Metagenomic Insights into Pollutants in Biorefinery and Dairy Wastewater: rDNA Dominance and Electricity Generation in Double Chamber Microbial Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
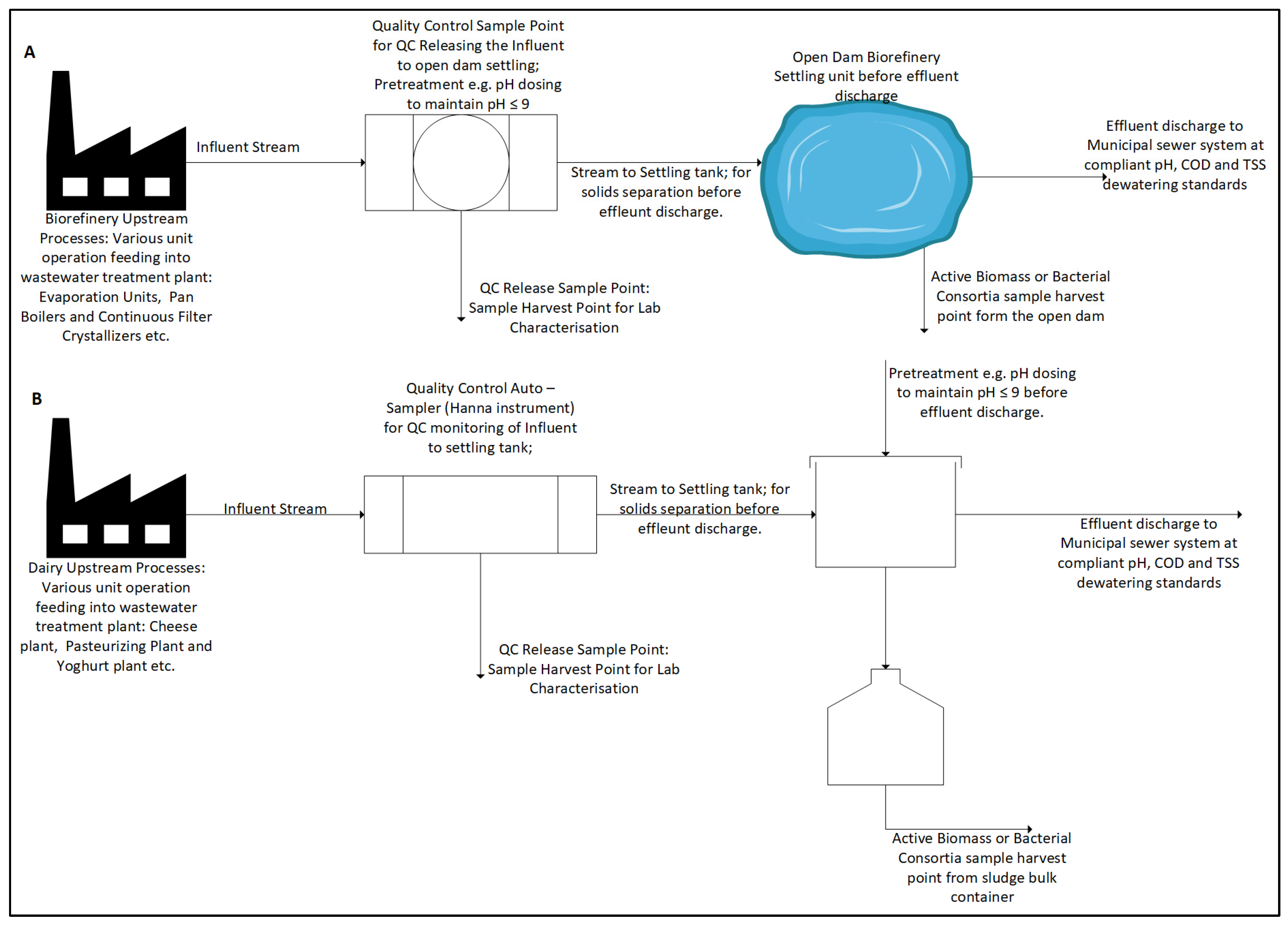
2.1. Wastewater Sample Characterisation Analytical Approach Before MFC Treatment
2.2. Biomass Harvesting, Culture Preparation, and Incubation Process Before MFC Inoculation
2.3. Biomass Analytical Characterisation Methods and Instruments
2.3.1. SEM/EDX and Light Preparation
2.3.2. Biomass rDNA Sequencing and Cloning Procedures
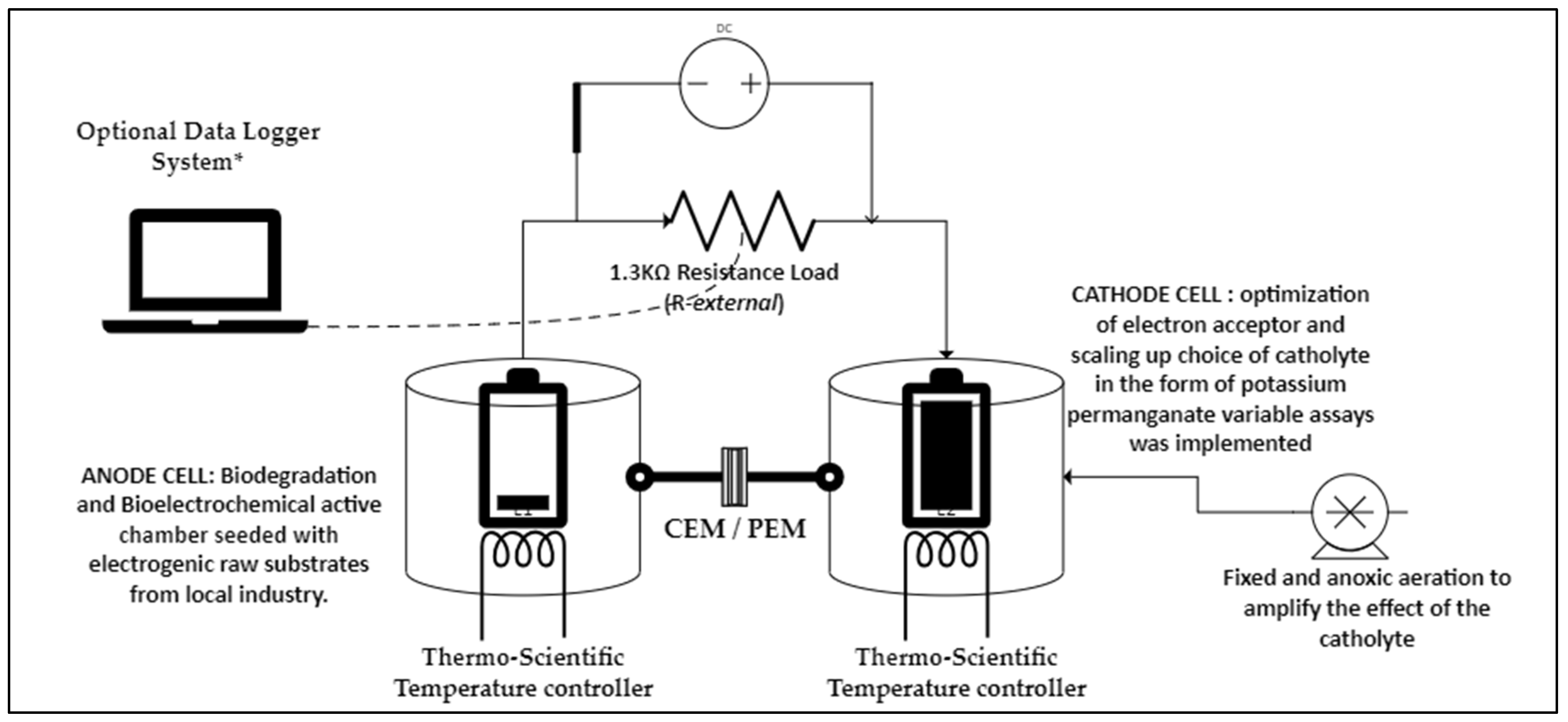
2.4. MFC Operation Design and Conditions
