Biochar for Soil Amendment: Applications, Benefits, and Environmental Impacts
Abstract
1. Introduction
2. Biochar Production
| Feedstock | Pyrolysis Temperature (°C) | Yield (%) | C (%) | N (%) | H (%) | S (%) | O (%) | Ash (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Coconut husk | 500 | 45.0 | 79.8 | 0.4 | 2.2 | 0.1 | 7.4 | 10.1 | [11] |
| Orange bagasse | 500 | 34.0 | 72.1 | 2.6 | 1.8 | 0.1 | 7.3 | 16.1 | [11] |
| Peanut shell | 300 | 36.9 | 68.3 | 1.9 | 3.9 | 0.1 | 25.9 | 1.2 | [12] |
| 550 | - | 67.4 | 1.3 | 29.2 | - | 11.6 | 6.7 | [13] | |
| 700 | 21.9 | 83.8 | 1.1 | 1.8 | 0 | 13.3 | 8.9 | [12] | |
| Pig manure | 300–700 | 63.0–42.8 | - | 2.9–6.1 | - | - | - | - | [14] |
| Pine wood | 500 | 30.0 | 88.2 | 0.5 | 2.7 | 0.1 | 6.1 | 2.5 | [11] |
| Pine wood | 300–700 | 45.5–23.2 | - | 0.1–0.9 | - | - | - | 0.4 | [14] |
| Rice straw | 300–700 | 45.2–30.6 | 69.6–81.1 | 0.1–0.9 | - | - | - | - | [14] |
| Sewage sludge | 300–700 | - | - | 6.1–0.9 | - | - | - | - | [14] |
| Sorghum bagasse | 350 | 38.9 | 62.6 | - | - | - | 13.1 | - | [15] |
| 700 | 27.1 | 75.8 | - | - | - | 0.8 | - | [15] | |
| Soybean stover | 300 | 37.0 | 68.8 | 1.9 | 4.3 | 0 | 25.0 | 10.4 | [12] |
| 700 | 21.9 | 83.8 | 1.1 | 1.8 | 0 | 13.3 | 8.9 | ||
| Wheat straw | 300 | 35.9 | 53.1 | 0.9 | 3.7 | 0.7 | 23.9 | 17.7 | [16] |
| 500 | 26.7 | 55.7 | 0.9 | 2.0 | 0.9 | 16.6 | 24.0 | ||
| 700 | 23.9 | 57.7 | 0.7 | 1.2 | 0.8 | 7.9 | 31.7 | ||
| Wood | 450 | - | 82.7 | 0.5 | 2.9 | - | 8.3 | 3.0 | [17] |
3. Biochar Engineering
3.1. Chemical Method
3.2. Physical Method
| Raw Material | Biochar Modification | Plant Studied/Active Matrix | Result | References |
|---|---|---|---|---|
| Chemical modification | ||||
| Peanut Shell | P | Pseudostellaria heterophlla/ Soil | Increase in Cd2+ removal by 73%phos, root length density by 61.1%, and yield by up to 301%. | [53] |
| Peanut Shell | MgO | Rice plant/ Soil | Increase in PO43− adsorption by 20%, rice biomass by 8%. | [54] |
| Sulfur–iron | Soil | Cd2+ removal up to 29.71%, increased bacterial abundance. | [55] | |
| Fe | Soil | Atrazine reduced at a rate of 100 mg L−1 and bacterial diversity was well maintained in contaminated soil. | [56] | |
| Pine needle | Sulfur | Water | Hg2+ adsorption was 0.349 g mg−1 min−1. | [57] |
| Oil Palm dry bunches | Chitosan | Soil | Herbicide imazapic adsorption increased by 23%; imazapyr enhanced by 78%. | [58] |
| Rice husk | Chitosan | Soil | Imazapic adsorption increased by 11%, and imazapyr enhanced by 31%. | [58] |
| Physical modification | ||||
| Coconut shell | HCl and ultrasonication | Soil | Cd2+, Ni2+, Zn2+ removal efficiency of 30.1%, 57.2%, and 12.7%, respectively. The bacterial community increased by 150%. | [59] |
| Wood | UV irradiation | NA | Adsorption of toluene increased from 12.80 mg g−1 to 54.60 mg g−1. | [60] |
| Microalgae | Steam activation | Water | Adsorption of Cu2+ by steam activation increased by 4-folds compared to the KOH-modified biochar.. | [61] |
| Bagasse | Ball milling | Water | Ni2+ adsorption increased by 6-folds compared to unmodified biochar. | [62] |
| Wheat straw (WS), coconut (CS), willow (WS) | Steam activation | Soil | PAHs reduced in WS, CS, and WS by 57%, 48%, and 47%, respectively. | [63] |
4. Characterization Methods
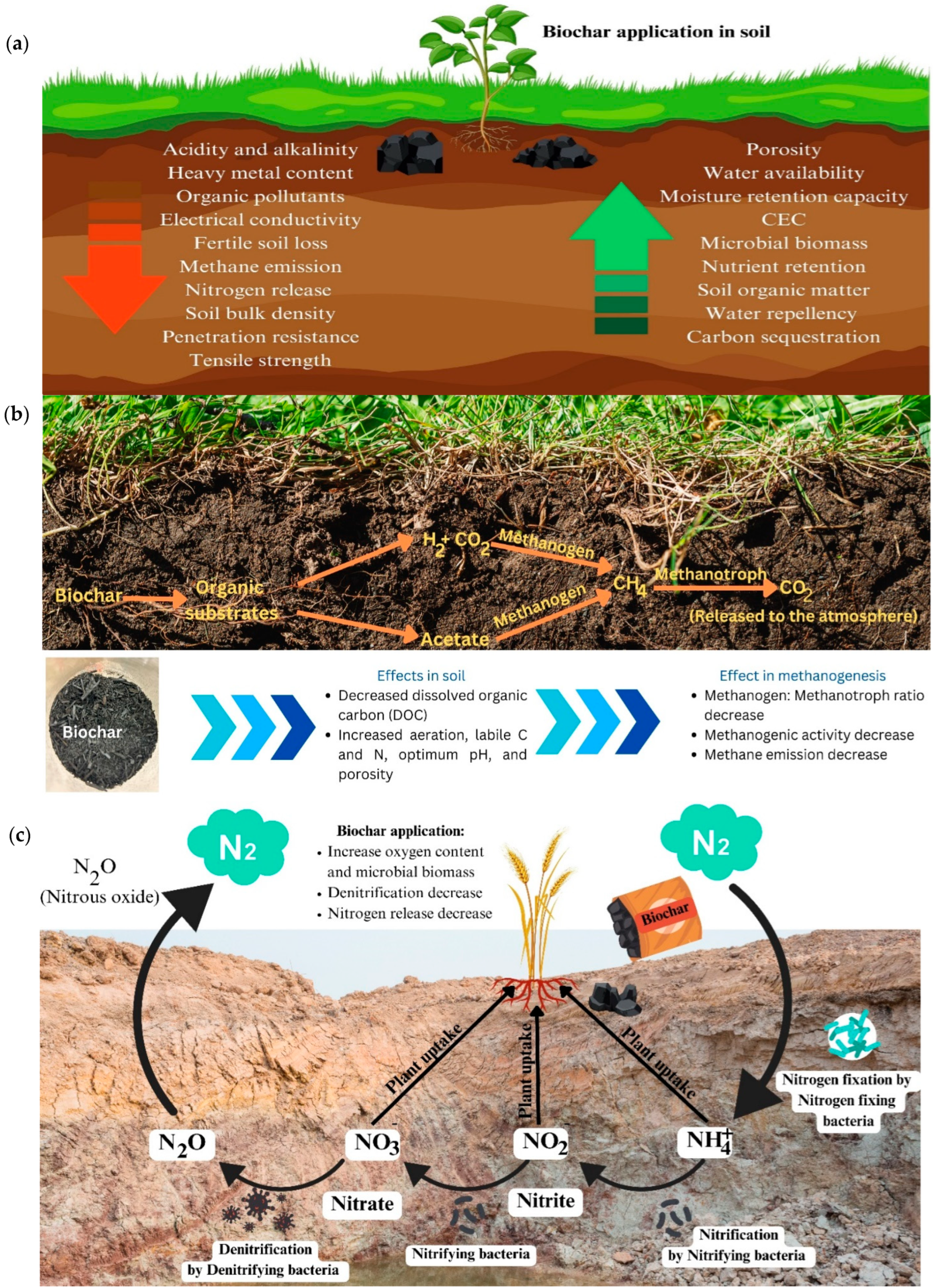
5. Application of Biochar
5.1. Bulk Density and Porosity
5.2. Tensile Strength and Particle Density
5.3. Water Repellency
5.4. pH Change
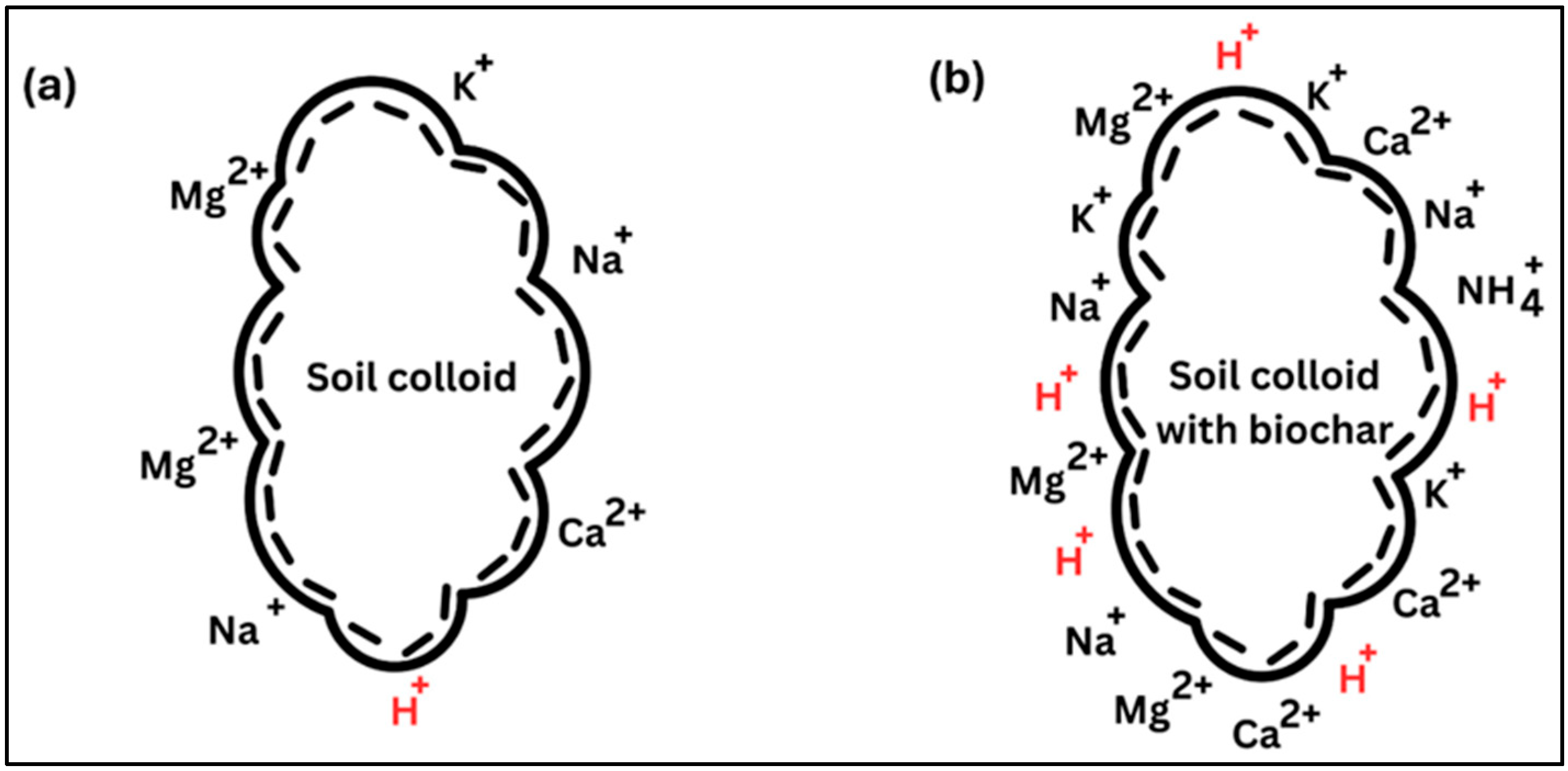
5.5. Cation Exchange Capacity
5.6. Organic Pollutants
5.7. Inorganic Pollutants
5.8. Microbial Communities
5.9. Carbon Sequestration
6. Techno–Economic Analysis
| Feedstock | Temperature and Yield | Features | Production Capacity (kg m−2) | NPV/IRR/MSP | Breakeven Period | Reference |
|---|---|---|---|---|---|---|
| Pine | 300 °C, 450 °C; Yield: 80% and 45% | Syngas converts to methanol | 10 | NPV: USD 0.220–0.280 kg−1 with 70% revenue from biochar and 30% from methanol production IRR: 14.2-10.1% (Shows moderate return) | - | [149] |
| Forest residues | Portable; ~680 °C–750 °C Yield: 13–21% BSI, 20% OK, 6.5% ACB | Power sources and production site distance considered | 0.02–0.038 | MSP for BSI is USD 3–6 kg−1, OK is USD 1.6 kg−1, and ACB is USD 0.5 kg−1 | 100 days | [151] |
| Grape residue | 500 °C Yield: 37% | Biochar production integrated into a biorefinery | 0.015 | NPV: USD 111.7 million (overall biorefinery) IRR: 34.3% (Shows high return) | 2.5 years | [152] |
| Tree pruning | 450–800 °C Yield: 20.20–29.17% | Investigating the economic feasibility of biochar systems | 0.121 | NPV: USD 3,119,448 IRR: 22.35% (Shows high return) | 8 years | [161] |
7. Life Cycle Assessment
| Feedstock | Pyrolysis | Methodology | Biochar Application to Soil | Impact Categories | Results | Reference |
|---|---|---|---|---|---|---|
| Winter oilseed rape straw | 400 °C and 800 °C | IPCC 2013 manual calculation | 0.1 kg m−2 | Carbon footprint: 100 yr, 20 yr | Reduction in GHG 400 °C: 73%; 800 °C: 83% | [146,168] |
| Oat Waste and willow wood | - | IPCC 2013 (GaBi) | 0.0025 kg–0.02 kg m−2 | Carbon footprint: 100 yr | Reduction of 0.050 kg CO2eq to 0.390 kg CO2eq | [168,169] |
| Miscanthus | Slow pyrolysis (Temperature is unknown) | IPCC 2013 (Simapro) | 0.5 kg m−2 | Carbon footprint: 100 yr | −0.737 kg CO2eq kg−1; biochar contributes 50% carbon sink in soil | [168,170] |
| Tomato plant waste | Intermediate pyrolysis (Temperature 400 °C) | IPCC 2013 (Simapro) | 0.1 kg m−2 with yield of 35%, 40% and 45% | Carbon footprint | At 80% stable C and 45% yield, kg CO2eq kg−1 biochar is −0.156. At 20% stable, C carbon sequestration is absent | [168,171] |
| Paddy rice, maize | Vertical kiln at 350–500 °C | IPCC 2013 | 2 kg m−2 | Carbon footprint | 2.037–4.129 kg CO2eq m−2 for paddy rice; 2.858–3.949 kg CO2eq m−2 for maize | [168,172] |
| Rice straw | Top-lift (TLUD) drum oven (Temperature unknown) | IPCC 2013 | 0.05 kg m−2 | Carbon footprint | 610 kg CO2eq in spring and 122 kg CO2eq in summer | [168,173] |
8. Optimal Biochar Application Rates
| Application Rate | Time of Application | Affected Species | Effect of Application | Reference |
|---|---|---|---|---|
| 0%, 2%, 4%, 8% wheat straw biochar | 4 months | Tomato plant | Photosynthetic rate of 17.08 ± 0.19 µmol m−2 s−1, increasing yield by 14%. | [179] |
