Constraint of Different Knee Implant Designs Under Anterior–Posterior Shear Forces and Internal–External Rotation Moments in Human Cadaveric Knees
Abstract
1. Introduction
2. Materials and Methods
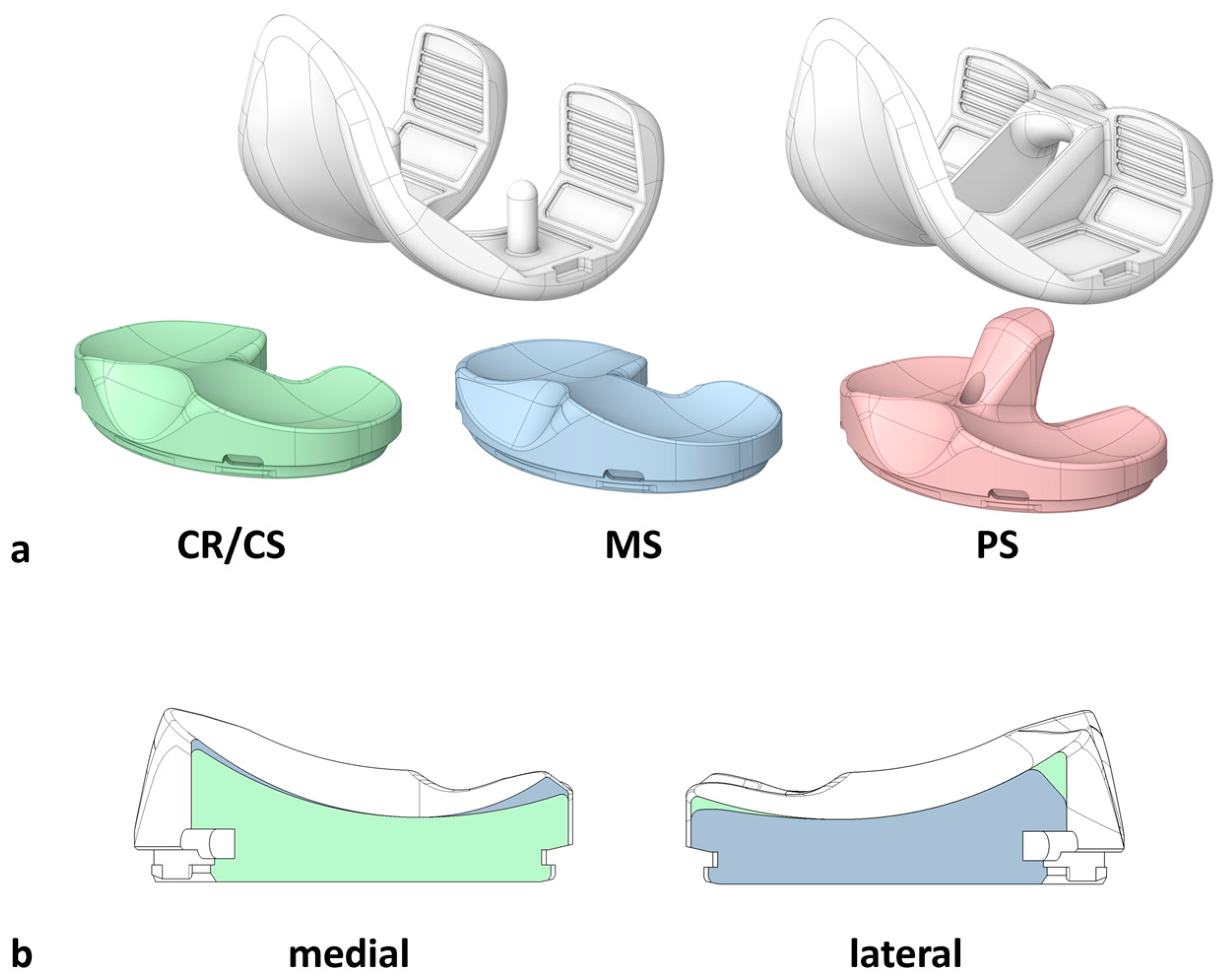
2.1. Specimen Preparation
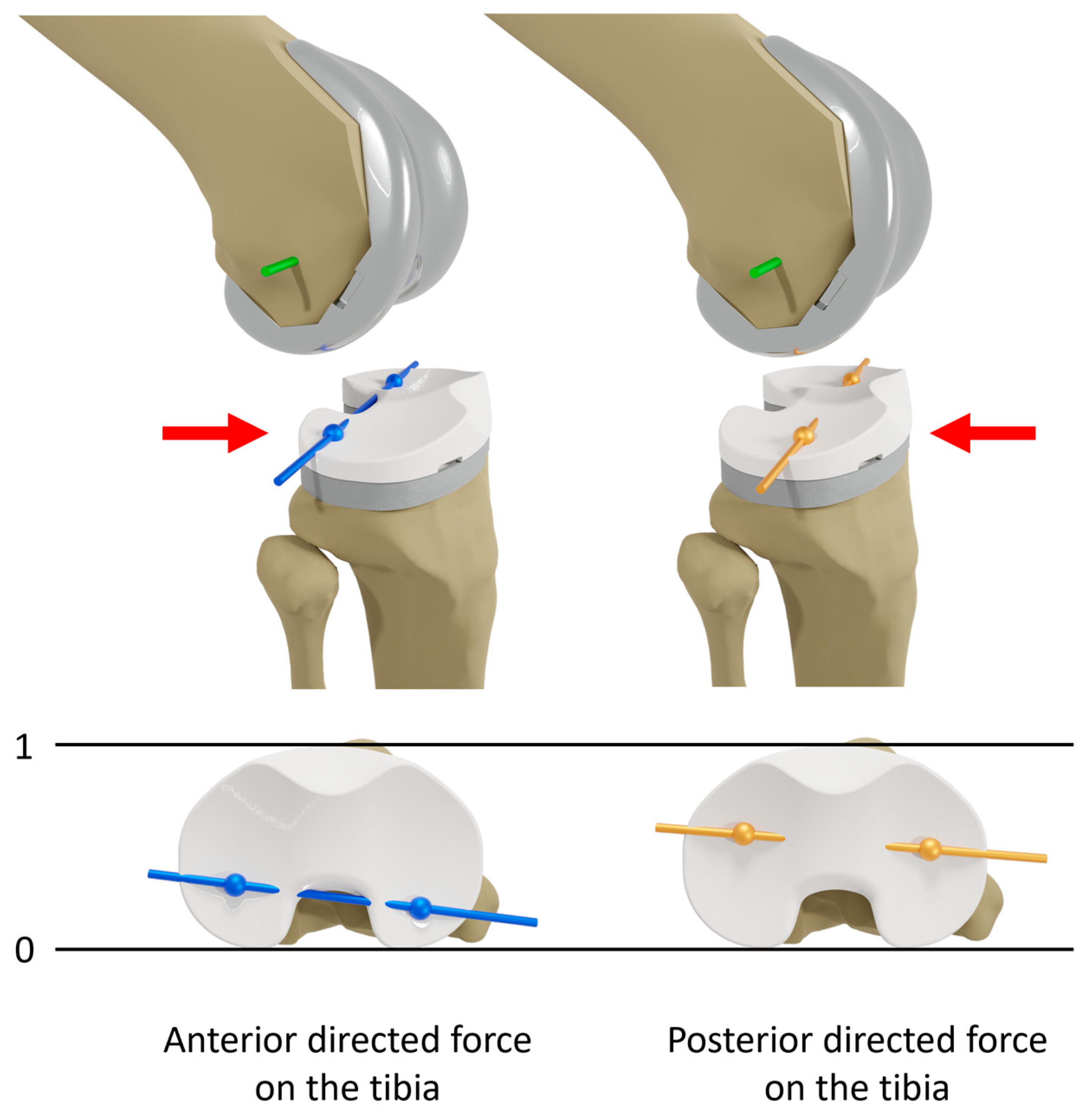
2.2. Experimental Testing
2.3. Data Analysis
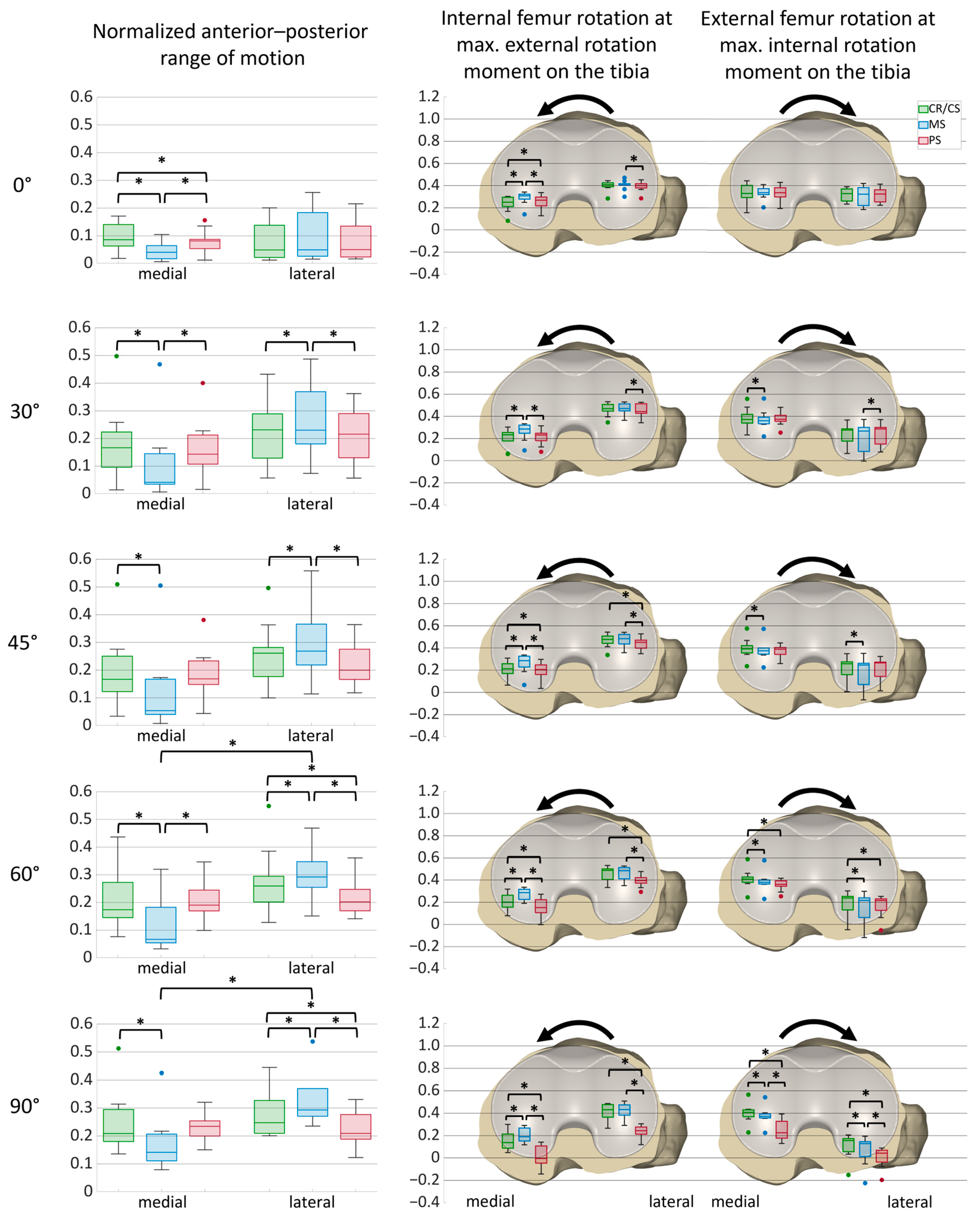
3. Results
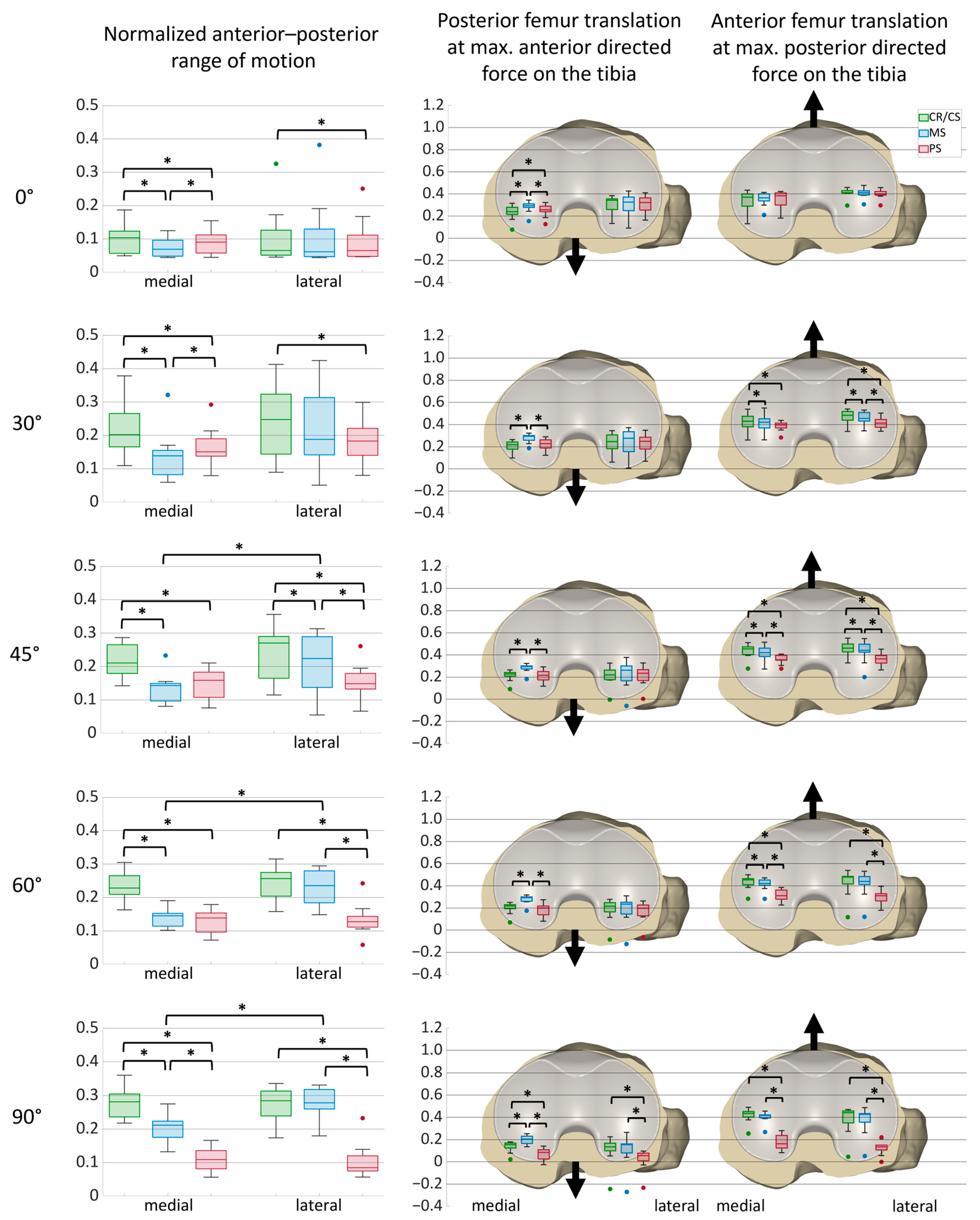
3.1. Anterior–Posterior Shear Forces
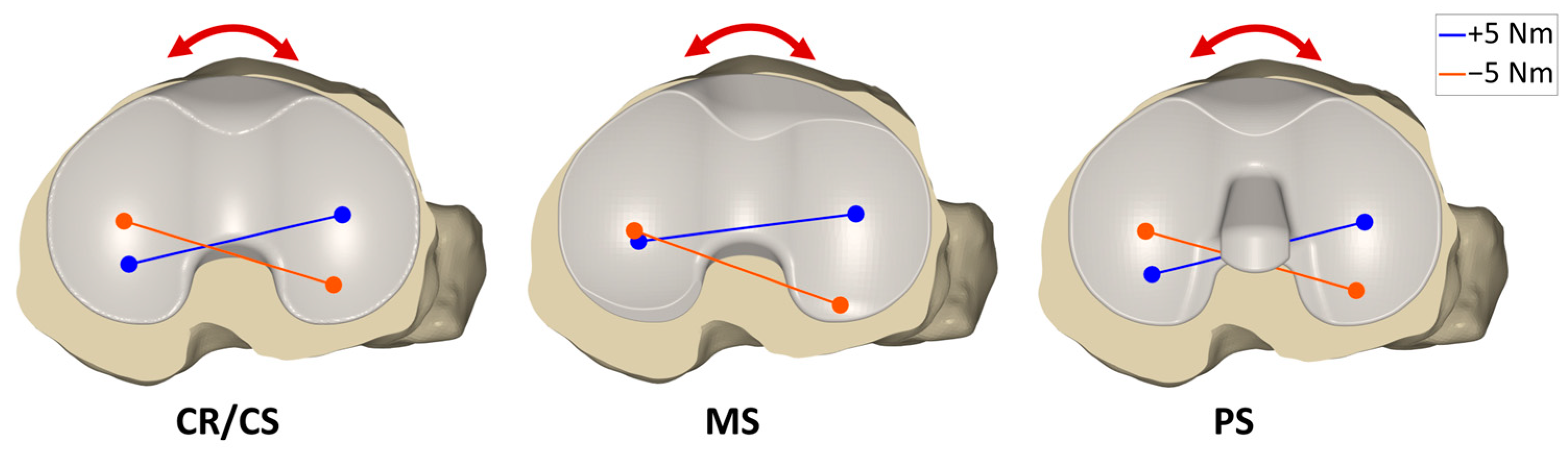
3.2. Internal–External Rotation Moments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Flexion Angle | Condyle | Median | p-Value | p-Value Medial vs. Lateral | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CR/CS | MS | PS | CR/CS vs. MS | CR/CS vs. PS | MS vs. PS | CR/CS | MS | PS | ||
| 0° flexion | Medial | 0.10 | 0.07 | 0.09 | 0.005 * | 0.037 * | 0.005 * | 0.756 | 0.824 | 1.000 |
| Lateral | 0.07 | 0.06 | 0.07 | 0.563 | 0.037 * | 0.824 | ||||
