
- Temporal Domain (25 metrics): Traditional statistical measures of R-R interval variability, including Mean NN, SDNN, RMSSD, pNN50, pNN20, SDANN (1-, 2-, and 5-min segments), coefficient of variation measures, percentile-based indices, and geometric measures (HTI, TINN).
- Frequency Domain (6 metrics): Power frequency analysis yielding low frequency (LF, 0.04–0.15 Hz), high frequency (HF, 0.15–0.4 Hz), total power (TP, 0–0.4 Hz), LF/HF ratio, and normalized units (LFnu, HFnu).
- Complexity/Information Domain (54 metrics): Nonlinear dynamics measures including Poincaré plot indices (SD1, SD2), entropy measures (approximate entropy [ApEn], sample entropy [SampEn], Shannon entropy, fuzzy entropy, multiscale entropy variants), detrended fluctuation analysis (DFA) scaling exponents (α1, α2), multifractal DFA parameters, correlation dimensions, fractal dimensions (Higuchi, Katz), complexity indices (CSI, CVI), and Lempel-Ziv complexity. Entropy rate measures the information generation rate (for details, see the respective subsection).
- Specialized Domain (9 metrics): Additional cardiac measures including heart rate turbulence parameters, coefficient of variation, temporal variability indices, and frequency characteristics (centroid frequency, bandwidth).
2.6. Statistical Methods
3. Results
3.1. Group Characteristics
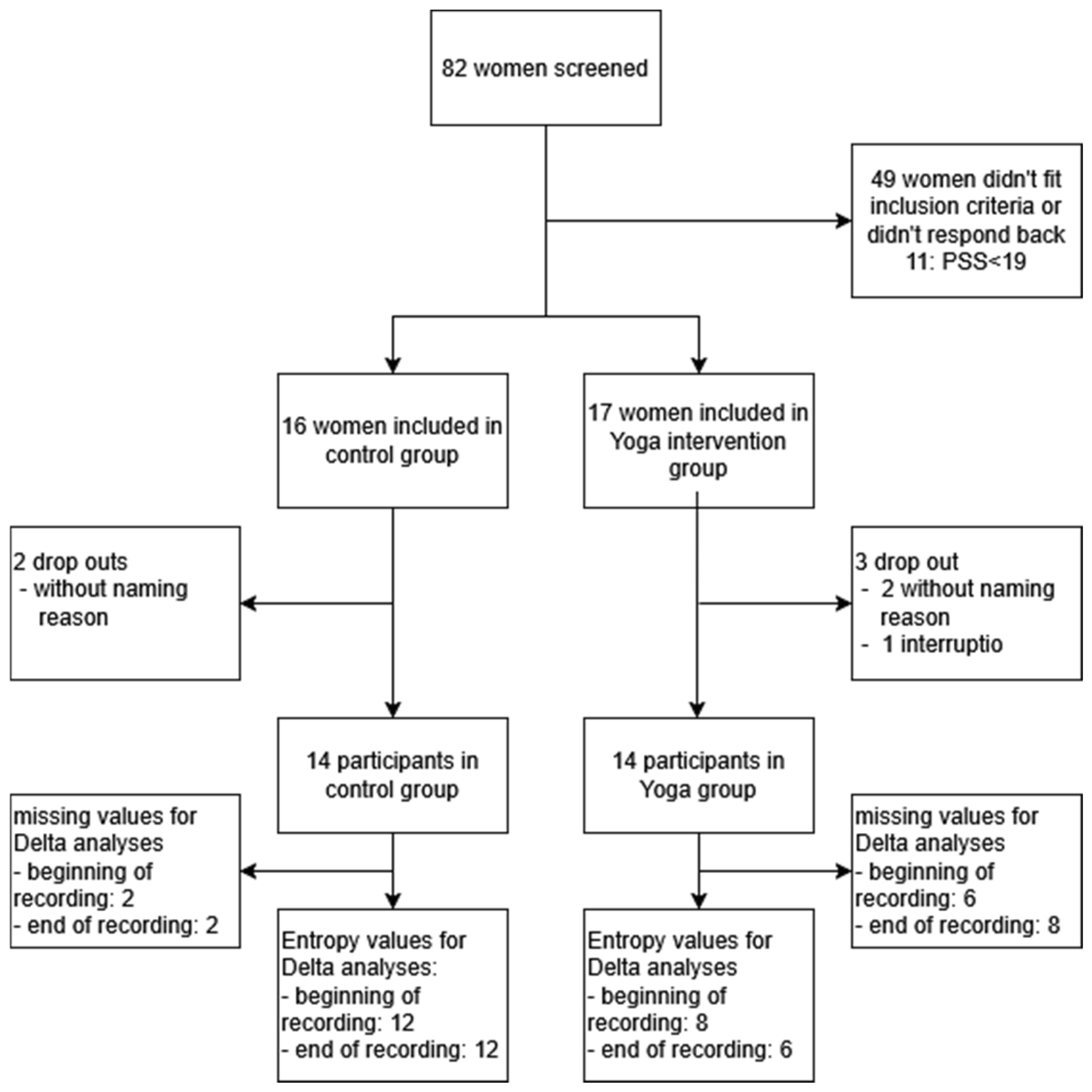
3.2. Data Quality
3.3. Primary Outcomes
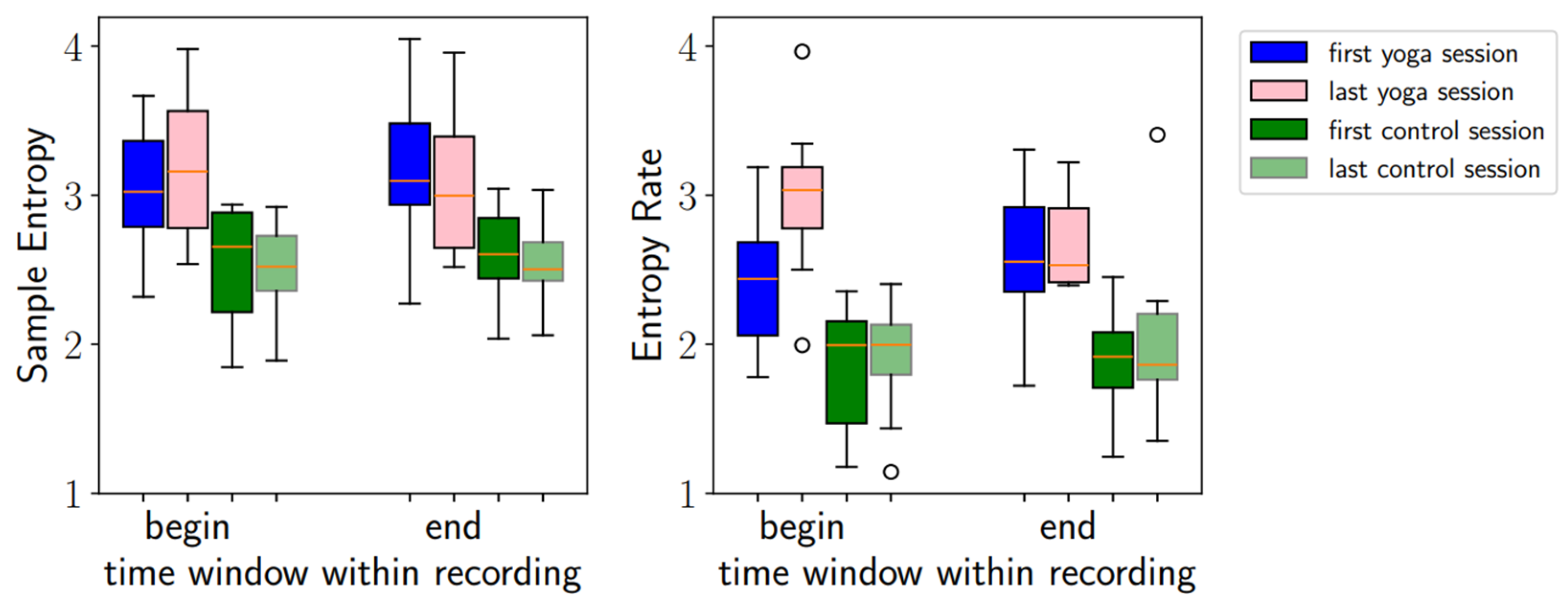
3.3.1. Sample Entropy and Entropy Rate
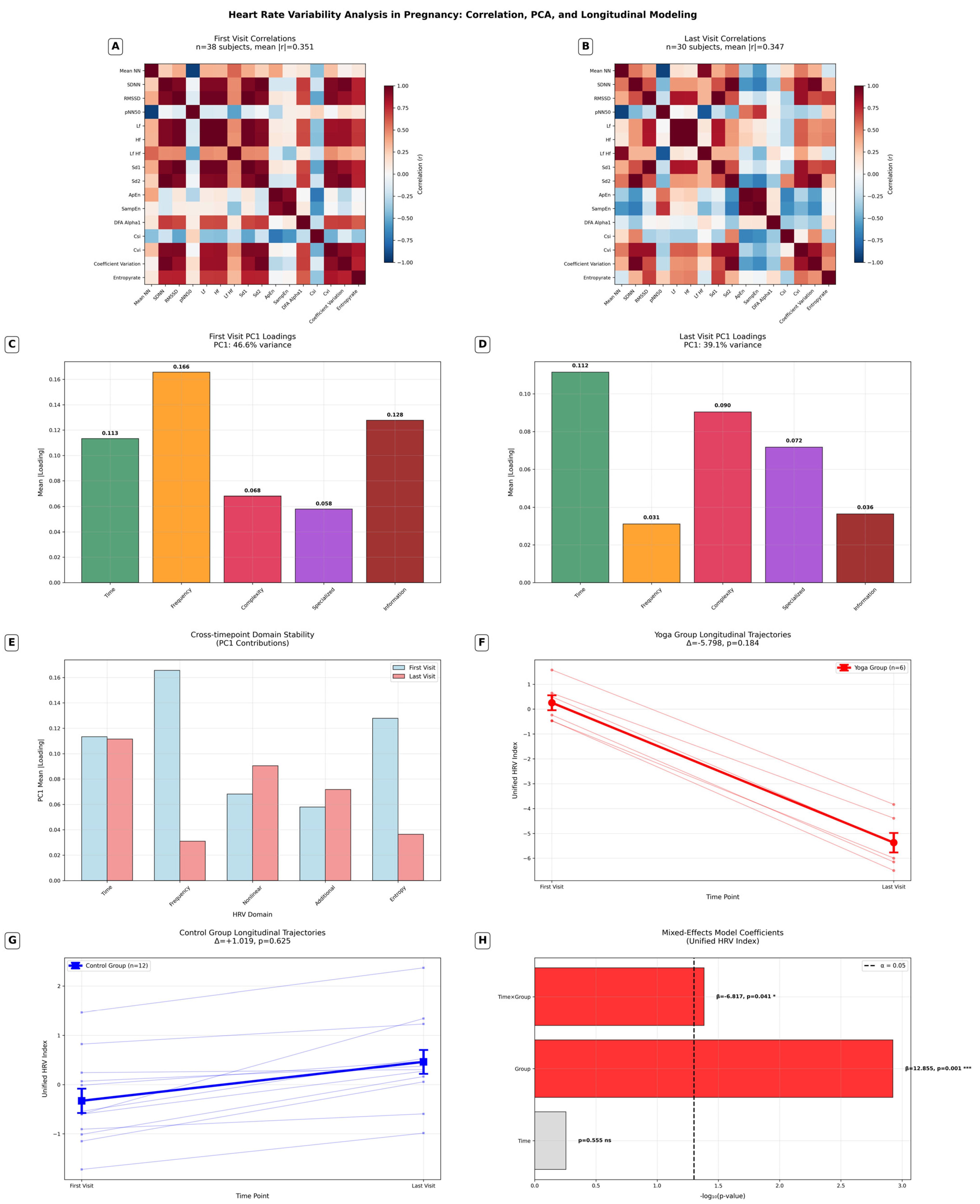
3.3.2. Comprehensive HRV Analysis
- Correlation network simplification: a 36% reduction in high correlations during pregnancy progression (454→290 pairs).