| 0%, 4%, 8% Conocarpus biochar | 80 days | Tomato plant | Yield increases by 14% to 43.3%. | [180] |
| 0–47.25 t ha−1 | 5 years | Maize | Increase in organic phosphorus by 12.8% to 66.6%. | [181] |
| 1.6 kg m−2 of biochar and fertilizer | 4 years | Wheat | Increase in yield by 16.3% outperforming fertilizers alone by up to 31.2%. | [182] |
9. Integrating Biochar with Organic Composts
| Application Rate | Plant Studied | Effect of Application | Reference |
|---|---|---|---|
| 20% oak biochar-blended compost | Grape | Increase in N by 44%, K+ by 26%, and microbial respiration by 26%. Weight of the fruit increases by 16%. | [187] |
| Cow manure Biochar + Compost (5 tons each) | Maize | 60% irrigation leads to an increase in yield by 107%. | [188] |
| 9% Willow wood Biochar- compost blend | Maize | Increase in yield by 20%. | [189] |
| 2% Grape pomace biochar–compost | Maize | Increase in biomass yield by 155%. | [190] |
| 2% Rice husk biochar–compost | Maize | Increase in biomass yield by 5-fold. | [190] |
| 2 t Acacia biochar in 10 t compost | Nitisol | Increase in yield by 60% and 54% in different soil groups. | [191] |
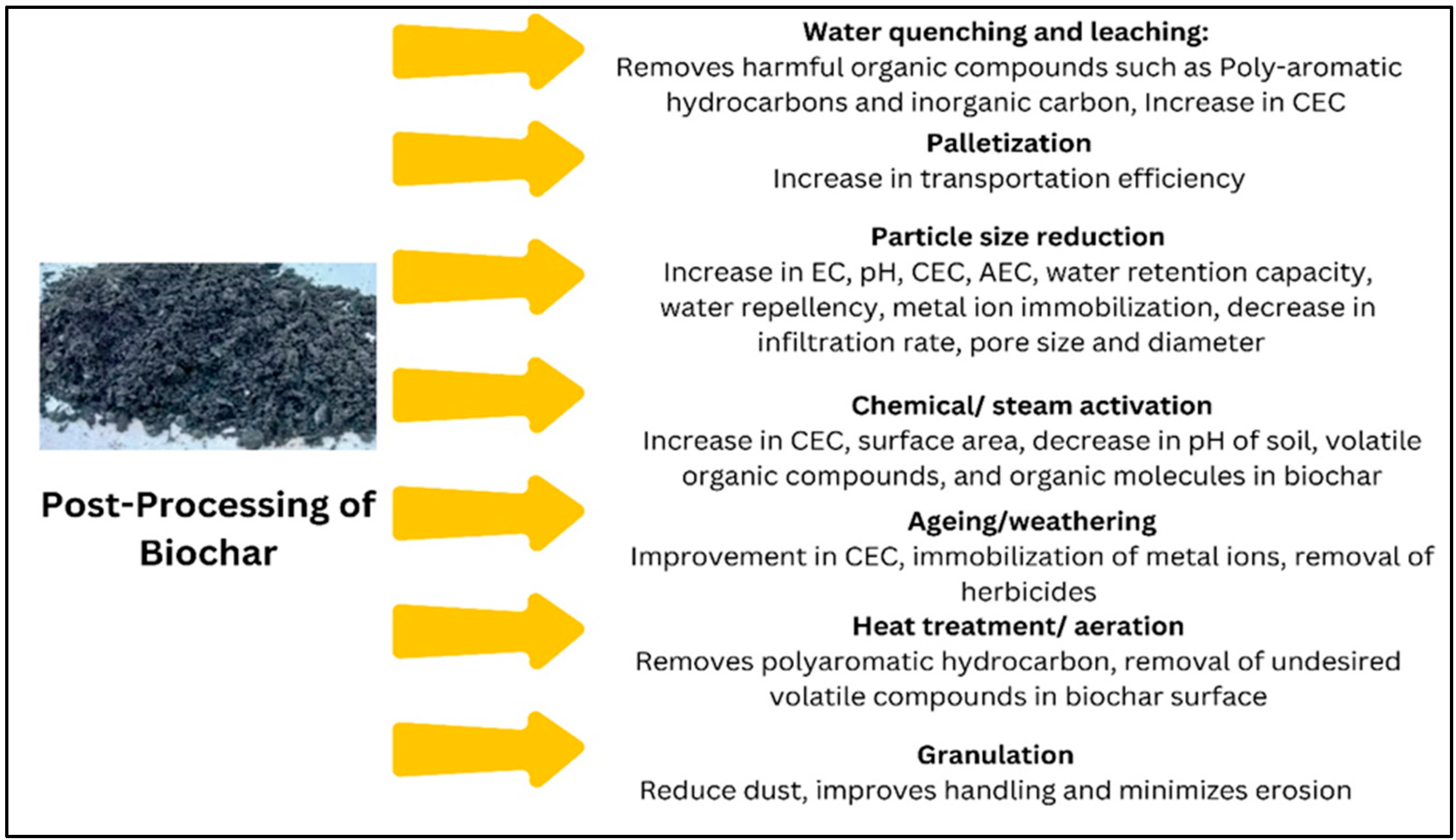
10. Post-Processing of Biochar
11. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AEC | Anion Exchange Capacity |
| AMF | Arbuscular Mycorrhizal Fungi |
| BNP | Biochar Nanoparticle |
| BET | Brunauer–Emmett–Teller |
| CEC | Cation Exchange Capacity (cmol kg−1) |
| FTIR | Fourier Transform Infrared Spectroscopy |
| GHG | Greenhouse Gases |
| ICP-MS | Inductively Coupled Plasma-Mass Spectrometry |
| ICP-OES | Inductively Coupled Plasma- Optical Emission Spectroscopy |
| IRR | Internal Rate of Return |
| LCA | Life Cycle Assessment |
| LCI | Life Cycle Inventory |
| MSP | Minimum Selling Price |
| NBC | Nitrogen-Doped Biochar |
| NPV | Net Present Value |
| PAH | Polycyclic Aromatic Hydrocarbon |
| PCB | Polychlorinated Biphenyl |
| PCDD/F | Dibenzo-P-Dioxins/Dibenzofuran |
| SEM-EDX | Scanning Electron Microscopy-Energy Dispersive X-Ray |
| TEA | Techno-Economic Assessment |
| TGA | Thermogravimetric Analysis |
| TKN | Total Kjeldahl Nitrogen |
| TN | Total Nitrogen |
| UV | Ultraviolet |
| XPS | X-ray Photoelectron Spectroscopy |
References
- Shaaban, M.; Van Zwieten, L.; Bashir, S.; Younas, A.; Núñez-Delgado, A.; Chhajro, M.A.; Kubar, K.A.; Ali, U.; Rana, M.S.; Mehmood, M.A. A concise review of biochar application to agricultural soils to improve soil conditions and fight pollution. J. Environ. Manag. 2018, 228, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, J.; Turnhout, E.; Vasquez, I.M.; Rittl, T.F.; Arts, B.; Kuyper, T.W. The promises of the Amazonian soil: Shifts in discourses of Terra Preta and biochar. J. Environ. Policy Plan. 2019, 21, 623–635. [Google Scholar] [CrossRef]
- Soudani, A.; Youcef, L.; Chebbi, M.; Bulgariu, L.; Patel, N. Agricultural waste–based biochars for sustainable removal of heavy metals from stabilized landfill leachate. Environ. Sci. Pollut. Res. 2024, 31, 57733–57747. [Google Scholar] [CrossRef]
- Babu, K.K.B.S.; Nataraj, M.; Tayappa, M.; Vyas, Y.; Mishra, R.K.; Acharya, B. Production of biochar from waste biomass using slow pyrolysis: Studies of the effect of pyrolysis temperature and holding time on biochar yield and properties. Mater. Sci. Energy Technol. 2024, 7, 318–334. [Google Scholar] [CrossRef]
- Al-Rumaihi, A.; Shahbaz, M.; Mckay, G.; Mackey, H.; Al-Ansari, T. A review of pyrolysis technologies and feedstock: A blending approach for plastic and biomass towards optimum biochar yield. Renew. Sustain. Energy Rev. 2022, 167, 112715. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E.; Cornelis, W.; Benis, K.Z. Understanding the physicochemical structure of biochar affected by feedstock, pyrolysis conditions, and post-pyrolysis modification methods–A meta-analysis. J. Environ. Chem. Eng. 2024, 12, 114885. [Google Scholar] [CrossRef]
- Huang, M.; Fan, L.; Chen, J.; Jiang, L.; Zou, Y. Continuous applications of biochar to rice: Effects on nitrogen uptake and utilization. Sci. Rep. 2018, 8, 11461. [Google Scholar] [CrossRef]
- Wong, J.W.; Ogbonnaya, U.O. Biochar porosity: A nature-based dependent parameter to deliver microorganisms to soils for land restoration. Environ. Sci. Pollut. Res. 2021, 28, 46894–46909. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Huang, Y.; Huang, L.; Li, Y.; Huang, Q.; Xu, G.; Müller, K.; Wang, H.; Ok, Y.S.; Liu, Z. The ratio of H/C is a useful parameter to predict adsorption of the herbicide metolachlor to biochars. Environ. Res. 2020, 184, 109324. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Development of biochar-based functional materials: Toward a sustainable platform carbon material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef] [PubMed]
- Gonzaga, M.I.S.; Mackowiak, C.; de Almeida, A.Q.; de Carvalho Junior, J.I.T.; Andrade, K.R. Positive and negative effects of biochar from coconut husks, orange bagasse and pine wood chips on maize (Zea mays L.) growth and nutrition. Catena 2018, 162, 414–420. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.-K.; Yang, J.E.; Ok, Y.S. Effects of pyrolysis temperature on soybean stover-and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef]
- Xu, C.-Y.; Hosseini-Bai, S.; Hao, Y.; Rachaputi, R.C.; Wang, H.; Xu, Z.; Wallace, H. Effect of biochar amendment on yield and photosynthesis of peanut on two types of soils. Environ. Sci. Pollut. Res. 2015, 22, 6112–6125. [Google Scholar]
- Wei, S.; Zhu, M.; Fan, X.; Song, J.; Li, K.; Jia, W.; Song, H. Influence of pyrolysis temperature and feedstock on carbon fractions of biochar produced from pyrolysis of rice straw, pine wood, pig manure and sewage sludge. Chemosphere 2019, 218, 624–631. [Google Scholar] [CrossRef]
- Lima, I.; Bigner, R.; Wright, M. Conversion of sweet sorghum bagasse into value-added biochar. Sugar Tech 2017, 19, 553–561. [Google Scholar] [CrossRef]
- Liu, L.; Fan, S. Removal of cadmium in aqueous solution using wheat straw biochar: Effect of minerals and mechanism. Environ. Sci. Pollut. Res. 2018, 25, 8688–8700. [Google Scholar] [CrossRef]