| 30° flexion | Medial | 0.20 | 0.14 | 0.15 | 0.004 * | 0.004 * | 0.045 * | 0.505 | 0.056 | 0.505 |
| Lateral | 0.25 | 0.19 | 0.18 | 0.056 | 0.009 * | 0.197 | ||||
| 45° flexion | Medial | 0.21 | 0.14 | 0.16 | 0.004 * | 0.004 * | 0.197 | 0.398 | 0.029 * | 0.625 |
| Lateral | 0.27 | 0.22 | 0.15 | 0.037 * | 0.007 * | 0.045 * | ||||
| 60° flexion | Medial | 0.23 | 0.15 | 0.14 | 0.004 * | 0.004 * | 0.505 | 0.351 | 0.005 * | 0.563 |
| Lateral | 0.26 | 0.24 | 0.13 | 0.266 | 0.005 * | 0.004 * | ||||
| 90° flexion | Medial | 0.28 | 0.21 | 0.11 | 0.004 * | 0.004 * | 0.004 * | 0.965 | 0.004 * | 0.266 |
| Lateral | 0.28 | 0.28 | 0.08 | 0.756 | 0.004 * | 0.004 * | ||||
| Flexion Angle | Position | Condyle | Median | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| CR/CS | MS | PS | CR/CS vs. MS | CR/CS vs. PS | MS vs. PS | |||
| 0° flexion | Anterior force | Medial | 0.24 | 0.29 | 0.26 | 0.005 * | 0.023 * | 0.009 * |
| Lateral | 0.34 | 0.33 | 0.32 | 0.965 | 0.505 | 0.824 | ||