- HRV domain restructuring: frequency measures decreased (−81.2%) while complexity measures increased (+32.8%) in late pregnancy.
- Baseline differences: significant initial difference of the Yoga group in the unified HRV index (p = 0.001).
- Differential trajectories: significant time × group interaction (p = 0.041), suggesting that Yoga modulated the pattern of HRV changes.
- Effect sizes: moderate for Yoga group changes (d = −0.521) versus small for controls (d = 0.106).
4. Discussion
4.1. Key Findings and Interpretation
4.1.1. SampEn and Entropy Rate
4.1.2. Comprehensive HRV Analysis
4.2. Relation to Previous Research
4.3. Strengths and Study Limitations
4.3.1. Strengths
4.3.2. Limitations
4.4. Implications and Future Research
4.4.1. Wearables and Feedback
4.4.2. Relation of Maternal Entropy and Fetal Health
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PS | Prenatal stress |
| HRV | Heart rate variability |
| ANS | Autonomic nervous system |
| HPA axis | Hypothalamic–Pituitary–Adrenal axis |
| aECG | Transabdominal electrocardiogram |
| mHR | Maternal heart rate |
| fHR | Fetal heart rate |
| FSI | Fetal stress index |
| PCA SampEn | Principal Component Analysis Sample Entropy |
| PC | Principal component |
Appendix A
| Rank | Metric Name | Category | Description |
|---|---|---|---|
| 1 | MeanNN | Time Domain | Time domain HRV metric: MeanNN |
| 2 | SDNN | Time Domain | Standard Deviation of Normal-to-Normal intervals |
| 3 | SDANN1 | Time Domain | SDANN–standard deviation of average NN in segments |
| 4 | SDNNI1 | Time Domain | SDNNI–mean of standard deviations in segments |
| 5 | SDANN2 | Time Domain | SDANN–standard deviation of average NN in segments |
| 6 | SDNNI2 | Time Domain | SDNNI–mean of standard deviations in segments |
| 7 | SDANN5 | Time Domain | SDANN–standard deviation of average NN in segments |
| 8 | SDNNI5 | Time Domain | SDNNI–mean of standard deviations in segments |
| 9 | RMSSD | Time Domain | Root Mean Square of Successive Differences |
| 10 | SDSD | Time Domain | Time domain HRV metric: SDSD |
| 11 | CVNN | Time Domain | Coefficient of variation measure |
| 12 | CVSD | Time Domain | Coefficient of variation measure |
| 13 | MedianNN | Time Domain | Time domain HRV metric: MedianNN |
| 14 | MadNN | Time Domain | Time domain HRV metric: MadNN |
| 15 | MCVNN | Time Domain | Time domain HRV metric: MCVNN |
| 16 | IQRNN | Time Domain | Time domain HRV metric: IQRNN |
| 17 | SDRMSSD | Time Domain | Time domain HRV metric: SDRMSSD |
| 18 | Prc20NN | Time Domain | Time domain HRV metric: Prc20NN |
| 19 | Prc80NN | Time Domain | Time domain HRV metric: Prc80NN |
| 20 | pNN50 | Time Domain | Percentage of NN intervals > 50 ms different |
| 21 | pNN20 | Time Domain | Percentage of NN intervals > 20 ms different |
| 22 | MinNN | Time Domain | Time domain HRV metric: MinNN |
| 23 | MaxNN | Time Domain | Time domain HRV metric: MaxNN |
| 24 | HTI | Time Domain | Time domain HRV metric: HTI |
| 25 | TINN | Time Domain | Triangular Interpolation of NN interval histogram |
| 26 | LF | Frequency Domain | Low Frequency power (0.04–0.15 Hz) |
| 27 | HF | Frequency Domain | High Frequency power (0.15–0.4 Hz) |
| 28 | TP | Frequency Domain | Total power |
| 29 | LF_HF | Frequency Domain | LF/HF ratio |
| 30 | LFnu | Frequency Domain | Low Frequency power normalized (0.04–0.15 Hz) |
| 31 | HFnu | Frequency Domain | High Frequency power normalized (0.15–0.4 Hz) |
| 32 | SD1 | Nonlinear | Poincaré plot geometric measure: SD1 |
| 33 | SD2 | Nonlinear | Poincaré plot geometric measure: SD2 |
| 34 | SD1SD2 | Nonlinear | Poincaré plot geometric measure: SD1SD2 |
| 35 | S | Nonlinear | Nonlinear HRV metric: S |
| 36 | CSI | Nonlinear | Nonlinear HRV metric: CSI |
| 37 | CVI | Nonlinear | Nonlinear HRV metric: CVI |
| 38 | CSI_Modified | Nonlinear | Nonlinear HRV metric: CSI_Modified |
| 39 | PIP | Nonlinear | Nonlinear HRV metric: PIP |
| 40 | IALS | Nonlinear | Nonlinear HRV metric: IALS |
| 41 | PSS | Nonlinear | Nonlinear HRV metric: PSS |
| 42 | PAS | Nonlinear | Nonlinear HRV metric: PAS |
| 43 | GI | Nonlinear | Nonlinear HRV metric: GI |
| 44 | SI | Nonlinear | Nonlinear HRV metric: SI |