- Sika, M.; Hardie, A. Effect of pine wood biochar on ammonium nitrate leaching and availability in a S outh A frican sandy soil. Eur. J. Soil Sci. 2014, 65, 113–119. [Google Scholar]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Bourke, J.; Manley-Harris, M.; Fushimi, C.; Dowaki, K.; Nunoura, T.; Antal, M.J. Do all carbonized charcoals have the same chemical structure? 2. A model of the chemical structure of carbonized charcoal. Ind. Eng. Chem. Res. 2007, 46, 5954–5967. [Google Scholar] [CrossRef]
- Shaaban, A.; Se, S.-M.; Dimin, M.; Juoi, J.M.; Husin, M.H.M.; Mitan, N.M.M. Influence of heating temperature and holding time on biochars derived from rubber wood sawdust via slow pyrolysis. J. Anal. Appl. Pyrolysis 2014, 107, 31–39. [Google Scholar] [CrossRef]
- Almutairi, A.A.; Ahmad, M.; Rafique, M.I.; Al-Wabel, M.I. Variations in composition and stability of biochars derived from different feedstock types at varying pyrolysis temperature. J. Saudi Soc. Agric. Sci. 2023, 22, 25–34. [Google Scholar] [CrossRef]
- Praveen, S.; Jegan, J.; Bhagavathi Pushpa, T.; Gokulan, R.; Bulgariu, L. Biochar for removal of dyes in contaminated water: An overview. Biochar 2022, 4, 10. [Google Scholar] [CrossRef]
- Li, S.; Tasnady, D. Biochar for soil carbon sequestration: Current knowledge, mechanisms, and future perspectives. C 2023, 9, 67. [Google Scholar] [CrossRef]
- Aslam, Z.; Khalid, M.; Aon, M. Impact of biochar on soil physical properties. Sch. J. Agric. Sci 2014, 4, 280–284. [Google Scholar]
- Usowicz, B.; Lipiec, J.; Łukowski, M.; Marczewski, W.; Usowicz, J. The effect of biochar application on thermal properties and albedo of loess soil under grassland and fallow. Soil Tillage Res. 2016, 164, 45–51. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Wu, Y.; Cheng, H.; Pan, D.; Zhang, L.; Li, W.; Song, Y.; Bian, Y.; Jiang, X.; Han, J. Potassium hydroxide-modified algae-based biochar for the removal of sulfamethoxazole: Sorption performance and mechanisms. J. Environ. Manag. 2021, 293, 112912. [Google Scholar]
- Ye, H.; Yu, K.; Li, B.; Guo, J. Study on adsorption properties and mechanism of sodium hydroxide–modified ball-milled biochar to dislodge lead (II) and MB from water. Biomass Convers. Biorefin. 2024, 14, 15989–16003. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, Q.; Li, M.; Niu, D.; Sang, W.; Verpoort, F. Investigating the sorption behavior of cadmium from aqueous solution by potassium permanganate-modified biochar: Quantify mechanism and evaluate the modification method. Environ. Sci. Pollut. Res. 2018, 25, 8330–8339. [Google Scholar] [CrossRef]
- El-Nemr, M.A.; Abdelmonem, N.M.; Ismail, I.M.; Ragab, S.; El Nemr, A. Ozone and ammonium hydroxide modification of biochar prepared from Pisum sativum peels improves the adsorption of copper (II) from an aqueous medium. Environ. Process. 2020, 7, 973–1007. [Google Scholar] [CrossRef]
- Shen, Y.; Guo, J.-Z.; Bai, L.-Q.; Chen, X.-Q.; Li, B. High effective adsorption of Pb (II) from solution by biochar derived from torrefaction of ammonium persulphate pretreated bamboo. Bioresour. Technol. 2021, 323, 124616. [Google Scholar] [CrossRef]
- Kaur, J.; Chaudhary, S.; Bhalla, A. Recent Advances in Modification of Biochar for Removal of Emerging Contaminants from Water Bodies. In Occurrence, Distribution and Toxic Effects of Emerging Contaminantsx; CRC Press: Boca Raton, FL, USA, 2024; pp. 168–200. [Google Scholar]
- Wang, T.; Zhang, D.; Fang, K.; Zhu, W.; Peng, Q.; Xie, Z. Enhanced nitrate removal by physical activation and Mg/Al layered double hydroxide modified biochar derived from wood waste: Adsorption characteristics and mechanisms. J. Environ. Chem. Eng. 2021, 9, 105184. [Google Scholar] [CrossRef]
- Yin, Q.; Ren, H.; Wang, R.; Zhao, Z. Evaluation of nitrate and phosphate adsorption on Al-modified biochar: Influence of Al content. Sci. Total Environ. 2018, 631, 895–903. [Google Scholar] [CrossRef]
- Wang, H.; Teng, H.; Wang, X.; Xu, J.; Sheng, L. Physicochemical modification of corn straw biochar to improve performance and its application of constructed wetland substrate to treat city tail water. J. Environ. Manag. 2022, 310, 114758. [Google Scholar] [CrossRef]
- Vu, T.M.; Doan, D.P.; Van, H.T.; Nguyen, T.V.; Vigneswaran, S.; Ngo, H.H. Removing ammonium from water using modified corncob-biochar. Sci. Total Environ. 2017, 579, 612–619. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Fan, S. Preparation of KOH and H3PO4 modified biochar and its application in methylene blue removal from aqueous solution. Processes 2019, 7, 891. [Google Scholar] [CrossRef]
- Dong, H.; Deng, J.; Xie, Y.; Zhang, C.; Jiang, Z.; Cheng, Y.; Hou, K.; Zeng, G. Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr (VI) removal from aqueous solution. J. Hazard. Mater. 2017, 332, 79–86. [Google Scholar] [CrossRef]
- Tan, G.; Sun, W.; Xu, Y.; Wang, H.; Xu, N. Sorption of mercury (II) and atrazine by biochar, modified biochars and biochar based activated carbon in aqueous solution. Bioresour. Technol. 2016, 211, 727–735. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, Y.; Zheng, Y.; Yang, Y.; Huang, J.; Chen, H.; Quan, G.; Gao, B. Potassium permanganate modification of hydrochar enhances sorption of Pb (II), Cu (II), and Cd (II). Bioresour. Technol. 2023, 386, 129482. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Xiang, Y.; Wang, P.; Zhang, J.; Zhang, F.; Wei, J.; Luo, L.; Lei, M.; Tang, L. Modification of biochar derived from sawdust and its application in removal of tetracycline and copper from aqueous solution: Adsorption mechanism and modelling. Bioresour. Technol. 2017, 245, 266–273. [Google Scholar] [CrossRef]
- Mo, Z.; Shi, Q.; Zeng, H.; Lu, Z.; Bi, J.; Zhang, H.; Rinklebe, J.; Lima, E.C.; Rashid, A.; Shahab, A. Efficient removal of Cd (II) from aqueous environment by potassium permanganate-modified eucalyptus biochar. Biomass Convers. Biorefin. 2024, 14, 77–89. [Google Scholar]
- Cuong, D.V.; Wu, P.-C.; Chen, L.-I.; Hou, C.-H. Active MnO2/biochar composite for efficient As (III) removal: Insight into the mechanisms of redox transformation and adsorption. Water Res. 2021, 188, 116495. [Google Scholar]
- Anderson, N.; Gu, H.; Bergman, R. Comparison of novel biochars and steam activated carbon from mixed conifer mill residues. Energies 2021, 14, 8472. [Google Scholar] [CrossRef]