2.5. Advanced Statistical Analysis and R Statistical Software
3. Results and Discussions
3.1. Statistical Classification of the Three Wastewater Streams Used in This by Welch Student t-Test and ANOVA Mean Average Test: Biorefinery Wastewater—TH; Dairy Wastewater—(CL) and MIXED-STREAM—(MX)
3.2. Taxonomy and Characterisation of the 3-Complex Substrates Based on Organic Constituents as Viable Electron Donor Before MFC Treatment Stage
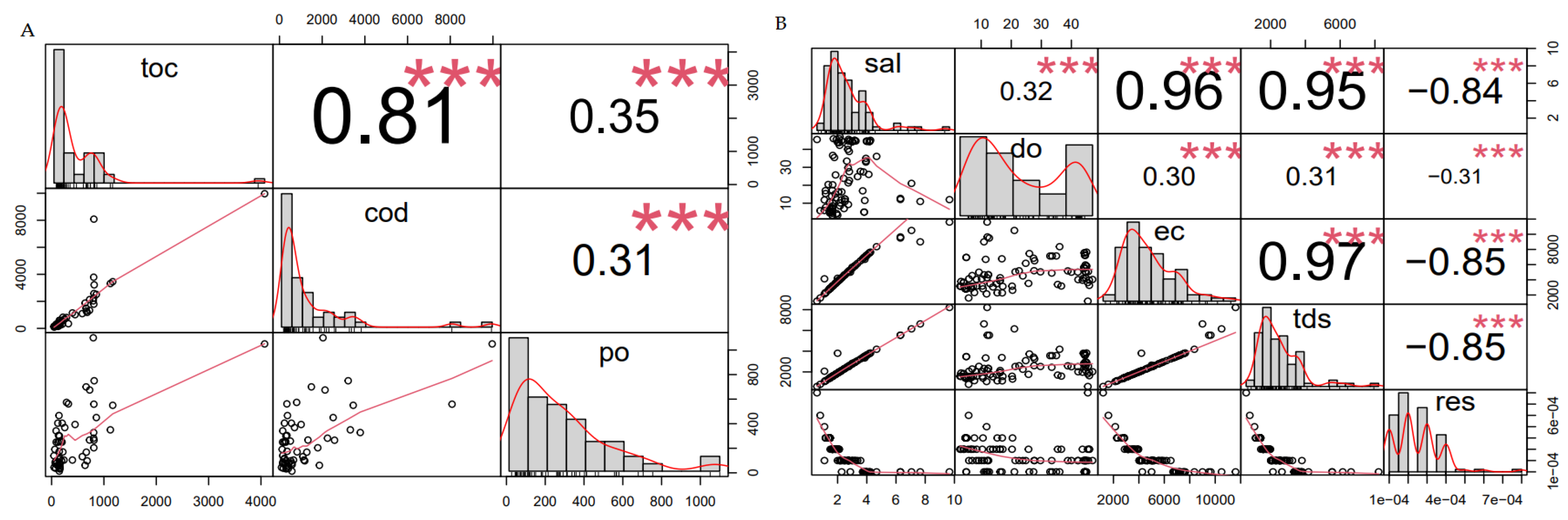
3.2.1. Total Organic Carbon (TOC) vs. Chemical Oxygen Demand (COD) Profile for All Wastewater Streams Harvested from the Wastewater Treatment Plant
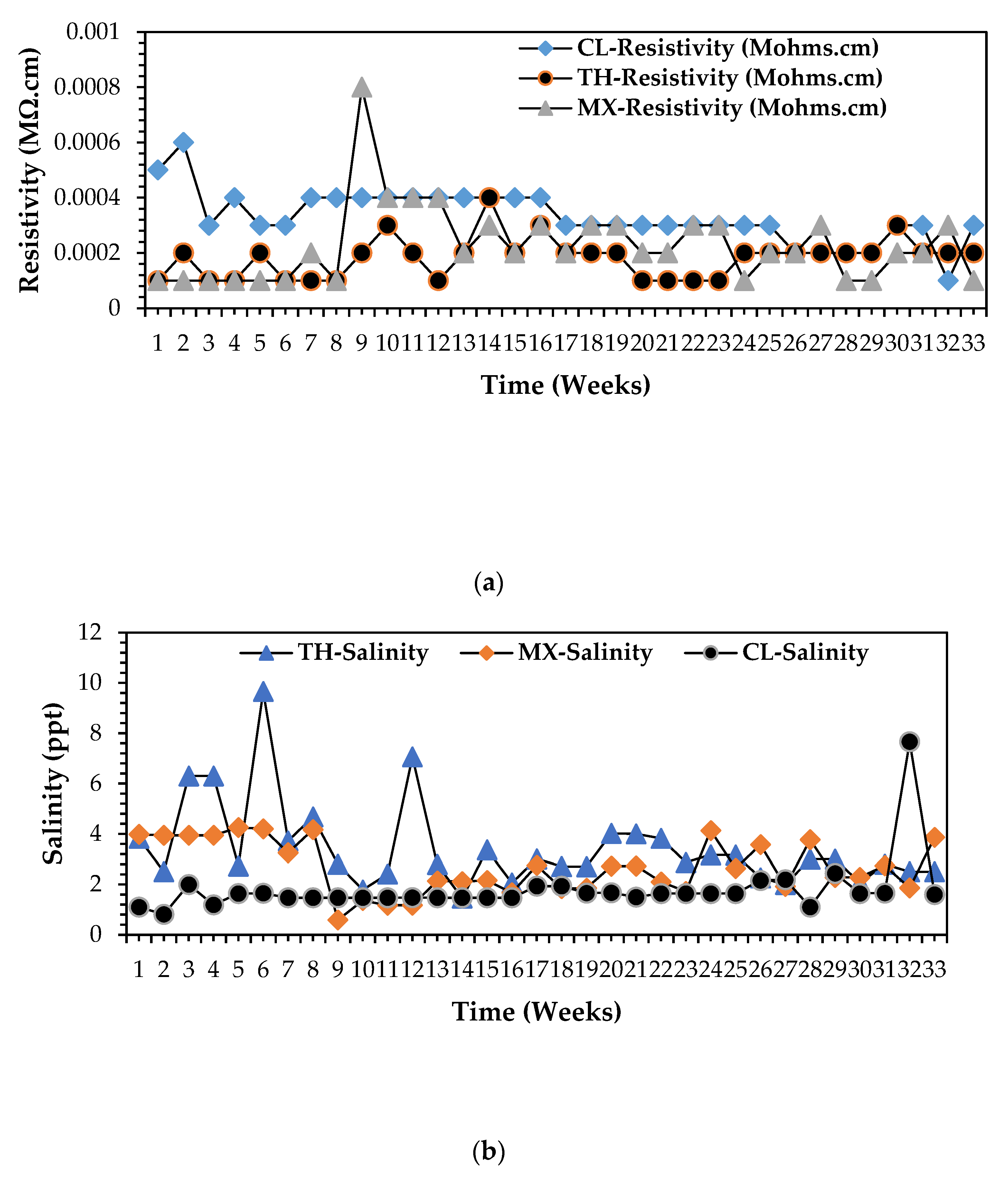