| Posterior force | Medial | 0.37 | 0.36 | 0.38 | 0.120 | 0.197 | 0.100 | |
| Lateral | 0.41 | 0.41 | 0.40 | 0.756 | 0.398 | 0.100 | ||
| 30° flexion | Anterior force | Medial | 0.22 | 0.30 | 0.23 | 0.004 * | 0.168 | 0.007 * |
| Lateral | 0.25 | 0.28 | 0.25 | 0.563 | 0.756 | 0.563 | ||
| Posterior force | Medial | 0.43 | 0.42 | 0.40 | 0.045 * | 0.018 * | 0.056 | |
| Lateral | 0.48 | 0.46 | 0.41 | 0.037 * | 0.005 * | 0.007 * | ||
| 45° flexion | Anterior force | Medial | 0.23 | 0.29 | 0.22 | 0.004 * | 0.689 | 0.005 * |
| Lateral | 0.22 | 0.26 | 0.23 | 0.625 | 0.625 | 0.266 | ||
| Posterior force | Medial | 0.46 | 0.42 | 0.36 | 0.014 * | 0.007 * | 0.011 * | |
| Lateral | 0.46 | 0.44 | 0.36 | 0.011 * | 0.004 * | 0.011 * | ||
| 60° flexion | Anterior force | Medial | 0.22 | 0.29 | 0.20 | 0.004 * | 0.142 | 0.004 * |
| Lateral | 0.22 | 0.23 | 0.20 | 0.625 | 0.068 | 0.056 | ||
| Posterior force | Medial | 0.45 | 0.42 | 0.31 | 0.029 * | 0.004 * | 0.005 * | |