- Shao, J.; Zhang, J.; Zhang, X.; Feng, Y.; Zhang, H.; Zhang, S.; Chen, H. Enhance SO2 adsorption performance of biochar modified by CO2 activation and amine impregnation. Fuel 2018, 224, 138–146. [Google Scholar] [CrossRef]
- Hassaan, M.A.; Yılmaz, M.; Helal, M.; El-Nemr, M.A.; Ragab, S.; El Nemr, A. Isotherm and kinetic investigations of sawdust-based biochar modified by ammonia to remove methylene blue from water. Sci. Rep. 2023, 13, 12724. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Zhao, H.; Lyu, H.; Wang, L.; Huang, H.; Nan, Q.; Tang, J. UV modification of biochar for enhanced hexavalent chromium removal from aqueous solution. Environ. Sci. Pollut. Res. 2018, 25, 10808–10819. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Meng, X.; Zhang, Y.; Huang, Y. Effects of modification and magnetization of rice straw derived biochar on adsorption of tetracycline from water. Bioresour. Technol. 2020, 311, 123455. [Google Scholar] [CrossRef] [PubMed]
- Peter, A.; Chabot, B.; Loranger, E. Enhanced activation of ultrasonic pre-treated softwood biochar for efficient heavy metal removal from water. J. Environ. Manag. 2021, 290, 112569. [Google Scholar] [CrossRef]
- Inyang, M.; Gao, B.; Zimmerman, A.; Zhang, M.; Chen, H. Synthesis, characterization, and dye sorption ability of carbon nanotube–biochar nanocomposites. Chem. Eng. J. 2014, 236, 39–46. [Google Scholar] [CrossRef]
- Ashebir, H.; Tibebu, S.; Bedada, D.; Fito, J.; Kassahun, E.; Worku, A. Advanced methylene blue adsorption with a tailored biochar/graphene oxide/magnetite nanocomposite: Characterization, optimization, and reusability. Biomass Convers. Biorefin. 2025, 15, 15885–15906. [Google Scholar] [CrossRef]
- Hou, X.; Dong, H.; Li, Y.; Xiao, J.; Dong, Q.; Xiang, S.; Chu, D. Activation of persulfate by graphene/biochar composites for phenol degradation: Performance and nonradical dominated reaction mechanism. J. Environ. Chem. Eng. 2023, 11, 109348. [Google Scholar] [CrossRef]
- Ng, C.W.W.; Wang, Y.C.; Ni, J.J.; So, P.S. Effects of phosphorus-modified biochar as a soil amendment on the growth and quality of Pseudostellaria heterophylla. Sci. Rep. 2022, 12, 7268. [Google Scholar] [CrossRef]
- Wu, L.; Wei, C.; Zhang, S.; Wang, Y.; Kuzyakov, Y.; Ding, X. MgO-modified biochar increases phosphate retention and rice yields in saline-alkaline soil. J. Clean. Prod. 2019, 235, 901–909. [Google Scholar] [CrossRef]
- Wu, C.; Shi, L.; Xue, S.; Li, W.; Jiang, X.; Rajendran, M.; Qian, Z. Effect of sulfur-iron modified biochar on the available cadmium and bacterial community structure in contaminated soils. Sci. Total Environ. 2019, 647, 1158–1168. [Google Scholar] [CrossRef]
- Tao, Y.; Hu, S.; Han, S.; Shi, H.; Yang, Y.; Li, H.; Jiao, Y.; Zhang, Q.; Akindolie, M.S.; Ji, M. Efficient removal of atrazine by iron-modified biochar loaded Acinetobacter lwoffii DNS32. Sci. Total Environ. 2019, 682, 59–69. [Google Scholar] [CrossRef]
- Jeon, C.; Solis, K.L.; An, H.-R.; Hong, Y.; Igalavithana, A.D.; Ok, Y.S. Sustainable removal of Hg (II) by sulfur-modified pine-needle biochar. J. Hazard. Mater. 2020, 388, 122048. [Google Scholar] [CrossRef]
- Yavari, S.; Abualqumboz, M.; Sapari, N.; Hata-Suhaimi, H.-A.; Nik-Fuaad, N.-Z.; Yavari, S. Sorption of imazapic and imazapyr herbicides on chitosan-modified biochars. Int. J. Environ. Sci. Technol. 2020, 17, 3341–3350. [Google Scholar] [CrossRef]
- Liu, H.; Xu, F.; Xie, Y.; Wang, C.; Zhang, A.; Li, L.; Xu, H. Effect of modified coconut shell biochar on availability of heavy metals and biochemical characteristics of soil in multiple heavy metals contaminated soil. Sci. Total Environ. 2018, 645, 702–709. [Google Scholar] [CrossRef]
- Qin, L.; Shin, D. Effects of UV light treatment on functional group and its adsorption capacity of biochar. Energies 2023, 16, 5508. [Google Scholar] [CrossRef]
- Park, S.H.; Cho, H.J.; Ryu, C.; Park, Y.-K. Removal of copper (II) in aqueous solution using pyrolytic biochars derived from red macroalga Porphyra tenera. J. Ind. Eng. Chem. 2016, 36, 314–319. [Google Scholar] [CrossRef]
- Lyu, H.; Gao, B.; He, F.; Zimmerman, A.R.; Ding, C.; Huang, H.; Tang, J. Effects of ball milling on the physicochemical and sorptive properties of biochar: Experimental observations and governing mechanisms. Environ. Pollut. 2018, 233, 54–63. [Google Scholar] [CrossRef]
- Kołtowski, M.; Hilber, I.; Bucheli, T.D.; Oleszczuk, P. Effect of steam activated biochar application to industrially contaminated soils on bioavailability of polycyclic aromatic hydrocarbons and ecotoxicity of soils. Sci. Total Environ. 2016, 566, 1023–1031. [Google Scholar] [CrossRef]
- Patwardhan, S.B.; Pandit, S.; Gupta, P.K.; Jha, N.K.; Rawat, J.; Joshi, H.C.; Priya, K.; Gupta, M.; Lahiri, D.; Nag, M. Recent advances in the application of biochar in microbial electrochemical cells. Fuel 2022, 311, 122501. [Google Scholar] [CrossRef]
- Jirka, S.; Tomlinson, T. 2013 State of the Biochar Industry; International Biochar Initiative: Washington, DC, USA, 2014. [Google Scholar]
- ASTM D1762-84; Standard Test Method for Chemical Analysis of Wood Charcoal. ASTM International: West Conshohocken, PA, USA, 2007.
- TMECC; Test Methods for the Examination of Composting and Compost. US Composting Council and US Department of Agriculture: Bethesda, MD, USA, 2001.
- ASTM D6556; Standard Test Method for Carbon 1 Black—Total and External Surface Area by Nitrogen Adsorption. ASTM International: West Conshohocken, PA, USA, 2009.
- US EPA 8270; Method 8270 D Semivolatile Organic Compounds by Gas Chromatography/Mass Spectrometry (GC/MS). US EPA: Washington, DC, USA, 2007.
- US EPA 8290; METHOD 8290A Polychlorinated Dibenzo-P-Dioxins (PCDDs) and Polychlorinated Dibenzofurans (PCDFs) by High Resolution Gas Chromatography/High Resolution Mass Spectrometry (HRGC/HRMS). US EPA: Washington, DC, USA, 2007.
- US EPA 8082; Method 8082 A Polychlorinated Biphenyls (PCBs) by Gas Chromatography. US EPA: Washington, DC, USA, 2007.
- US EPA 8275; Method 8275A Semivolatile Organic Compounds (PAHs and PCBs) in Soils/Sludges and Solid Wastes Using Thermal Extraction/Gas Chromatography/Mass Spectrometry (TE/GC/MS). US EPA: Washington, DC, USA, 1996.
- US EPA 7471; Method 7471B Mercury in Solid or Semisolid Waste (Manual Cold-Vapor Technique). US EPA: Washington, DC, USA, 2007.