3.2.2. Salinity Taxonomical Classification and Profile for All Wastewater Streams, Harvested from the Wastewater Treatment Plant
3.2.3. ORP Taxonomical Profiles for All Streams Harvested from Wastewater Treatment Plant
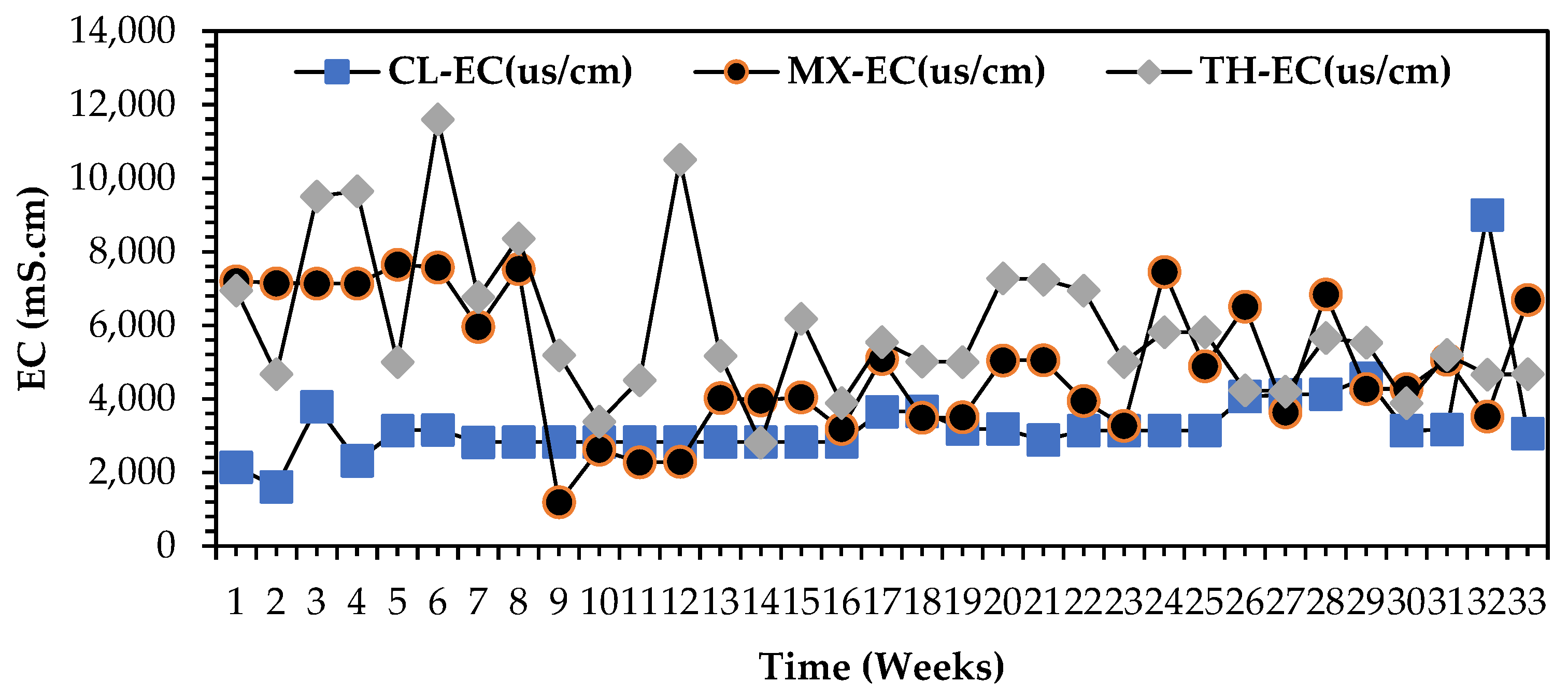
3.2.4. Taxonomic Classification of Electrical Conductivity (EC) Profiles for All Wastewater Streams
3.2.5. Comparison of the Present Study Wastewater Classification Profiles of Other Studies
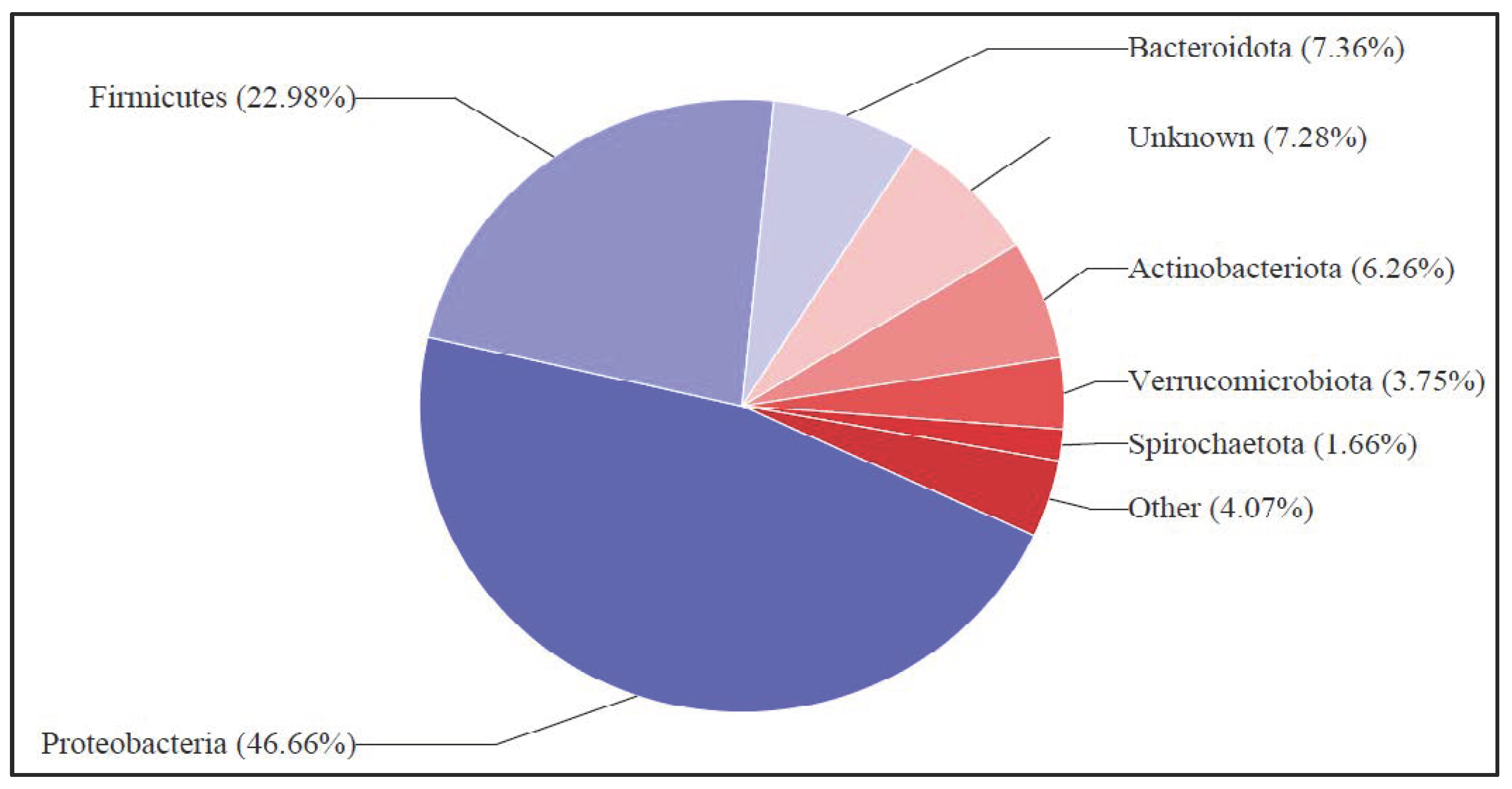
3.3. Taxonomy on Biomass rDNA Sequencing and Analysis of Phylum’s Class as Viable Bioelectrochemical Inoculate, After Wastewater Treament Palnt Harvest
3.3.1. Biorefinery (Tongaat Hullet) Biomass–Phylum Classification Blueprint for Sugar Biorefinery Biomass from Wastewater Treatment Plant
3.3.2. Biorefinery (Sugar Mill) Biomass Taxonomical Graphical Classifications
3.3.3. Dairy (Clover) Biomass—Phylum Classification and Taxonomical Blueprint, After Harvest from Wastewater Treatment Plant
3.3.4. Dairy Biomass Samples 1 and 2 FEG SEM-EDX Analysis via Zeiss Ultra
3.3.5. Comparison of the Three-Wastewater Substrates and Their Dominant Phylum for DCMFC Treatment and Production of Bioelectricity
3.3.6. Comparative Summary of Findings of Current Paper vs. Previous Studies on MFCs
4. Conclusions and Recommendations
4.1. Conclusions
4.2. Recommendations
- i.