| Lateral | 0.48 | 0.44 | 0.31 | 0.142 | 0.005 * | 0.005 * | ||
| 90° flexion | Anterior force | Medial | 0.15 | 0.20 | 0.08 | 0.005 * | 0.004 * | 0.004 * |
| Lateral | 0.14 | 0.15 | 0.06 | 0.894 | 0.005 * | 0.007 * | ||
| Posterior force | Medial | 0.43 | 0.42 | 0.16 | 0.083 | 0.004 * | 0.004 * | |
| Lateral | 0.44 | 0.43 | 0.14 | 0.351 | 0.004 * | 0.004 * | ||
| Flexion Angle | Condyle | Median | p-Value | p-Value Medial vs. Lateral | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CR/CS | MS | PS | CR/CS vs. MS | CR/CS vs. PS | MS vs. PS | CR/CS | MS | PS | ||
| 0° flexion | Medial | 0.09 | 0.04 | 0.08 | 0.006 * | 0.041 * | 0.006 * | 0.476 | 0.308 | 1.000 |
| Lateral | 0.05 | 0.05 | 0.05 | 0.067 | 1.00 | 0.154 | ||||
| 30° flexion | Medial | 0.17 | 0.04 | 0.14 | 0.006 * | 0.541 | 0.019 * | 0.308 | 0.083 | 0.308 |
| Lateral | 0.23 | 0.23 | 0.22 | 0.032 * | 0.308 | 0.011 * | ||||
| 45° flexion | Medial | 0.17 | 0.05 | 0.17 | 0.006 * | 0.919 | 0.053 | 0.185 | 0.067 | 0.262 |