- Baronti, S.; Alberti, G.; Delle Vedove, G.; Di Gennaro, F.; Fellet, G.; Genesio, L.; Miglietta, F.; Peressotti, A.; Vaccari, F.P. The biochar option to improve plant yields: First results from some field and pot experiments in Italy. Ital. J. Agron. 2010, 5, 3–12. [Google Scholar] [CrossRef]
- Zavalloni, C.; Alberti, G.; Biasiol, S.; Delle Vedove, G.; Fornasier, F.; Liu, J.; Peressotti, A. Microbial mineralization of biochar and wheat straw mixture in soil: A short-term study. Appl. Soil Ecol. 2011, 50, 45–51. [Google Scholar] [CrossRef]
- Kookana, R.S.; Sarmah, A.K.; Van Zwieten, L.; Krull, E.; Singh, B. Biochar application to soil: Agronomic and environmental benefits and unintended consequences. Adv. Agron. 2011, 112, 103–143. [Google Scholar]
- Burrell, L.D.; Zehetner, F.; Rampazzo, N.; Wimmer, B.; Soja, G. Long-term effects of biochar on soil physical properties. Geoderma 2016, 282, 96–102. [Google Scholar] [CrossRef]
- Gamage, D.V.; Mapa, R.; Dharmakeerthi, R.; Biswas, A. Effect of rice-husk biochar on selected soil properties in tropical Alfisols. Soil Res. 2016, 54, 302–310. [Google Scholar] [CrossRef]
- Głąb, T.; Palmowska, J.; Zaleski, T.; Gondek, K. Effect of biochar application on soil hydrological properties and physical quality of sandy soil. Geoderma 2016, 281, 11–20. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. Soil Res. 2007, 45, 629–634. [Google Scholar] [CrossRef]
- Githinji, L. Effect of biochar application rate on soil physical and hydraulic properties of a sandy loam. Arch. Agron. Soil Sci. 2014, 60, 457–470. [Google Scholar] [CrossRef]
- Devereux, R.C.; Sturrock, C.J.; Mooney, S.J. The effects of biochar on soil physical properties and winter wheat growth. Earth Environ. Sci. Trans. R. Soc. Edinburgh 2012, 103, 13–18. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, X.; Tang, C.; Muhammad, N.; Wu, J.; Brookes, P.C.; Xu, J. Potential role of biochars in decreasing soil acidification-a critical review. Sci. Total Environ. 2017, 581, 601–611. [Google Scholar] [CrossRef]
- Lataf, A.; Jozefczak, M.; Vandecasteele, B.; Viaene, J.; Schreurs, S.; Carleer, R.; Yperman, J.; Marchal, W.; Cuypers, A.; Vandamme, D. The effect of pyrolysis temperature and feedstock on biochar agronomic properties. J. Anal. Appl. Pyrolysis 2022, 168, 105728. [Google Scholar] [CrossRef]
- Takaya, C.; Fletcher, L.; Singh, S.; Anyikude, K.; Ross, A. Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere 2016, 145, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Regelink, I.C.; Stoof, C.R.; Rousseva, S.; Weng, L.; Lair, G.J.; Kram, P.; Nikolaidis, N.P.; Kercheva, M.; Banwart, S.; Comans, R.N. Linkages between aggregate formation, porosity and soil chemical properties. Geoderma 2015, 247, 24–37. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhu, L.-X.; Zhang, H.-P.; Li, X.-Y.; Shen, Y.-F.; Li, S.-Q. Soil amendment with biochar increases maize yields in a semi-arid region by improving soil quality and root growth. Crop Pasture Sci. 2016, 67, 495–507. [Google Scholar] [CrossRef]
- Lu, S.-G.; Sun, F.-F.; Zong, Y.-T. Effect of rice husk biochar and coal fly ash on some physical properties of expansive clayey soil (Vertisol). Catena 2014, 114, 37–44. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, K.; Chen, B. Linking hydrophobicity of biochar to the water repellency and water holding capacity of biochar-amended soil. Environ. Pollut. 2019, 253, 779–789. [Google Scholar] [CrossRef]
- Wang, Z.; Han, L.; Sun, K.; Jin, J.; Ro, K.S.; Libra, J.A.; Liu, X.; Xing, B. Sorption of four hydrophobic organic contaminants by biochars derived from maize straw, wood dust and swine manure at different pyrolytic temperatures. Chemosphere 2016, 144, 285–291. [Google Scholar] [CrossRef]
- Page-Dumroese, D.S.; Robichaud, P.R.; Brown, R.E.; Tirocke, J.M. Water repellency of two forest soils after biochar addition. Trans. ASABE 2015, 58, 335–342. [Google Scholar] [CrossRef]
- Geng, N.; Kang, X.; Yan, X.; Yin, N.; Wang, H.; Pan, H.; Yang, Q.; Lou, Y.; Zhuge, Y. Biochar mitigation of soil acidification and carbon sequestration is influenced by materials and temperature. Ecotoxicol. Environ. Saf. 2022, 232, 113241. [Google Scholar] [CrossRef]
- Waters, B.M.; Amundsen, K.; Graef, G. Gene expression profiling of iron deficiency chlorosis sensitive and tolerant soybean indicates key roles for phenylpropanoids under alkalinity stress. Front. Plant Sci. 2018, 9, 323680. [Google Scholar] [CrossRef]
- Antonangelo, J.A.; Culman, S.; Zhang, H. Comparative analysis and prediction of cation exchange capacity via summation: Influence of biochar type and nutrient ratios. Front. Soil Sci. 2024, 4, 1371777. [Google Scholar] [CrossRef]
- Omara, P.; Singh, H.; Singh, K.; Sharma, L.; Otim, F.; Obia, A. Short-term effect of field application of biochar on cation exchange capacity, pH, and electrical conductivity of sandy and clay loam temperate soils. Technol. Agron. 2023, 3, 16. [Google Scholar] [CrossRef]
- Sharma, A.; Weindorf, D.C.; Wang, D.; Chakraborty, S. Characterizing soils via portable X-ray fluorescence spectrometer: 4. Cation exchange capacity (CEC). Geoderma 2015, 239, 130–134. [Google Scholar] [CrossRef]
- Ramos, F.T.; Dores, E.F.d.C.; Weber, O.L.d.S.; Beber, D.C.; Campelo, J.H., Jr.; Maia, J.C.d.S. Soil organic matter doubles the cation exchange capacity of tropical soil under no-till farming in Brazil. J. Sci. Food Agric. 2018, 98, 3595–3602. [Google Scholar] [CrossRef] [PubMed]
- Nkoh, J.N.; Baquy, M.A.-A.; Mia, S.; Shi, R.; Kamran, M.A.; Mehmood, K.; Xu, R. A critical-systematic review of the interactions of biochar with soils and the observable outcomes. Sustainability 2021, 13, 13726. [Google Scholar] [CrossRef]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.-M.; Dallmeyer, I.; Garcia-Perez, M. Influence of feedstock source and pyrolysis temperature on biochar bulk and surface properties. Biomass Bioenergy 2016, 84, 37–48. [Google Scholar] [CrossRef]
- Zama, E.F.; Zhu, Y.-G.; Reid, B.J.; Sun, G.-X. The role of biochar properties in influencing the sorption and desorption of Pb (II), Cd (II) and As (III) in aqueous solution. J. Clean. Prod. 2017, 148, 127–136. [Google Scholar] [CrossRef]
- Gai, X.; Wang, H.; Liu, J.; Zhai, L.; Liu, S.; Ren, T.; Liu, H. Effects of feedstock and pyrolysis temperature on biochar adsorption of ammonium and nitrate. PLoS ONE 2014, 9, e113888. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- Jones, D.; Edwards-Jones, G.; Murphy, D. Biochar mediated alterations in herbicide breakdown and leaching in soil. Soil Biol. Biochem. 2011, 43, 804–813. [Google Scholar] [CrossRef]
- Khorram, M.S.; Sarmah, A.K.; Yu, Y. The effects of biochar properties on fomesafen adsorption-desorption capacity of biochar-amended soil. Water Air Soil Pollut. 2018, 229, 60. [Google Scholar] [CrossRef]
- Gámiz, B.; Velarde, P.; Spokas, K.A.; Hermosín, M.C.; Cox, L. Biochar soil additions affect herbicide fate: Importance of application timing and feedstock species. J. Agric. Food Chem. 2017, 65, 3109–3117. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Zou, Z.; Li, X.; Zhang, L.; Zhang, L.; Fu, J.; Wenyan, H. Biochar changed the distribution of imidacloprid in a plant–soil–groundwater system. Chemosphere 2022, 307, 136213. [Google Scholar] [CrossRef]
- Apolloni, F.; Menegazzo, F.; Bittencourt, C.; Signoretto, M. Hazelnut shells and rice husks activated biochars for the adsorption of atrazine and terbuthylazine. Next Energy 2025, 7, 100291. [Google Scholar] [CrossRef]
- You, X.; Jiang, H.; Zhao, M.; Suo, F.; Zhang, C.; Zheng, H.; Sun, K.; Zhang, G.; Li, F.; Li, Y. Biochar reduced Chinese chive (Allium tuberosum) uptake and dissipation of thiamethoxam in an agricultural soil. J. Hazard. Mater. 2020, 390, 121749. [Google Scholar] [CrossRef]
- Clay, S.A.; Krack, K.K.; Bruggeman, S.A.; Papiernik, S.; Schumacher, T.E. Maize, switchgrass, and ponderosa pine biochar added to soil increased herbicide sorption and decreased herbicide efficacy. J. Environ. Sci. Health B 2016, 51, 497–507. [Google Scholar] [CrossRef]
- Szewczuk-Karpisz, K.; Tomczyk, A.; Celińska, M.; Sokołowska, Z.; Kuśmierz, M. Carboxin and diuron adsorption mechanism on sunflower husks biochar and goethite in the single/mixed pesticide solutions. Materials 2021, 14, 2584. [Google Scholar] [CrossRef]
- Wang, P.; Liu, X.; Yu, B.; Wu, X.; Xu, J.; Dong, F.; Zheng, Y. Characterization of peanut-shell biochar and the mechanisms underlying its sorption for atrazine and nicosulfuron in aqueous solution. Sci. Total Environ. 2020, 702, 134767. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Singh, T.; Azad, D.; Adhikari, H.; Verma, A. Role of biochar as a sustainable sorbent for fipronil removal from aqueous and soil environments. In Microbiology-2.0 Update for a Sustainable Future; Springer: Singapore, 2024; pp. 187–207. [Google Scholar]