- Biorefinery and mixed wastewater streams emerge as highly viable options for serving as reliable anolyte and inoculum sources in operating this benchtop DCMFC unit utilising purely raw industrial wastewater as an electrogenic bacterial community and efficient anolyte.
- ii.
- The heterotrophs classified in the morphology section, specifically Proteobacteria and Bacteroidetes, are considered as viable sources of electrons for operating this benchtop DCMFC unit in subsequent experiments of this study. Biorefinery wastewater stands out for its reliability, renewability, and sustainability in terms of being the primary DCMFC bioelectricity source.
- iii.
- Organic removal or biodegradable contaminants as shown in Figure 11a, b, demonstrated that in all three different wastewater sources, the overall percentage removal was achieved within a short span of time, specifically, within 72 h of treatment incubation in the DCMFC technology. Complete 100% removal was observed with mixed wastewater substrates within the same 72 h treatment period.
4.3. Future Perspectives
- i.
- Expansion of genetically engineered microorganisms for tailored applications.
- ii.
- Real-time signal processing improvements with MATLAB/SIMSCAPE-simulated empirical algorithms.
- iii.
- Focus on optimising the bioenergy capacity of the MFC unit via integration with power boosting electrical components towards meeting the national grid connection IEEE standards for the practicality and applicability of this technology in fighting the current energy and freshwater scarcity in South Africa.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gómez-Rubio, V. ggplot2—Elegant Graphics for Data Analysis (2nd Edition). J. Stat. Softw. 2017, 77, 1–3. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Dairy Products, 17th ed.; American Public Health Association: Washington, DC, USA, 2004; Available online: https://scirp.org/reference/referencespapers.aspx?referenceid=1456317 (accessed on 19 July 2022).
- Pichtel, J. Waste Management Practices: Municipal, Hazardous, and Industrial; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Hemati, A.; Nobaharan, K.; Amirifar, A.; Moghiseh, E.; Asgari Lajayer, B. Municipal Waste Management: Current Research and Future Challenges. In Sustainable Management and Utilization of Sewage Sludge; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Tompe, A.S.; Wagh, C.H. A review on different methods of dairy wastewater treatment. Int. J. Recent Res. Asp. 2017, 4, 443–445. [Google Scholar]
- Radwan, M. A Short Review on Effective Dairy Wastewater Treatment Techniques a Short Review on Effective Dairy Wastewater Treatment Techniques. Int. J. Eng. Res. Technol. (IJERT) 2020, 9. [Google Scholar]
- Sisay, E.J.; László, Z. Trend and Novel Possibilities of Dairy Wastewater Treatment by Membrane Filtration. J. Eng. Sci. Technol. Rev. 2021, 14, 46–55. [Google Scholar] [CrossRef]
- Demirel, B.; Yenigun, O.; Onay, T.T. Anaerobic treatment of dairy wastewaters: A review. Process Biochem. 2005, 40, 2583–2595. [Google Scholar] [CrossRef]
- Sarkar, B.; Chakrabarti, P.P.; Vijaykumar, A.; Kale, V. Wastewater treatment in dairy industries—Possibility of reuse. Desalination 2006, 195, 141–152. [Google Scholar] [CrossRef]
- Sinha, S.; Srivastava, A.; Mehrotra, T.; Singh, R. A Review on the Dairy Industry Wastewater Characteristics, Its Impact on Environment and Treatment Possibilities. In Emerging Issues in Ecology and Environmental Science: Case Studies from India; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Evangelista, C.; Basiricò, L.; Bernabucci, U. An Overview on the Use of Near Infrared Spectroscopy (NIRS) on Farms for the Management of Dairy Cows. Agriculture 2021, 11, 296. [Google Scholar] [CrossRef]
- Ally, B.; Syde, S.; Rasdi, I. Municipal solid waste management of Zanzibar: Current practice, the challenges, and the future. Int. J. Curr. Res. Acad. Rev. 2014, 1, 5–19. [Google Scholar]
- Kumar, S. Municipal Solid Waste Management in India: Present Practices and Future Challenge; Neeri: Nagpur, India, 2005. [Google Scholar]
- Patil, I. Visualizations with statistical details: The “ggstatsplot” approach. J. Open-Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- Whalen, P.A.; Whalen, P.J.; Cairns, J.E. ATP monitoring technology for microbial growth control in potable water systems. In Optics and Photonics in Global Homeland Security II; SPIE: Bellingham, WA, USA, 2006; Volume 6203, pp. 116–127. Available online: https://www.spiedigitallibrary.org/conference-proceedings-of-spie/6203/62030N/ATP-monitoring-technology-for-microbial-growth-control-in-potable-water/10.1117/12.673425.full (accessed on 12 February 2024).