| 45 | AI | Nonlinear | Nonlinear HRV metric: AI |
| 46 | PI | Nonlinear | Nonlinear HRV metric: PI |
| 47 | C1d | Nonlinear | Nonlinear HRV metric: C1d |
| 48 | C1a | Nonlinear | Nonlinear HRV metric: C1a |
| 49 | SD1d | Nonlinear | Poincaré plot geometric measure: SD1d |
| 50 | SD1a | Nonlinear | Poincaré plot geometric measure: SD1a |
| 51 | C2d | Nonlinear | Nonlinear HRV metric: C2d |
| 52 | C2a | Nonlinear | Nonlinear HRV metric: C2a |
| 53 | SD2d | Nonlinear | Poincaré plot geometric measure: SD2d |
| 54 | SD2a | Nonlinear | Poincaré plot geometric measure: SD2a |
| 55 | Cd | Nonlinear | Nonlinear HRV metric: Cd |
| 56 | Ca | Nonlinear | Nonlinear HRV metric: Ca |
| 57 | SDNNd | Nonlinear | Poincaré plot geometric measure: SDNNd |
| 58 | SDNNa | Nonlinear | Poincaré plot geometric measure: SDNNa |
| 59 | DFA_alpha1 | Nonlinear | Nonlinear HRV metric: DFA_alpha1 |
| 60 | MFDFA_alpha1_Width | Nonlinear | Multifractal Detrended Fluctuation Analysis parameter |
| 61 | MFDFA_alpha1_Peak | Nonlinear | Multifractal Detrended Fluctuation Analysis parameter |
| 62 | MFDFA_alpha1_Mean | Nonlinear | Multifractal Detrended Fluctuation Analysis parameter |
| 63 | MFDFA_alpha1_Max | Nonlinear | Multifractal Detrended Fluctuation Analysis parameter |
| 64 | MFDFA_alpha1_Delta | Nonlinear | Multifractal Detrended Fluctuation Analysis parameter |
| 65 | MFDFA_alpha1_Asymmetry | Nonlinear | Multifractal Detrended Fluctuation Analysis parameter |
| 66 | MFDFA_alpha1_Fluctuation | Nonlinear | Multifractal Detrended Fluctuation Analysis parameter |
| 67 | MFDFA_alpha1_Increment | Nonlinear | Multifractal Detrended Fluctuation Analysis parameter |
| 68 | DFA_alpha2 | Nonlinear | Nonlinear HRV metric: DFA_alpha2 |
| 69 | MFDFA_alpha2_Width | Nonlinear | Multifractal Detrended Fluctuation Analysis parameter |
| 70 | MFDFA_alpha2_Peak | Nonlinear | Multifractal Detrended Fluctuation Analysis parameter |
| 71 | MFDFA_alpha2_Mean | Nonlinear | Multifractal Detrended Fluctuation Analysis parameter |
| 72 | MFDFA_alpha2_Max | Nonlinear | Multifractal Detrended Fluctuation Analysis parameter |
| 73 | MFDFA_alpha2_Delta | Nonlinear | Multifractal Detrended Fluctuation Analysis parameter |
| 74 | MFDFA_alpha2_Asymmetry | Nonlinear | Multifractal Detrended Fluctuation Analysis parameter |
| 75 | MFDFA_alpha2_Fluctuation | Nonlinear | Multifractal Detrended Fluctuation Analysis parameter |
| 76 | MFDFA_alpha2_Increment | Nonlinear | Multifractal Detrended Fluctuation Analysis parameter |
| 77 | ApEn | Nonlinear | Approximate Entropy–complexity measure |
| 78 | SampEn | Nonlinear | Sample Entropy–regularity measure |
| 79 | ShanEn | Nonlinear | Entropy measure: ShanEn |
| 80 | FuzzyEn | Nonlinear | Entropy measure: FuzzyEn |
| 81 | MSEn | Nonlinear | Entropy measure: MSEn |
| 82 | CMSEn | Nonlinear | Entropy measure: CMSEn |
| 83 | RCMSEn | Nonlinear | Entropy measure: RCMSEn |
| 84 | CD | Nonlinear | Nonlinear HRV metric: CD |
| 85 | HFD | Nonlinear | Nonlinear HRV metric: HFD |
| 86 | KFD | Nonlinear | Nonlinear HRV metric: KFD |
| 87 | LZC | Nonlinear | Nonlinear HRV metric: LZC |
| 88 | EntropyRate | Nonlinear | Novel entropy rate measure–our methodological contribution |
| 89 | additional_hrt_turbulence_onset | Other | HRV metric: additional_hrt_turbulence_onset |
| 90 | additional_hrt_turbulence_slope | Other | HRV metric: additional_hrt_turbulence_slope |
| 91 | additional_coefficient_variation | Other | HRV metric: additional_coefficient_variation |
| 92 | additional_temporal_variability | Other | HRV metric: additional_temporal_variability |
| 93 | additional_spectral_centroid | Other | HRV metric: additional_spectral_centroid |
| 94 | additional_spectral_bandwidth | Other | HRV metric: additional_spectral_bandwidth |
References
- Keller, A.; Litzelman, K.; Wisk, L.E.; Maddox, T.; Cheng, E.R.; Creswell, P.D.; Witt, W.P. Does the perception that stress affects health matter? The association with health and mortality. Health Psychol. 2012, 31, 677–684. [Google Scholar] [CrossRef]
- Chandra, M.; Paray, A.A. Natural Physiological Changes During Pregnancy. Yale J. Biol. Med. 2024, 97, 85–92. [Google Scholar] [CrossRef]