- Kumari, U.; Banerjee, T.; Singh, N. Evaluating ash and biochar mixed biomixtures for atrazine and fipronil degradation. Environ. Technol. Innov. 2021, 23, 101745. [Google Scholar] [CrossRef]
- Jacob, M.M.; Ponnuchamy, M.; Kapoor, A.; Sivaraman, P. Bagasse based biochar for the adsorptive removal of chlorpyrifos from contaminated water. J. Environ. Chem. Eng. 2020, 8, 103904. [Google Scholar] [CrossRef]
- Tang, X.-Y.; Huang, W.-D.; Guo, J.-J.; Yang, Y.; Tao, R.; Feng, X. Use of Fe-impregnated biochar to efficiently sorb chlorpyrifos, reduce uptake by Allium fistulosum L., and enhance microbial community diversity. J. Agric. Food Chem. 2017, 65, 5238–5243. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, B.; Shen, J.; Yan, P.; Kang, J.; Wang, W.; Bi, L.; Zhu, X.; Li, Y.; Wang, S. Preparation of novel N-doped biochar and its high adsorption capacity for atrazine based on π–π electron donor-acceptor interaction. J. Hazard. Mater. 2022, 432, 128757. [Google Scholar] [CrossRef]
- Ban, S.-E.; Lee, E.-J.; Lim, D.-J.; Kim, I.-S.; Lee, J.-W. Evaluation of sulfuric acid-pretreated biomass-derived biochar characteristics and its diazinon adsorption mechanism. Bioresour. Technol. 2022, 348, 126828. [Google Scholar] [CrossRef]
- Ha, N.T.H.; Toan, N.C.; Kajitvichyanukul, P. Enhanced paraquat removal from contaminated water using cell-immobilized biochar. Clean Technol. Environ. Policy 2022, 24, 1073–1085. [Google Scholar] [CrossRef]
- Bashir, S.; Zhu, J.; Fu, Q.; Hu, H. Cadmium mobility, uptake and anti-oxidative response of water spinach (Ipomoea aquatic) under rice straw biochar, zeolite and rock phosphate as amendments. Chemosphere 2018, 194, 579–587. [Google Scholar] [CrossRef]
- Bashir, S.; Hussain, Q.; Akmal, M.; Riaz, M.; Hu, H.; Ijaz, S.S.; Iqbal, M.; Abro, S.; Mehmood, S.; Ahmad, M. Sugarcane bagasse-derived biochar reduces the cadmium and chromium bioavailability to mash bean and enhances the microbial activity in contaminated soil. J. Soils Sed. 2018, 18, 874–886. [Google Scholar] [CrossRef]
- Lu, K.; Yang, X.; Shen, J.; Robinson, B.; Huang, H.; Liu, D.; Bolan, N.; Pei, J.; Wang, H. Effect of bamboo and rice straw biochars on the bioavailability of Cd, Cu, Pb and Zn to Sedum plumbizincicola. Agric. Ecosyst. Environ. 2014, 191, 124–132. [Google Scholar] [CrossRef]
- Yang, F.; Du, Q.; Sui, L.; Cheng, K. One-step fabrication of artificial humic acid-functionalized colloid-like magnetic biochar for rapid heavy metal removal. Bioresour. Technol. 2021, 328, 124825. [Google Scholar] [CrossRef]
- Fu, H.; Ma, S.; Xu, S.; Duan, R.; Cheng, G.; Zhao, P. Hierarchically porous magnetic biochar as an efficient amendment for cadmium in water and soil: Performance and mechanism. Chemosphere 2021, 281, 130990. [Google Scholar] [CrossRef]
- Xu, Y.; Seshadri, B.; Sarkar, B.; Wang, H.; Rumpel, C.; Sparks, D.; Farrell, M.; Hall, T.; Yang, X.; Bolan, N. Biochar modulates heavy metal toxicity and improves microbial carbon use efficiency in soil. Sci. Total Environ. 2018, 621, 148–159. [Google Scholar] [CrossRef]
- Xing, Y.; Luo, X.; Liu, S.; Wan, W.; Huang, Q.; Chen, W. A novel eco-friendly recycling of food waste for preparing biofilm-attached biochar to remove Cd and Pb in wastewater. J. Clean. Prod. 2021, 311, 127514. [Google Scholar] [CrossRef]
- Aguayo-Villarreal, I.; Bonilla-Petriciolet, A.; Muñiz-Valencia, R. Preparation of activated carbons from pecan nutshell and their application in the antagonistic adsorption of heavy metal ions. J. Mol. Liq. 2017, 230, 686–695. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, R.; Li, R.; Ali, A.; Chen, A.; Zhang, Z. Enhanced aqueous Cr (VI) removal using chitosan-modified magnetic biochars derived from bamboo residues. Chemosphere 2020, 261, 127694. [Google Scholar] [CrossRef]
- Long, J.; Tan, D.; Deng, S.; Li, B.; Ding, D.; Lei, M. Antimony accumulation and iron plaque formation at different growth stages of rice (Oryza sativa L.). Environ. Pollut. 2019, 249, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jia, C.; Gan, Y.; Wang, S. Impact of biochars on the iron plaque formation and the antimony accumulation in rice seedings. Bull. Environ. Contam. Toxicol. 2022, 109, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Wilfong, W.C.; Kail, B.W.; Wang, Q.; Shi, F.; Shipley, G.; Tarka, T.J.; Gray, M.L. Stable immobilized amine sorbents for heavy metal and REE removal from industrial wastewaters. Environ. Sci. Water Res. Technol. 2020, 6, 1286–1299. [Google Scholar] [CrossRef]
- Hadjittofi, L.; Charalambous, S.; Pashalidis, I. Removal of trivalent samarium from aqueous solutions by activated biochar derived from cactus fibres. J. Rare Earths 2016, 34, 99–104. [Google Scholar] [CrossRef]
- Li, H.; Jiang, Q.; Li, R.; Zhang, B.; Zhang, J.; Zhang, Y. Passivation of lead and cerium in soil facilitated by biochar-supported phosphate-doped ferrihydrite: Mechanisms and microbial community evolution. J. Hazard. Mater. 2022, 436, 129090. [Google Scholar] [CrossRef] [PubMed]
- Komnitsas, K.; Zaharaki, D.; Bartzas, G.; Alevizos, G. Adsorption of scandium and neodymium on biochar derived after low-temperature pyrolysis of sawdust. Minerals 2017, 7, 200. [Google Scholar] [CrossRef]
- Maestrini, B.; Herrmann, A.M.; Nannipieri, P.; Schmidt, M.W.; Abiven, S. Ryegrass-derived pyrogenic organic matter changes organic carbon and nitrogen mineralization in a temperate forest soil. Soil Biol. Biochem. 2014, 69, 291–301. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Pokharel, P.; Ma, Z.; Chang, S.X. Biochar increases soil microbial biomass with changes in extra-and intracellular enzyme activities: A global meta-analysis. Biochar 2020, 2, 65–79. [Google Scholar] [CrossRef]
- Quilliam, R.S.; Glanville, H.C.; Wade, S.C.; Jones, D.L. Life in the ‘charosphere’–Does biochar in agricultural soil provide a significant habitat for microorganisms? Soil Biol. Biochem. 2013, 65, 287–293. [Google Scholar] [CrossRef]
- Zhang, M.; Riaz, M.; Zhang, L.; El-Desouki, Z.; Jiang, C. Biochar induces changes to basic soil properties and bacterial communities of different soils to varying degrees at 25 mm rainfall: More effective on acidic soils. Front. Microbiol. 2019, 10, 1321. [Google Scholar] [CrossRef]
- DeForest, J.L.; Otuya, R.K. Soil nitrification increases with elevated phosphorus or soil pH in an acidic mixed mesophytic deciduous forest. Soil Biol. Biochem. 2020, 142, 107716. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, J.-L. Effects of biochar on soil microbial diversity and community structure in clay soil. Ann. Microbiol. 2022, 72, 35. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, Y.; Xiang, Y.; Zhang, R.; Lu, H. Responses of soil microbial community structure changes and activities to biochar addition: A meta-analysis. Sci. Total Environ. 2018, 643, 926–935. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Chang, S.X.; Jiang, X.; Song, Y. Biochar increases soil microbial biomass but has variable effects on microbial diversity: A meta-analysis. Sci. Total Environ. 2020, 749, 141593. [Google Scholar] [CrossRef]
- Deshoux, M.; Sadet-Bourgeteau, S.; Gentil, S.; Prévost-Bouré, N.C. Effects of biochar on soil microbial communities: A meta-analysis. Sci. Total Environ. 2023, 902, 166079. [Google Scholar] [CrossRef]
- Abujabhah, I.S.; Bound, S.A.; Doyle, R.; Bowman, J.P. Effects of biochar and compost amendments on soil physico-chemical properties and the total community within a temperate agricultural soil. Appl. Soil Ecol. 2016, 98, 243–253. [Google Scholar] [CrossRef]
- Warnock, D.D.; Mummey, D.L.; McBride, B.; Major, J.; Lehmann, J.; Rillig, M.C. Influences of non-herbaceous biochar on arbuscular mycorrhizal fungal abundances in roots and soils: Results from growth-chamber and field experiments. Appl. Soil Ecol. 2010, 46, 450–456. [Google Scholar] [CrossRef]
- Thers, H.; Djomo, S.N.; Elsgaard, L.; Knudsen, M.T. Biochar potentially mitigates greenhouse gas emissions from cultivation of oilseed rape for biodiesel. Sci. Total Environ. 2019, 671, 180–188. [Google Scholar] [CrossRef]
- Enebe, M.C.; Ray, R.L.; Griffin, R.W. The impacts of biochar on carbon sequestration, soil processes, and microbial communities: A review. Biochar 2025, 7, 1–26. [Google Scholar] [CrossRef]
- Mishra, R.K.; Kumar, D.J.P.; Narula, A.; Chistie, S.M.; Naik, S.U. Production and beneficial impact of biochar for environmental application: A review on types of feedstocks, chemical compositions, operating parameters, techno-economic study, and life cycle assessment. Fuel 2023, 343, 127968. [Google Scholar] [CrossRef]