- Metcalf, L.; Eddy, H.P.; Tchobanoglous, G. Wastewater Energy: Treatment and Reuse; McGraw-Hill: New York, NY, USA, 2004. [Google Scholar]
- Slavov, A.K. General characteristics and treatment possibilities of dairy wastewater—A review. Food Technol. Biotechnol. 2017, 55, 14–28. [Google Scholar] [CrossRef]
- Jungmeier, G. The Biorefinery Fact Sheet. Int. J. Life Cycle Assess. 2017, 23. [Google Scholar]
- Logan, B.E.; Rabaey, K. Conversion of Wastes into Bioelectricity and Chemicals by Using Microbial Electrochemical Technologies. Science 2012, 337, 686–690. Available online: https://www.science.org/doi/10.1126/science.1217412 (accessed on 6 October 2022). [CrossRef] [PubMed]
- Min, B.; Logan, B.E. Continuous Electricity Generation from Domestic Wastewater and Organic Substrates in a Flat Plate Microbial Fuel Cell. Environ. Sci. Technol. 2004, 38, 5809–5814. Available online: https://pubs.acs.org/doi/abs/10.1021/es0491026 (accessed on 21 January 2022). [CrossRef] [PubMed]
- Ahn, Y.; Logan, B.E. Altering anode thickness to improve power production in microbial fuel cells with different electrode distances. Energy Fuels 2013, 27, 271–276. [Google Scholar] [CrossRef]
- Gadhe, A.; Sonawane, S.S.; Varma, M.N. Kinetic analysis of biohydrogen production from complex dairy wastewater under optimized condition. Int. J. Hydrogen Energy 2014, 39, 1306–1314. [Google Scholar] [CrossRef]
- Min, B.; Kim, J.R.; Oh, S.E.; Regan, J.M.; Logan, B.E. Electricity generation from swine wastewater using microbial fuel cells. Water Res. 2005, 39, 4961–4968. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Wallack, M.J.; Kim, K.Y.; He, W.; Feng, Y.; Saikaly, P.E. Assessment of Microbial Fuel Cell Configurations and Power Densities. Environ. Sci. Technol. Lett. 2015, 2, 206–214. Available online: https://pubs.acs.org/sharingguidelines (accessed on 26 July 2023). [CrossRef]
- Mutombo, N.M.A.; Numbi, B.P. Development of a Linear Regression Model Based on the Most Influential Predictors for a Research Office Cooling Load. Energies 2022, 15, 5097. [Google Scholar] [CrossRef]
- Ahn, Y.; Logan, B.E. Effectiveness of domestic wastewater treatment using microbial fuel cells at ambient and mesophilic temperatures. Bioresour. Technol. 2010, 101, 469–475. [Google Scholar] [CrossRef]
- Logan, B.E.; Regan, J.M. Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol. 2006, 14, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Wang, X.; Logan, B.E. High performance flow through microbial fuel cells with anion exchange membrane. J. Power Sources 2020, 475, 228633. [Google Scholar] [CrossRef]
- Standard Methods for Examination of Water and Wastewater—ProQuest [Internet]. Available online: https://www.proquest.com/openview/85626245e0feccc3314bcce5a84957f3/1?pq-origsite=gscholar&cbl=54817 (accessed on 26 July 2023).
- Rice, E.W.; Baird, R.B.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Jadhav, G.S.; Ghangrekar, M.M. Performance of microbial fuel cell subjected to variation in pH, temperature, external load, and substrate concentration. Bioresour. Technol. 2009, 100, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Finley, S.D.; Broadbelt, L.J.; Hatzimanikatis, V. Thermodynamic Analysis of Biodegradation Pathways. Biotechnol. Bioeng. 2009, 103, 532–541. Available online: https://onlinelibrary.wiley.com/doi/10.1002/bit.22285 (accessed on 26 July 2023). [CrossRef] [PubMed]
- Khan, T.A.; Liaquat, R.; Zeshan Khoja, A.H.; Bano, A. Biological carbon capture, growth kinetics and biomass composition of novel microalgal species. Bioresour. Technol. Rep. 2022, 17, 100982. [Google Scholar] [CrossRef]
- Rabaey, K.; Rodríguez, J.; Blackall, L.L.; Keller, J.; Gross, P.; Batstone, D.; Verstraete, W.; Nealson, K.H. Microbial ecology meets electrochemistry: Electricity-driven and driving communities. ISME J. 2007, 1, 9–18. [Google Scholar] [CrossRef]
- Fito, J.; Tefera, N.; Van Hulle, S.W.H. Sugarcane biorefineries wastewater: Bioremediation technologies for environmental sustainability. Chem. Biol. Technol. Agric. 2019, 6, 6. [Google Scholar] [CrossRef]
- Morphology of Biomass from Sugar Wastewater Treatment. Available online: https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Morphology+of+Biomass+from+Sugar+wastewater+treatment+plant+by+SEM-EDX&btnG= (accessed on 19 July 2023).
- Hossain, N.; Zaini, J.; Mahlia, T.M.I.; Azad, A.K. Elemental, morphological, and thermal analysis of mixed microalgae species from drain water. Renew. Energy 2019, 131, 617–624. [Google Scholar] [CrossRef]
- Goel, P.K. Water Pollution: Causes, Effects and Control. Available online: https://books.google.co.za/books?hl=en&lr=&id=4R9CYYoiFCcC&oi=fnd&pg=PA2&dq=Goel+PK+2006&ots=fKkvz-YRgv&sig=UEvjQMCmHzBIwJKf8Vg2jzg2opU&redir_esc=y#v=onepage&q=Goel%20PK%202006&f=false (accessed on 26 July 2023).
- Wastewater Engineering: Treatment and Reuse—ProQuest [Internet]. Available online: https://www.proquest.com/openview/82d18bbd088cd47b8eee58569f8f6a36/1?pq-origsite=gscholar&cbl=25142 (accessed on 26 July 2023).
- Bryant, B.; Hall, B. Municipal and Industrial Waste: Sources, Management Practices and Future Challenges; Nova Science Publishers: Hauppauge, NY, USA, 2018. [Google Scholar]
- Wastewater Engineering: Treatment, Disposal, and Reuse|Titel [Internet]. Available online: https://library.wur.nl/WebQuery/titel/1979505 (accessed on 26 July 2023).