| Lateral | 0.26 | 0.27 | 0.20 | 0.014 * | 0.053 | 0.011 * | ||||
| 60° flexion | Medial | 0.17 | 0.07 | 0.19 | 0.006 * | 0.919 | 0.006 * | 0.154 | 0.014 * | 0.308 |
| Lateral | 0.26 | 0.29 | 0.20 | 0.006 * | 0.041 * | 0.008 * | ||||
| 90° flexion | Medial | 0.21 | 0.14 | 0.23 | 0.006 * | 0.541 | 0.067 | 0.126 | 0.025 * | 0.541 |
| Lateral | 0.25 | 0.29 | 0.21 | 0.006 * | 0.041 * | 0.008 * | ||||
| Flexion Angle | Position | Condyle | Median | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| CR/CS | MS | PS | CR/CS vs. MS | CR/CS vs. PS | MS vs. PS | |||
| 0° flexion | External moment | Medial | 0.25 | 0.31 | 0.27 | 0.006 * | 0.032 * | 0.006 * |
| Lateral | 0.41 | 0.41 | 0.40 | 0.760 | 0.221 | 0.032 * | ||
| Internal moment | Medial | 0.33 | 0.34 | 0.34 | 0.683 | 0.541 | 0.683 | |
| Lateral | 0.33 | 0.32 | 0.32 | 0.541 | 0.185 | 0.760 | ||
| 30° flexion | External moment | Medial | 0.23 | 0.29 | 0.23 | 0.008 * | 0.221 | 0.006 * |