- Shabangu, S.; Woolf, D.; Fisher, E.M.; Angenent, L.T.; Lehmann, J. Techno-economic assessment of biomass slow pyrolysis into different biochar and methanol concepts. Fuel 2014, 117, 742–748. [Google Scholar] [CrossRef]
- Nematian, M.; Keske, C.; Ng’ombe, J.N. A techno-economic analysis of biochar production and the bioeconomy for orchard biomass. Waste Manag. 2021, 135, 467–477. [Google Scholar] [CrossRef]
- Sahoo, K.; Upadhyay, A.; Runge, T.; Bergman, R.; Puettmann, M.; Bilek, E. Life-cycle assessment and techno-economic analysis of biochar produced from forest residues using portable systems. Int. J. Life Cycle Assess. 2021, 26, 189–213. [Google Scholar] [CrossRef]
- Jin, Q.; O’Keefe, S.F.; Stewart, A.C.; Neilson, A.P.; Kim, Y.-T.; Huang, H. Techno-economic analysis of a grape pomace biorefinery: Production of seed oil, polyphenols, and biochar. Food Bioprod. Process. 2021, 127, 139–151. [Google Scholar] [CrossRef]
- Li, W.; Dumortier, J.; Dokoohaki, H.; Miguez, F.E.; Brown, R.C.; Laird, D.; Wright, M.M. Regional techno-economic and life-cycle analysis of the pyrolysis-bioenergy-biochar platform for carbon-negative energy. Biofuels Bioprod. Biorefining 2019, 13, 1428–1438. [Google Scholar] [CrossRef]
- Jayakumar, A.; Morrisset, D.; Koutsomarkos, V.; Wurzer, C.; Hadden, R.M.; Lawton, L.; Edwards, C.; Mašek, O. Systematic evaluation of pyrolysis processes and biochar quality in the operation of low-cost flame curtain pyrolysis kiln for sustainable biochar production. Curr. Res. Environ. Sustain. 2023, 5, 100213. [Google Scholar] [CrossRef]
- Smebye, A.B.; Sparrevik, M.; Schmidt, H.P.; Cornelissen, G. Life-cycle assessment of biochar production systems in tropical rural areas: Comparing flame curtain kilns to other production methods. Biomass Bioenergy 2017, 101, 35–43. [Google Scholar]
- Akom, M.; Fanyin-Martin, A.; Oti-Boateng, C.; Otoo, E.; Dawoe, E. Yield and cost of biochar produced by a locally fabricated reactor. J. Agric. Environ. Sci. 2020, 9, 2334–2412. [Google Scholar] [CrossRef]
- Granatstein, D.; Kruger, C.; Collins, H.; Garcia-Perez, M.; Yoder, J. Use of Biochar from the Pyrolysis of Waste Organic Material as a Soil Amendment; Center for Sustaining Agriculture and Natural Resources: Wenatchee, WA, USA, 2009. [Google Scholar]
- Brown, T.R.; Wright, M.M.; Brown, R.C. Estimating profitability of two biochar production scenarios: Slow pyrolysis vs fast pyrolysis. Biofuels Bioprod. Biorefining 2011, 5, 54–68. [Google Scholar] [CrossRef]
- Hu, M.; Guo, K.; Zhou, H.; Zhu, W.; Deng, L.; Dai, L. Techno-economic assessment of swine manure biochar production in large-scale piggeries in China. Energy 2024, 308, 133037. [Google Scholar] [CrossRef]
- Pandit, N.R.; Schmidt, H.P.; Mulder, J.; Hale, S.E.; Husson, O.; Cornelissen, G. Nutrient effect of various composting methods with and without biochar on soil fertility and maize growth. Arch. Agron. Soil Sci. 2020, 66, 250–265. [Google Scholar] [CrossRef]
- Fawzy, S.; Osman, A.I.; Mehta, N.; Moran, D.; Ala’a, H.; Rooney, D.W. Atmospheric carbon removal via industrial biochar systems: A techno-economic-environmental study. J. Clean. Prod. 2022, 371, 133660. [Google Scholar]
- Matuštík, J.; Hnátková, T.; Kočí, V. Life cycle assessment of biochar-to-soil systems: A review. J. Clean. Prod. 2020, 259, 120998. [Google Scholar]
- Kavindi, G.A.G.; Tang, L.; Sasaki, Y. Assessing GHG Emission Reduction in Biomass-Derived Biochar Production via Slow Pyrolysis: A Cradle-to-gate LCA Approach. Resour. Conserv. Recycl. 2025, 212, 107900. [Google Scholar]
- Carvalho, J.; Nascimento, L.; Soares, M.; Valério, N.; Ribeiro, A.; Faria, L.; Silva, A.; Pacheco, N.; Araújo, J.; Vilarinho, C. Life cycle assessment (LCA) of biochar production from a circular economy perspective. Processes 2022, 10, 2684. [Google Scholar] [CrossRef]
- Liu, K.; Fang, L.; Li, F.; Hou, D.; Liu, C.; Song, Y.; Ran, Q.; Pang, Y.; Du, Y.; Yuan, Y. Sustainability assessment and carbon budget of chemical stabilization based multi-objective remediation of Cd contaminated paddy field. Sci. Total Environ. 2022, 819, 152022. [Google Scholar]
- Brassard, P.; Godbout, S.; Hamelin, L. Framework for consequential life cycle assessment of pyrolysis biorefineries: A case study for the conversion of primary forestry residues. Renew. Sustain. Energy Rev. 2021, 138, 110549. [Google Scholar] [CrossRef]
- Yang, Q.; Mašek, O.; Zhao, L.; Nan, H.; Yu, S.; Yin, J.; Li, Z.; Cao, X. Country-level potential of carbon sequestration and environmental benefits by utilizing crop residues for biochar implementation. Appl. Energy 2021, 282, 116275. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis; IPCC: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Uusitalo, V.; Leino, M. Neutralizing global warming impacts of crop production using biochar from side flows and buffer zones: A case study of oat production in the boreal climate zone. J. Clean. Prod. 2019, 227, 48–57. [Google Scholar] [CrossRef]
- Bartocci, P.; Bidini, G.; Saputo, P.; Fantozzi, F. Biochar pellet carbon footprint. Chem. Eng 2016, 50, 217–222. [Google Scholar]
- Llorach-Massana, P.; Lopez-Capel, E.; Peña, J.; Rieradevall, J.; Montero, J.I.; Puy, N. Technical feasibility and carbon footprint of biochar co-production with tomato plant residue. Waste Manag. 2017, 67, 121–130. [Google Scholar] [CrossRef]
- Xu, X.; Cheng, K.; Wu, H.; Sun, J.; Yue, Q.; Pan, G. Greenhouse gas mitigation potential in crop production with biochar soil amendment—A carbon footprint assessment for cross-site field experiments from China. Gcb Bioenergy 2019, 11, 592–605. [Google Scholar] [CrossRef]
- Mohammadi, A.; Cowie, A.; Mai, T.L.A.; de la Rosa, R.A.; Brandão, M.; Kristiansen, P.; Joseph, S. Quantifying the greenhouse gas reduction benefits of utilising straw biochar and enriched biochar. Energy Procedia 2016, 97, 254–261. [Google Scholar] [CrossRef]
- Rafiq, M.K.; Joseph, S.D.; Li, F.; Bai, Y.; Shang, Z.; Rawal, A.; Hook, J.M.; Munroe, P.R.; Donne, S.; Taherymoosavi, S. Pyrolysis of attapulgite clay blended with yak dung enhances pasture growth and soil health: Characterization and initial field trials. Sci. Total Environ. 2017, 607, 184–194. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, S.; Wang, J.; Ding, X. Phosphorus retention using iron (II/III) modified biochar in saline-alkaline soils: Adsorption, column and field tests. Environ. Pollut. 2020, 261, 114223. [Google Scholar] [CrossRef]
- Wei, M.; Liu, X.; He, Y.; Xu, X.; Wu, Z.; Yu, K.; Zheng, X. Biochar inoculated with Pseudomonas putida improves grape (Vitis vinifera L.) fruit quality and alters bacterial diversity. Rhizosphere 2020, 16, 100261. [Google Scholar] [CrossRef]
- Pan, D.; Liu, C.; Yu, H.; Li, F. A paddy field study of arsenic and cadmium pollution control by using iron-modified biochar and silica sol together. Environ. Sci. Pollut. Res. 2019, 26, 24979–24987. [Google Scholar] [CrossRef]
- Wang, L.; Ok, Y.S.; Tsang, D.C.; Alessi, D.S.; Rinklebe, J.; Mašek, O.; Bolan, N.S.; Hou, D. Biochar composites: Emerging trends, field successes and sustainability implications. Soil Use Manag. 2022, 38, 14–38. [Google Scholar] [CrossRef]
- She, D.; Sun, X.; Gamareldawla, A.H.; Nazar, E.A.; Hu, W.; Edith, K.; Yu, S.E. Benefits of soil biochar amendments to tomato growth under saline water irrigation. Sci. Rep. 2018, 8, 14743. [Google Scholar] [CrossRef] [PubMed]
- Usman, A.R.A.; Al-Wabel, M.I.; Abdulaziz, A.-H.; Mahmoud, W.-A.; El-Naggar, A.H.; Ahmad, M.; Abdulelah, A.-F.; Abdulrasoul, A.-O. Conocarpus biochar induces changes in soil nutrient availability and tomato growth under saline irrigation. Pedosphere 2016, 26, 27–38. [Google Scholar] [CrossRef]
- Cao, D.; Chen, W.; Yang, P.; Lan, Y.; Sun, D. Spatio-temporal variabilities of soil phosphorus pool and phosphorus uptake with maize stover biochar amendment for 5 years of maize. Environ. Sci. Pollut. Res. 2020, 27, 36350–36361. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Sun, B.; Wu, S.; Feng, H.; Gao, M.; Zhang, B.; Liu, Y. After-effects of straw and straw-derived biochar application on crop growth, yield, and soil properties in wheat (Triticum aestivum L.)-maize (Zea mays L.) rotations: A four-year field experiment. Sci. Total Environ. 2021, 780, 146560. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, J.; Han, L.; Ma, S.; Sun, X.; Huang, G. Role and multi-scale characterization of bamboo biochar during poultry manure aerobic composting. Bioresour. Technol. 2017, 241, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, G.; Sun, H.; Zhou, S.; Zou, G. Straw biochar hastens organic matter degradation and produces nutrient-rich compost. Bioresour. Technol. 2016, 200, 876–883. [Google Scholar] [CrossRef]