- Khumalo, S.M.; Bakare, B.F.; Tetteh, E.K.; Rathilal, S. Sequencing batch reactor performance evaluation on orthophosphates and COD removal from brewery wastewater. Fermentation 2022, 8, 296. [Google Scholar] [CrossRef]
- Abed, H.; Alisawi, O. Performance of wastewater treatment during variable temperature. Appl. Water Sci. 2020, 10, 89. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Srinivasan, S.; Appleby, A.J.; Martin, C.R. Temperature Dependence of the Electrode Kinetics of Oxygen Reduction at the Platinum/Nafion® Interface—A Microelectrode Investigation. J. Electrochem. Soc. 1992, 139, 2530–2537. Available online: https://iopscience.iop.org/article/10.1149/1.2221258 (accessed on 12 January 2025). [CrossRef]
- Wan, T.; Bai, Y.; Wang, T.; Wei, Z. BPNN-based optimal strategy for dynamic energy optimization with providing proper thermal comfort under the different outdoor air temperatures. Appl. Energy 2022, 313, 118899. [Google Scholar] [CrossRef]
- Gonzalez del Campo, A.; Lobato, J.; Cañizares, P.; Rodrigo, M.A.; Fernandez Morales, F.J. Short-term effects of temperature and COD in a microbial fuel cell. Appl. Energy 2013, 101, 213–217. [Google Scholar] [CrossRef]
- Newbury, D.E.; Ritchie, N.W.M. Performing elemental microanalysis with high accuracy and high precision by scanning electron microscopy/silicon drift detector energy-dispersive X-ray spectrometry (SEM/SDD-EDS). J. Mater. Sci. 2014, 50, 493–518. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhou, B.; Budai, A.; Jeng, A.; Hao, X.; Wei, D.; Zhang, Y.; Rasse, D. Study of Biochar Properties by Scanning Electron Microscope—Energy Dispersive X-Ray Spectroscopy (SEM-EDX). Commun. Soil Sci. Plant Anal. 2016, 47, 593–601. [Google Scholar] [CrossRef]
- Rana, M.S.; Prajapati, S.K. Multifarious applications of nanoparticles in microalgae for bioenergy generation: State-of-the-art review. J. Environ. Chem. Eng. 2023, 11, 109145. [Google Scholar] [CrossRef]
- Pancha, I.; Chokshi, K.; Ghosh, T.; Paliwal, C.; Maurya, R.; Mishra, S. Bicarbonate supplementation enhanced biofuel production potential as well as nutritional stress mitigation in the microalgae Scenedesmus sp. CCNM 1077. Bioresour. Technol. 2015, 193, 315–323. [Google Scholar] [CrossRef]
- Okolodkov, Y.B.; Huerta-Quintanilla, D.A. A low voltage scanning electron microscopy study of the theca of marine dinoflagellates. J. Adv. Microsc. Res. 2012, 7, 170–175. [Google Scholar] [CrossRef]
- Kurchania, A.K. Biomass Energy. Biomass Conversion: The Interface of Biotechnology, Chemistry and Materials Science; 2012; pp. 91–122, 9783642284182.
- Mahata, C.; Mishra, S.; Dhar, S.; Ray, S.; Mohanty, K.; Das, D. Utilization of dark fermentation effluent for algal cultivation in a modified airlift photobioreactor for biomass and biocrude production. J. Environ. Manag. 2023, 330, 117121. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Wang, L.Y.; Mbadinga, S.M.; Liu, J.F.; Yang, S.Z.; Gu, J.D.; Mu, B.Z. Anaerolineaceae and Methanosaeta turned to be the dominant microorganisms in alkanes-dependent methanogenic culture after long-term of incubation. AMB Express 2015, 5, 37. Available online: http://web.expasy.org/translate/ (accessed on 25 July 2023). [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Islam, N.; Parisa, T.A.; Rafa, N.; Bokhari, A.; Klemeš, J.J.; Mahlia, T.M.I. Insights into the development of microbial fuel cells for generating biohydrogen, bioelectricity, and treating wastewater. Energy 2022, 254, 124163. [Google Scholar] [CrossRef]
- Nwaokocha, C.N.; Giwa, S.O.; Layeni, A.T.; Kuye, S.I.; Samuel, O.D.; Ogunbona, C.K.; Adebayo, J.K.; Sosanya, A.; Babalola, A. Microbial fuel cell: Bio-energy production from Nigerian corn starch wastewater using iron electrodes. Mater. Today Proc. 2020, 46, 5565–5569. [Google Scholar] [CrossRef]
- Lu, N.; Zhou, S.G.; Zhuang, L.; Zhang, J.T.; Ni, J.R. Electricity generation from starch processing wastewater using microbial fuel cell technology. Biochem. Eng. J. 2009, 43, 246–251. [Google Scholar] [CrossRef]
- Angenent, L.T.; Karim, K.; Al-Dahhan, M.H.; Wrenn, B.A.; Domíguez-Espinosa, R. Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol. 2004, 22, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, X.; Logan, B.E.; Lee, H. Brewery Wastewater Treatment Using Air-Cathode Microbial Fuel Cells Microbial fuel cell for sustainable WW treatment View project Phosphorus recovery via vivianite from wastewater View project Brewery wastewater treatment using air-cathode microbial fuel cells. Appl. Microbiol. Biotechnol. 2008, 78, 873–880. Available online: https://www.researchgate.net/publication/5606547 (accessed on 6 October 2022). [PubMed]
- Di Lorenzo, M.; Scott, K.; Curtis, T.P.; Head, I.M. Effect of increasing anode surface area on the performance of a single chamber microbial fuel cell. Chem. Eng. J. 2010, 156, 40–48. [Google Scholar] [CrossRef]
- Ieropoulos, I.; Greenman, J.; Melhuish, C. Improved energy output levels from small-scale Microbial Fuel Cells. Bioelectrochemistry 2010, 78, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Franks, A.E.; Nevin, K.P. Microbial fuel cells, a current review. Energies 2010, 3, 899–919. [Google Scholar] [CrossRef]
- Katuri, K.P.; Scott, K.; Head, I.M.; Picioreanu, C.; Curtis, T.P. Microbial fuel cells meet with external resistance. Bioresour. Technol. 2011, 102, 2758–2766. [Google Scholar] [CrossRef] [PubMed]
- Jafary, T.; Asghar Ghoreyshi, A.; Darzi Najafpour, G.; Fatemi, S.; Rahimnejad, M.; Ghoreyshi, A.A. Investigation on Performance of Microbial Fuel Cells Based on Carbon Sources and Kinetic Models. 2012. Available online: https://onlinelibrary.wiley.com/doi/10.1002/er.2994 (accessed on 12 January 2025).