| Lateral | 0.47 | 0.47 | 0.44 | 0.610 | 0.083 | 0.025 * | ||
| Internal moment | Medial | 0.37 | 0.36 | 0.37 | 0.041 * | 0.919 | 0.083 | |
| Lateral | 0.28 | 0.27 | 0.29 | 0.053 | 1.000 | 0.041 * | ||
| 45° flexion | External moment | Medial | 0.21 | 0.29 | 0.21 | 0.011 * | 0.041 * | 0.006 * |
| Lateral | 0.48 | 0.48 | 0.45 | 0.683 | 0.011 * | 0.006 * | ||
| Internal moment | Medial | 0.39 | 0.38 | 0.39 | 0.006 * | 0.262 | 0.359 | |
| Lateral | 0.26 | 0.25 | 0.27 | 0.011 * | 0.760 | 0.083 | ||
| 60° flexion | External moment | Medial | 0.20 | 0.29 | 0.15 | 0.008 * | 0.006 * | 0.006 * |
| Lateral | 0.49 | 0.49 | 0.40 | 0.185 | 0.006 * | 0.006 * | ||
| Internal moment | Medial | 0.41 | 0.38 | 0.36 | 0.006 * | 0.019 * | 0.610 | |
| Lateral | 0.24 | 0.22 | 0.22 | 0.006 * | 0.019 * | 0.308 | ||
| 90° flexion | External moment | Medial | 0.14 | 0.19 | 0.00 | 0.008 * | 0.006 * | 0.006 * |
| Lateral | 0.43 | 0.43 | 0.24 | 0.067 | 0.006 * | 0.006 * | ||