- Aziz, H.A.; Yahya, H.D.B.; Ang, W.W.; Lau, Y. Global prevalence of depression, anxiety, and stress symptoms in different trimesters of pregnancy: A meta-analysis and meta-regression. J. Psychiatr. Res. 2025, 181, 528–546. [Google Scholar] [CrossRef]
- Grace, T.; Bulsara, M.; Robinson, M.; Hands, B. The Impact of Maternal Gestational Stress on Motor Development in Late Childhood and Adolescence: A Longitudinal Study. Child. Dev. 2016, 87, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Babineau, V.; Fonge, Y.N.; Miller, E.S.; Grobman, W.A.; Ferguson, P.L.; Hunt, K.J.; Vena, J.E.; Newman, R.B.; Guille, C.; Tita, A.T.N.; et al. Associations of Maternal Prenatal Stress and Depressive Symptoms With Childhood Neurobehavioral Outcomes in the ECHO Cohort of the NICHD Fetal Growth Studies: Fetal Growth Velocity as a Potential Mediator. J. Am. Acad. Child. Adolesc. Psychiatry 2022, 61, 1155–1167. [Google Scholar] [CrossRef] [PubMed]
- Tuovinen, S.; Lahti-Pulkkinen, M.; Girchenko, P.; Heinonen, K.; Lahti, J.; Reynolds, R.M.; Hämäläinen, E.; Villa, P.M.; Kajantie, E.; Laivuori, H.; et al. Maternal antenatal stress and mental and behavioral disorders in their children. J. Affect. Disord. 2021, 278, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Nishigori, T.; Ogata, Y.; Suzuki, T.; Sato, A.; Murata, T.; Kyozuka, H.; Yamaguchi, A.; Metoki, H.; Shinohara, Y.; et al. Maternal prenatal psychological distress and motor/cognitive development in two-year-old offspring: The Japan Environment and Children’s Study. J. Dev. Orig. Health Dis. 2023, 14, 389–401. [Google Scholar] [CrossRef]
- Rakers, F.; Rupprecht, S.; Dreiling, M.; Bergmeier, C.; Witte, O.W.; Schwab, M. Transfer of maternal psychosocial stress to the fetus. Neurosci. Biobehav. Rev. 2017, 117, 185–197. [Google Scholar] [CrossRef]
- Zimmermann, P.; Antonelli, M.C.; Sharma, R.; Müller, A.; Zelgert, C.; Fabre, B.; Wenzel, N.; Wu, H.T.; Frasch, M.G.; Lobmaier, S.M. Prenatal stress perturbs fetal iron homeostasis in a sex specific manner. Sci. Rep. 2022, 12, 9341. [Google Scholar] [CrossRef]
- Sharma, R.; Frasch, M.G.; Zelgert, C.; Zimmermann, P.; Fabre, B.; Wilson, R.; Waldenberger, M.; MacDonald, J.W.; Bammler, T.K.; Lobmaier, S.M.; et al. Maternal-fetal stress and DNA methylation signatures in neonatal saliva: An epigenome-wide association study. Clin. Epigenetics 2022, 14, 87. [Google Scholar] [CrossRef]
- Glover, V.; O’Connor, T.G.; O’Donnell, K. Prenatal stress and the programming of the HPA axis. Neurosci. Biobehav. Rev. 2010, 35, 17–22. [Google Scholar] [CrossRef]
- Kapoor, A.; Dunn, E.; Kostaki, A.; Andrews, M.H.; Matthews, S.G. Fetal programming of hypothalamo-pituitary-adrenal function: Prenatal stress and glucocorticoids. J. Physiol. 2006, 572, 31–44. [Google Scholar] [CrossRef]
- Semeia, L.; Bauer, I.; Sippel, K.; Hartkopf, J.; Schaal, N.K.; Preissl, H. Impact of maternal emotional state during pregnancy on fetal heart rate variability. Compr. Psychoneuroendocrinol. 2023, 14, 100181. [Google Scholar] [CrossRef]
- DiPietro, J.A.; Kivlighan, K.T.; Costigan, K.A.; Rubin, S.E.; Shiffler, D.E.; Henderson, J.L.; Pillion, J.P. Prenatal antecedents of newborn neurological maturation. Child. Dev. 2010, 81, 115–130. [Google Scholar] [CrossRef]
- Lobmaier, S.M.; Müller, A.; Zelgert, C.; Shen, C.; Su, P.C.; Schmidt, G.; Haller, B.; Berg, G.; Fabre, B.; Weyrich, J.; et al. Fetal heart rate variability responsiveness to maternal stress, non-invasively detected from maternal transabdominal ECG. Arch. Gynecol. Obstet. 2020, 301, 405–414. [Google Scholar] [CrossRef]
- Roux, S.G.; Garnier, N.B.; Abry, P.; Gold, N.; Frasch, M.G. Distance to Healthy Metabolic and Cardiovascular Dynamics From Fetal Heart Rate Scale-Dependent Features in Pregnant Sheep Model of Human Labor Predicts the Evolution of Acidemia and Cardiovascular Decompensation. Front. Pediatr. 2021, 9, 660476. [Google Scholar] [CrossRef] [PubMed]
- Granero-Belinchon, C.; Roux, S.G.; Abry, P.; Doret, M.; Garnier, N.B. Information Theory to Probe Intrapartum Fetal Heart Rate Dynamics. Entropy 2017, 19, 640. [Google Scholar] [CrossRef]