- Rahmani Eliato, T.; Pazuki, G.; Majidian, N. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects Potassium Permanganate as an Electron Receiver in a Microbial Fuel Cell Potassium Permanganate as an Electron Receiver in a Microbial Fuel Cell. 2016. Available online: https://www.tandfonline.com/action/journalInformation?journalCode=ueso20 (accessed on 12 January 2025).
- Mateo, S.; Rodrigo, M.; Fonseca, L.P.; Cañizares, P.; Fernandez-Morales, F.J. Oxygen availability effect on the performance of air-breathing cathode microbial fuel cell. Biotechnol. Prog. 2015, 31, 900–907. [Google Scholar] [CrossRef]
- Rimboud, M.; Desmond-Le Quemener, E.; Erable, B.; Bouchez, T.; Bergel, A. The current provided by oxygen-reducing microbial cathodes is related to the composition of their bacterial community. Bioelectrochemistry 2015, 102, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Mateo, S.; D’Angelo, A.; Scialdone, O.; Cañizares, P.; Rodrigo, M.A.; Fernandez-Morales, F.J. The influence of sludge retention time on mixed culture microbial fuel cell start-ups. Biochem. Eng. J. 2017, 123, 38–44. [Google Scholar] [CrossRef][Green Version]
- Shabangu, K.P.; Mthembu, N.; Chetty, M.; Bwapwa, J.K.; Bakare, B.F. A comparative analysis of organic substrates from industrial wastewater streams for enhanced electricity production using a double chamber microbial fuel cell (DCMFC). Energy Rep. 2024, 11, 3050–3063. [Google Scholar] [CrossRef]
- Shabangu, K.P.; Mthembu, N.; Chetty, M.; Bakare, B.F. Validation of RSM Predicted Optimum Scaling-Up Factors for Generating Electricity in a DCMFC: MATLAB Design and Simulation Model. Fermentation 2023, 9, 856. [Google Scholar] [CrossRef]
- Shabangu, K.P.; Chetty, M.; Bakare, B.F. Optimization and Modeling of a Dual-Chamber Microbial Fuel Cell (DCMFC) for Industrial Wastewater Treatment: A Box–Behnken Design Approach. Energies 2024, 17, 2740. [Google Scholar] [CrossRef]
- Shabangu, K.P.; Bakare, B.F.; Bwapwa, J.K. Microbial fuel cells for electrical energy: Outlook on scaling-up and application possibilities towards South African energy grid. Sustainability 2022, 14, 14268. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Ren, N.; Wang, H.; Lee, H.; Li, N.; Zhao, Q. Accelerated start-up of two-chambered microbial fuel cells: Effect of anodic positive poised potential. Electrochim. Acta 2009, 54, 1109–1114. [Google Scholar] [CrossRef]
- Ortiz-Martínez, V.M.; Salar-García, M.J.; de los Ríos, A.P.; Hernández-Fernández, F.J.; Egea, J.A.; Lozano, L.J. Developments in microbial fuel cell modeling. Chem. Eng. J. 2015, 271, 50–60. [Google Scholar] [CrossRef]
- Mateo, S.; Gonzalez del Campo, A.; Cañizares, P.; Lobato, J.; Rodrigo, M.A.; Fernandez, F.J. Bioelectricity generation in a self-sustainable Microbial Solar Cell. Bioresour. Technol. 2014, 159, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Xiao, J.; Tang, X. Microbial Fuel Cell-Based Organic Matter Sensors: Principles, Structures and Applications. Bioengineering 2023, 10, 886. [Google Scholar] [CrossRef]
- Lovecchio, N.; Di Meo, V.; Pietrelli, A. Customized Multichannel Measurement System for Microbial Fuel Cell Characterization. Bioengineering 2023, 10, 624. [Google Scholar] [CrossRef] [PubMed]




| Category | Doble Chamber Microbial Fuel Cell-Based on Industrial Organic Matter: Current Study | Customised Multichannel Measurement System: | Critical Findings in MFC-Based Organic Sensors: |
|---|---|---|---|
| Loveccho et al. [78] | Huang et al. [77] | ||
| Key Findings |
|
|
|
|
|
| |
|
|
| |
|
|
| |
| Optimisation |
|
|
|
|
|
| |
| Applications |
|
|
|
| Challenges |
|
|
|
| Contributions |
|
|
|
| Future Directions |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabangu, K.P.; Chetty, M.; Bakare, B.F. Metagenomic Insights into Pollutants in Biorefinery and Dairy Wastewater: rDNA Dominance and Electricity Generation in Double Chamber Microbial Fuel Cells. Bioengineering 2025, 12, 88. https://doi.org/10.3390/bioengineering12010088
Shabangu KP, Chetty M, Bakare BF. Metagenomic Insights into Pollutants in Biorefinery and Dairy Wastewater: rDNA Dominance and Electricity Generation in Double Chamber Microbial Fuel Cells. Bioengineering. 2025; 12(1):88. https://doi.org/10.3390/bioengineering12010088
Chicago/Turabian StyleShabangu, Khaya Pearlman, Manimagalay Chetty, and Babatunde Femi Bakare. 2025. "Metagenomic Insights into Pollutants in Biorefinery and Dairy Wastewater: rDNA Dominance and Electricity Generation in Double Chamber Microbial Fuel Cells" Bioengineering 12, no. 1: 88. https://doi.org/10.3390/bioengineering12010088
APA StyleShabangu, K. P., Chetty, M., & Bakare, B. F. (2025). Metagenomic Insights into Pollutants in Biorefinery and Dairy Wastewater: rDNA Dominance and Electricity Generation in Double Chamber Microbial Fuel Cells. Bioengineering, 12(1), 88. https://doi.org/10.3390/bioengineering12010088