- Xiao, R.; Awasthi, M.K.; Li, R.; Park, J.; Pensky, S.M.; Wang, Q.; Wang, J.J.; Zhang, Z. Recent developments in biochar utilization as an additive in organic solid waste composting: A review. Bioresour. Technol. 2017, 246, 203–213. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Changes in physical, chemical, and microbiological properties during the two-stage co-composting of green waste with spent mushroom compost and biochar. Bioresour. Technol. 2014, 171, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Monedero, M.A.; Cayuela, M.L.; Sánchez-García, M.; Vandecasteele, B.; D’Hose, T.; López, G.; Martínez-Gaitán, C.; Kuikman, P.J.; Sinicco, T.; Mondini, C. Agronomic evaluation of biochar, compost and biochar-blended compost across different cropping systems: Perspective from the European project FERTIPLUS. Agronomy 2019, 9, 225. [Google Scholar] [CrossRef]
- Zahra, M.B.; Aftab, Z.E.H.; Haider, M.S. Water productivity, yield and agronomic attributes of maize crop in response to varied irrigation levels and biochar–compost application. J. Sci. Food Agric. 2021, 101, 4591–4604. [Google Scholar] [CrossRef]
- Agegnehu, G.; Bass, A.M.; Nelson, P.N.; Bird, M.I. Benefits of biochar, compost and biochar–compost for soil quality, maize yield and greenhouse gas emissions in a tropical agricultural soil. Sci. Total Environ. 2016, 543, 295–306. [Google Scholar] [CrossRef]
- Manolikaki, I.; Diamadopoulos, E. Positive effects of biochar and biochar-compost on maize growth and nutrient availability in two agricultural soils. Commun. Soil Sci. Plant Anal. 2019, 50, 512–526. [Google Scholar] [CrossRef]
- Agegnehu, G.; Nelson, P.N.; Bird, M.I. The effects of biochar, compost and their mixture and nitrogen fertilizer on yield and nitrogen use efficiency of barley grown on a Nitisol in the highlands of Ethiopia. Sci. Total Environ. 2016, 569, 869–879. [Google Scholar] [CrossRef]
- Khorram, M.S.; Zhang, Q.; Lin, D.; Zheng, Y.; Fang, H.; Yu, Y. Biochar: A review of its impact on pesticide behavior in soil environments and its potential applications. J. Environ. Sci. 2016, 44, 269–279. [Google Scholar] [CrossRef]
- Chen, M.; Chen, X.; Xu, X.; Xu, Z.; Zhang, Y.; Song, B.; Tsang, D.C.; Xu, N.; Cao, X. Biochar colloids facilitate transport and transformation of Cr (VI) in soil: Active site competition coupling with reduction reaction. J. Hazard. Mater. 2022, 440, 129691. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, Y.; Zhao, H.; Li, Q. A comparative study on behavior of heavy metals in pyrochar and hydrochar from sewage sludge. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 565–571. [Google Scholar] [CrossRef]
- Godlewska, P.; Ok, Y.S.; Oleszczuk, P. The dark side of black gold: Ecotoxicological aspects of biochar and biochar-amended soils. J. Hazard. Mater. 2021, 403, 123833. [Google Scholar] [CrossRef]
- Pinelli, S.; Rossi, S.; Malcevschi, A.; Miragoli, M.; Corradi, M.; Selis, L.; Tagliaferri, S.; Rossi, F.; Cavallo, D.; Ursini, C.L. Biochar dust emission: Is it a health concern? Preliminary results for toxicity assessment. Environ. Toxicol. Pharmacol. 2024, 109, 104477. [Google Scholar] [CrossRef]
- Thomas, S. Post-processing of biochars to enhance plant growth responses: A review and meta-analysis. Biochar 2021, 3, 437–455. [Google Scholar] [CrossRef]
- Cornelissen, G.; Pandit, N.R.; Taylor, P.; Pandit, B.H.; Sparrevik, M.; Schmidt, H.P. Emissions and char quality of flame-curtain” Kon Tiki” Kilns for Farmer-Scale charcoal/biochar production. PLoS ONE 2016, 11, e0154617. [Google Scholar] [CrossRef]
- Sangani, M.F.; Abrishamkesh, S.; Owens, G. Physicochemical characteristics of biochars can be beneficially manipulated using post-pyrolyzed particle size modification. Bioresour. Technol. 2020, 306, 123157. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Y.; Mao, J.; Chen, B. Effects of biochar nanoparticles on seed germination and seedling growth. Environ. Pollut. 2020, 256, 113409. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Lian, F.; Han, Y.; Bao, Q.; Wang, Z.; Xing, B. The effect of biochar nanoparticles on rice plant growth and the uptake of heavy metals: Implications for agronomic benefits and potential risk. Sci. Total Environ. 2019, 656, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wu, Z.; Zhang, X.; Feng, L.; Xiong, Z. Dynamic responses of ammonia volatilization to different rates of fresh and field-aged biochar in a rice-wheat rotation system. Field Crops Res. 2019, 241, 107568. [Google Scholar] [CrossRef]
- Heitkötter, J.; Marschner, B. Interactive effects of biochar ageing in soils related to feedstock, pyrolysis temperature, and historic charcoal production. Geoderma 2015, 245, 56–64. [Google Scholar] [CrossRef]
- Manzoor, M.; Gul, S.; Khan, H. Influence of biochars on yield and nitrogen and phosphorus use efficiency of Pisum sativum under groundwater and wastewater irrigation in arid climate. Commun. Soil Sci. Plant Anal. 2019, 50, 1563–1579. [Google Scholar] [CrossRef]
- Gámiz, B.; Velarde, P.; Spokas, K.A.; Celis, R.; Cox, L. Changes in sorption and bioavailability of herbicides in soil amended with fresh and aged biochar. Geoderma 2019, 337, 341–349. [Google Scholar] [CrossRef]
- Intani, K.; Latif, S.; Islam, M.S.; Müller, J. Phytotoxicity of corncob biochar before and after heat treatment and washing. Sustainability 2018, 11, 30. [Google Scholar] [CrossRef]


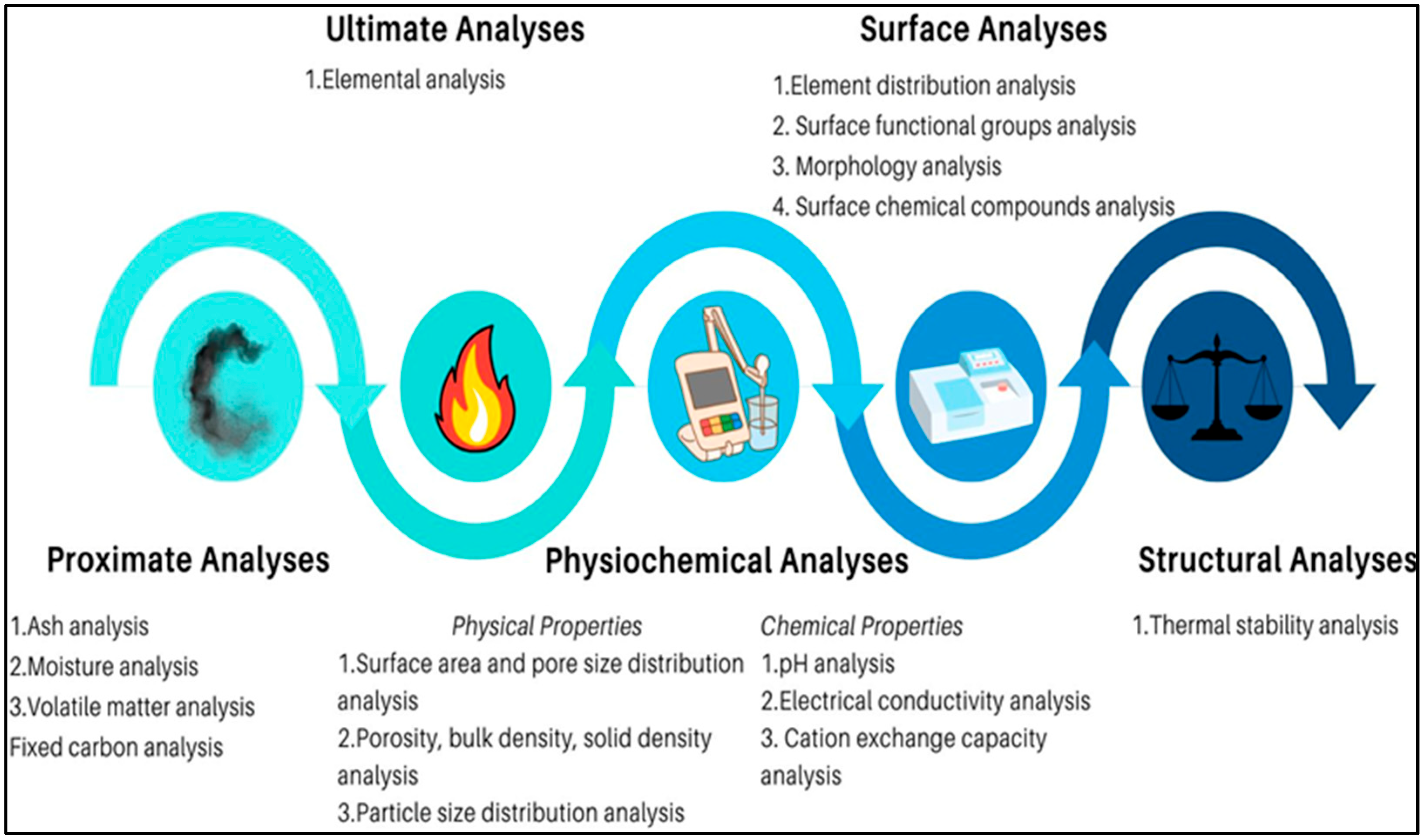
| Analyses Type | Parameter | Standard/Test Method | References |
|---|---|---|---|
| Proximate Analyses |
| ASTM D1762-84 | [66] |
| Chemical Analyses |
| TMECC (2001) and IBI | [65,67] |
| Physical Analyses |
| IBI | [65] |
| ASTM D6556 | [68] | |
| Surface Analyses |
| US EPA 8270 (2007) and IBI | [65,69] |
| US EPA 8290 (2007) | [70] | |
| US EPA 8082 (2007) or US EPA 8275 (1996) | [71,72] | |
| US EPA 7471 (2007) | [73] | |
| TMECC (2001) | [65] |
| Property | Effect of Biochar Application | Result | Reference |
|---|---|---|---|
| Bulk density | Reduction | Decreased by up to 28%. | [25,77,78] |
| Porosity | Increase | Increased by up to 24%. | [79] |
| Tensile strength | Reduction | Decreased by up to 242%. | [80] |
| Particle density | Reduction | Decreased by up to 39%. | [81] |
| Water repellency | Regulated according to need | Low-temperature pyrolyzed biochar was more hydrophobic than high-temperature biochar. | [79,82] |
| pH Change | Regulated according to need | Regulated pH in the soil and increased the bioavailability of nutrients. | [83] |
| CEC | Increase | Low-temperature pyrolyzed biochar exhibited more CEC than high-temperature pyrolyzed biochar. | [84,85] |
| Biochar Feedstock | Pyrolysis Temperature (°C) | CEC (cmol kg−1) | Reference |
|---|---|---|---|
| Douglas fir wood | 350 | 54.0 | [99] |
| 400 | 46.0 | ||
| 450 | 47.0 | ||
| 500 | 53.0 | ||
| 550 | 51.0 | ||
| 600 | 49.0 | ||
| Oak wood | 400 | 106.0 | [85] |
| 600 | 65.2 | ||
| Buckwheat husk | 450 | 11.5 | [100] |
| 550 | 10.1 | ||
| Peanut shells | 450 | 11.1 | [100] |
| 550 | 10.6 | ||
| Peat-based growing media | 450 | 54.0 | [84] |
| 600 | 11.0 | ||
| 750 | 8.0 | ||
| Woody green waste | 450 | 65.0 | [84] |
| 600 | 16.0 | ||
| Tree bark (Pinus pinaster) | 450 | 292.0 | [84] |
| 600 | 160.0 | ||
| Wheat straw | 500 | 5.1 | [101] |
| 600 | 1.3 | ||
| 700 | 0.5 | ||
| Corn straw | 500 | 68.6 | [101] |
| 600 | 20.1 | ||
| 700 | 19.0 | ||
| Peanut shell | 500 | 8.5 | [101] |
| 600 | 1.2 | ||
| 700 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokharel, U.; Neelgund, G.; Ray, R.L.; Balan, V.; Kumar, S. Biochar for Soil Amendment: Applications, Benefits, and Environmental Impacts. Bioengineering 2025, 12, 1137. https://doi.org/10.3390/bioengineering12111137
Pokharel U, Neelgund G, Ray RL, Balan V, Kumar S. Biochar for Soil Amendment: Applications, Benefits, and Environmental Impacts. Bioengineering. 2025; 12(11):1137. https://doi.org/10.3390/bioengineering12111137
Chicago/Turabian StylePokharel, Ujjwal, Gururaj Neelgund, Ram L. Ray, Venkatesh Balan, and Sandeep Kumar. 2025. "Biochar for Soil Amendment: Applications, Benefits, and Environmental Impacts" Bioengineering 12, no. 11: 1137. https://doi.org/10.3390/bioengineering12111137
APA StylePokharel, U., Neelgund, G., Ray, R. L., Balan, V., & Kumar, S. (2025). Biochar for Soil Amendment: Applications, Benefits, and Environmental Impacts. Bioengineering, 12(11), 1137. https://doi.org/10.3390/bioengineering12111137