- Lake, D.E.; Richman, J.S.; Griffin, M.P.; Moorman, J.R. Sample entropy analysis of neonatal heart rate variability. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R789–R797. [Google Scholar] [CrossRef] [PubMed]
- Visnovcova, Z.; Mestanik, M.; Javorka, M.; Mokra, D.; Gala, M.; Jurko, A.; Calkovska, A.; Tonhajzerova, I. Complexity and time asymmetry of heart rate variability are altered in acute mental stress. Physiol. Meas. 2014, 35, 1319–1334. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.; Kim, A.Y.; Jang, E.H.; Kim, S.; Choi, K.W.; Yu, H.Y.; Jeon, H.J. Entropy analysis of heart rate variability and its application to recognize major depressive disorder: A pilot study. Technol. Health Care 2019, 27 (Suppl. 1), 407–424. [Google Scholar] [CrossRef]
- Herry, C.L.; Burns, P.; Desrochers, A.; Fecteau, G.; Durosier, L.D.; Cao, M.; Seely, A.J.E.; Frasch, M.G. Vagal contributions to fetal heart rate variability: An omics approach. Physiol. Meas. 2019, 40, 065004. [Google Scholar] [CrossRef]
- Frasch, M.G. Heart Rate Variability Code: Does It Exist and Can We Hack It? Bioengineering 2023, 10, 822. [Google Scholar] [CrossRef]
- Lake, D.E.; Fairchild, K.D.; Moorman, J.R. Complex signals bioinformatics: Evaluation of heart rate characteristics monitoring as a novel risk marker for neonatal sepsis. J. Clin. Monit. Comput. 2014, 28, 329–339. [Google Scholar] [CrossRef]
- Wakefield, C.; Yao, L.; Self, S.; Frasch, M.G. Wearable technology for health monitoring during pregnancy: An observational cross-sectional survey study. Arch. Gynecol. Obstet. 2023, 308, 73–78. [Google Scholar] [CrossRef]
- Szaszkó, B.; Schmid, R.R.; Pomper, U.; Maiworm, M.; Laiber, S.; Tschenett, H.; Nater, U.M.; Ansorge, U. The influence of hatha yoga on stress, anxiety, and suppression: A randomized controlled trial. Acta Psychol. 2023, 241, 104075. [Google Scholar] [CrossRef]
- Lemay, V.; Hoolahan, J.; Buchanan, A. Impact of a Yoga and Meditation Intervention on Students’ Stress and Anxiety Levels. Am. J. Pharm. Educ. 2019, 83, 7001. [Google Scholar] [CrossRef]
- Pascoe, M.C.; Thompson, D.R.; Ski, C.F. Yoga, mindfulness-based stress reduction and stress-related physiological measures: A meta-analysis. Psychoneuroendocrinology 2017, 86, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, L.; Moran, P.; McGrath, N.; Eustace-Cook, J.; Daly, D. The characteristics and effectiveness of pregnancy yoga interventions: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2022, 22, 250. [Google Scholar] [CrossRef] [PubMed]
- Kwon, R.; Kasper, K.; London, S.; Haas, D.M. A systematic review: The effects of yoga on pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 250, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists; Society for Maternal–Fetal Medicine; Kilpatrick, S.K.; Ecker, J.L. Severe maternal morbidity: Screening and review. Am. J. Obstet. Gynecol. 2016, 215, B17–B22. [Google Scholar] [CrossRef]
- Kintraia, P.I.; Zarnadze, M.G.; Kintraia, N.P.; Kashakashvili, I.G. Development of daily rhythmicity in heart rate and locomotor activity in the human fetus. J. Circadian Rhythms. 2005, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Dickmeis, T. Glucocorticoids and the circadian clock. J. Endocrinol. 2009, 200, 3–22. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Klein, E.M.; Brähler, E.; Dreier, M.; Reinecke, L.; Müller, K.W.; Schmutzer, G.; Wölfling, K.; Beutel, M.E. The German version of the Perceived Stress Scale—Psychometric characteristics in a representative German community sample. BMC Psychiatry. 2016, 16, 159. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; Spence, D.W.; Srivastava, N.; Kanchibhotla, D.; Kumar, K.; Sharma, G.S.; Gupta, R.; Batmanabane, G. The Origin and Clinical Relevance of Yoga Nidra. Sleep. Vigil. 2022, 6, 61–84. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef]
- Spilka, J.; Roux, S.G.; Garnier, N.B.; Abry, P.; Goncalves, P.; Doret, M. Nearest-neighbor based wavelet entropy rate measures for intrapartum fetal heart rate variability. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2014, 2014, 2813–2816. [Google Scholar] [CrossRef]
- Gandrillon, O.; Gaillard, M.; Espinasse, T.; Garnier, N.B.; Dussiau, C.; Kosmider, O.; Sujobert, P. Entropy as a measure of variability and stemness in single-cell transcriptomics. Curr. Opin. Syst. Biol. 2021, 27, 100348. [Google Scholar] [CrossRef]
- Granero-Belinchón, C.; Roux, S.; Garnier, N. Un estimateur du taux d’entropie basé sur l’Information Mutuelle. In Colloque Gretsi; Université de Lyon: Lyon, France, 2017. [Google Scholar]
- Frasch, M.G. Comprehensive HRV estimation pipeline in Python using Neurokit2: Application to sleep physiology. MethodsX 2022, 9, 101782. [Google Scholar] [CrossRef]
- Satyapriya, M.; Nagendra, H.R.; Nagarathna, R.; Padmalatha, V. Effect of integrated yoga on stress and heart rate variability in pregnant women. Int. J. Gynaecol. Obstet. 2009, 104, 218–222. [Google Scholar] [CrossRef]
- Amrhein, V.; Greenland, S.; McShane, B. Scientists rise up against statistical significance. Nature 2019, 567, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.R.; Fontinha, H.; Thompson, J.; Couper, S.; Jani, D.; Mirjalili, A.; Bennet, L.; Stone, P. Maternal Cardiovascular Responses to Position Change in Pregnancy. Biology 2023, 12, 1268. [Google Scholar] [CrossRef] [PubMed]
- Rowan, S.P.; Lilly, C.L.; Claydon, E.A.; Wallace, J.; Merryman, K. Monitoring one heart to help two: Heart rate variability and resting heart rate using wearable technology in active women across the perinatal period. BMC Pregnancy Childbirth 2022, 22, 887. [Google Scholar] [CrossRef] [PubMed]
- Hayase, M.; Shimada, M. Effects of maternity yoga on the autonomic nervous system during pregnancy. J. Obstet. Gynaecol. Res. 2018, 44, 1887–1895. [Google Scholar] [CrossRef]
- Žebeljan, I.; Lučovnik, M.; Dinevski, D.; Lackner, H.K.; Moertl, M.G.; Vesenjak Dinevski, I.; Mujezinović, F. Effect of Prenatal Yoga on Heart Rate Variability and Cardio-Respiratory Synchronization: A Prospective Cohort Study. J. Clin. Med. 2022, 11, 5777. [Google Scholar] [CrossRef]
- Dietz, P.; Watson, E.D.; Sattler, M.C.; Ruf, W.; Titze, S.; van Poppel, M. The influence of physical activity during pregnancy on maternal, fetal or infant heart rate variability: A systematic review. BMC Pregnancy Childbirth 2016, 16, 326. [Google Scholar] [CrossRef]
- Malhotra, V.; Pathak, T.; Javed, D.; Wakode, S.; Thakare, A.; Shrivastava, R.; Chouhan, S.; Filho, F.J.C. Comparative Analysis of Heart Rate Variability Parameters between Surya Namaskar and Stationary Bike Exercise Groups. Int. J. Yoga. 2023, 16, 202–209. [Google Scholar] [CrossRef]
- Cramer, H.; Lauche, R.; Langhorst, J.; Dobos, G. Are Indian yoga trials more likely to be positive than those from other countries? A systematic review of randomized controlled trials. Contemp. Clin. Trials. 2015, 41, 269–272. [Google Scholar] [CrossRef]
- Yuan, L.; Yuan, Y.; Zhou, Z.; Bai, Y.; Wu, S. A Fetal ECG Monitoring System Based on the Android Smartphone. Sensors 2019, 19, 446. [Google Scholar] [CrossRef]
- Jasinski, S.R.; Rowan, S.; Presby, D.M.; Claydon, E.A.; Capodilupo, E.R. Wearable-derived maternal heart rate variability as a novel digital biomarker of preterm birth. PLoS ONE 2024, 19, e0295899. [Google Scholar] [CrossRef] [PubMed]

| Control N = 14 | Yoga N = 14 | p-Value | |
|---|---|---|---|
| Gestational age at inclusion (weeks), mean (SD) | 17.3 (3.4) | 15.9 (3.0) | 0.284 |
| Gestational age at first ECG (weeks), median (IQR)/mean (SD) | 21.0 (16.6–21.9) | 18.3 (3.5) | 0.283 |
| Gestational age at last ECG (weeks), mean (SD) | 35.3 (2.5) | 34.6 (3.6) | 0.612 |
| Maternal age at inclusion (years), mean (SD) | 35.2 (3.8) | 33.3 (3.1) | 0.157 |
| PSS at inclusion, mean (SD), median (IQR) | 24.86 (4.8) | 22.5 (20–28) | 0.828 |
| BMI at inclusion 1, mean (SD | 21.9 (2.5) | 21.9 (3.4) | 0.867 |
| Ethnicity European, n (%) | 13 (93) | 12 (86) | 0.219 |
| Marital status, n (%) Married In relationship | 11 (79) 3 (21) | 8 (57) 6 (43) | 0.420 |
| Single mom, n (%) | 0 (0) | 0 (0) | |
| Working, n (%) | 10 (71) | 12 (86) | 0.648 |
| Highest level of education: University degree, n (%) | 10 (71) | 14 (100) | 0.097 |
| Net household income, n (%) 1000–2500€ 2500–5000€ 5000–10,000€ >10,000€ | 0 (0) 5 (36) 7 (50) 2 (14) | 1 (7) 5 (36) 5 (36) 3 (21) | 0.675 |
| Parity, n (%) Primipara Multipara | 9 (64) 5 (36) | 13 (93) 1 (7) | 0.165 |
| Planned pregnancy, n (%) | 12 (86) | 12 (86) | 1.00 |
| Substance use (alcohol, tobacco, or drugs), n (%) | 0 (0) | 0 (0) | |
| Autoimmune disease, n (%) | 2 (14) | 1 (7) | 1.000 |
| Gestational diabetes, n (%) | 0 | 0 | |
| Arterial hypertension, n (%) | 1 (7) | 0 | 1.0 |
| Physical activity before pregnancy, n (%) | 12 (86) | 14 (100) | 0.481 |
| Yoga experience before study, n (%) | 10 (71) | 10 (71) | 1.000 |
| Physical activity at second-trimester screening (incl. Yoga) 2, n (%) | 7 (50) | 12 (100) | 0.005 |
| Physical activity at third-trimester screening (incl. Yoga), n (%) | 12 (86) | 14 (100) | 0.241 |
| Third trimester: Yoga practice (private or study-related), n (%) | 8 (57) | 14 (100) | 0.008 |
| Frequency of Yoga practice during third trimester, n (%) 0 min 1–30 min 31–60 min 61–90 min 91–120 min 181–210 min | 6 (43) 3 (21) 3 (21) 2 (14) 0 0 | 0 0 0 12 (86) 1 (7) 1 (7) | <0.001 |
| Sex of newborn: female, n (%) | 6 (43) | 8 (57) | 0.706 |
| total | valid | ||||
| begin | end | ||||
| Yoga | first session | 10 | 10 | 10 | |
| last session | 10 | 8 | 6 | ||
| delta | 10 | 8 | 6 | ← delta needs both first and last | |
| control | first session | 12 | 12 | 12 | |
| last session | 12 | 12 | 12 | ||
| delta | 12 | 12 | 12 | ||
| Sample Entropy | |||||
|---|---|---|---|---|---|
| First visit | |||||
| Yoga | Control | p-value | Cohen’s d | Effect size r | |
| Begin | n = 10 3.03 (0.43) | n = 12 2.55 (0.38) | 0.011 | 1.19 | 0.51 |
| End | n = 10 3.14 (0.59) | n = 12 2.59 (0.32) | 0.006 | 1.19 | 0.51 |
| Last visit | |||||
| Yoga | Control | p-value | Cohen’s d | Effect size r | |
| Begin | n = 8 3.19 (0.51) | n = 12 2.50 (0.33) | <0.001 | 1.69 | 0.64 |
| End | n = 6 3.09 (0.56) | n = 12 2.54 (0.27) | 0.030 | 1.43 | 0.58 |
| Entropy Rate | |||||
| First visit | |||||
| Yoga | Control | p-value | Cohen’s d | Effect size r | |
| Begin | n = 10 2.45 (0.49) | n = 12 1.85 (0.39) | 0.004 | 1.37 | 0.57 |
| End | n = 10 2.58 (0.53) | n = 12 1.88 (0.36) | <0.001 | 1.57 | 0.62 |
| Last visit | |||||
| Yoga | Control | p-value | Cohen’s d | Effect size r | |
| Begin | n = 8 2.98 (0.58) | n = 12 1.90 (0.36) | <0.001 | 2.36 | 0.76 |
| End | n = 6 2.68 (0.35) | n = 12 1.85 (1.7–2.1) | 0.003 | 0.66 | |
| Sample Entropy | |||||
|---|---|---|---|---|---|
| Yoga | Control | p-value | Cohen’s d | Effect size r | |
| Begin | n = 8 0.18 (0.32) | n = 12 −0.53 (0.34) | 0.072 | 2.14 | 0.73 |
| End | n = 6 0.08 (0.36) | n = 12 −0.05 (0.18) | 0.159 | 0.52 | 0.25 |
| Entropy Rate | |||||
| Yoga | Control | p-value | Cohen’s d | Effect size r | |
| Begin | n = 8 0.50 (0.67) | n = 12 0.05 (0.39) | 0.034 | 0.87 | 0.40 |
| End | n = 6 0.19 (0.57) | n = 12 0.11 (0.34) | 0.360 | 0.19 | 0.094 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayer, M.J.E.; Garnier, N.B.; Becker, C.; Antonelli, M.C.; Lobmaier, S.M.; Frasch, M.G. Heart Rate Variability Patterns Reflect Yoga Intervention in Chronically Stressed Pregnant Women: A Quasi-Randomized Controlled Trial. Bioengineering 2025, 12, 1141. https://doi.org/10.3390/bioengineering12111141
Mayer MJE, Garnier NB, Becker C, Antonelli MC, Lobmaier SM, Frasch MG. Heart Rate Variability Patterns Reflect Yoga Intervention in Chronically Stressed Pregnant Women: A Quasi-Randomized Controlled Trial. Bioengineering. 2025; 12(11):1141. https://doi.org/10.3390/bioengineering12111141
Chicago/Turabian StyleMayer, Marlene J. E., Nicolas B. Garnier, Clara Becker, Marta C. Antonelli, Silvia M. Lobmaier, and Martin G. Frasch. 2025. "Heart Rate Variability Patterns Reflect Yoga Intervention in Chronically Stressed Pregnant Women: A Quasi-Randomized Controlled Trial" Bioengineering 12, no. 11: 1141. https://doi.org/10.3390/bioengineering12111141
APA StyleMayer, M. J. E., Garnier, N. B., Becker, C., Antonelli, M. C., Lobmaier, S. M., & Frasch, M. G. (2025). Heart Rate Variability Patterns Reflect Yoga Intervention in Chronically Stressed Pregnant Women: A Quasi-Randomized Controlled Trial. Bioengineering, 12(11), 1141. https://doi.org/10.3390/bioengineering12111141