| Internal moment | Medial | 0.41 | 0.37 | 0.22 | 0.014 * | 0.006 * | 0.008 * | |
| Lateral | 0.16 | 0.13 | 0.04 | 0.006 * | 0.006 * | 0.011 * | ||
References
- Movassaghi, K.; Patel, A.; Ghulam-Jelani, Z.; Levine, B.R. Modern Total Knee Arthroplasty Bearing Designs and the Role of the Posterior Cruciate Ligament. Arthroplast. Today 2023, 21, 101130. [Google Scholar] [CrossRef] [PubMed]
- Grimberg, A.; Lützner, J.; Melsheimer, O.; Morlock, M.; Steinbrück, A. Endoprothesenregister Deutschland (EPRD) Jahresbericht 2023; EPRD Deutsche Endoprothesenregister gGmbH: Berlin, Germany, 2023. [Google Scholar] [CrossRef]
- American Joint Replacement Registry (AJRR): 2024 Annual Report; American Academy of Orthopaedic Surgeons: Rosemont, IL, USA, 2024.
- Sabatini, L.; Risitano, S.; Parisi, G.; Tosto, F.; Indelli, P.F.; Atzori, F.; Massè, A. Medial Pivot in Total Knee Arthroplasty: Literature Review and Our First Experience. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2018, 11, 1179544117751431. [Google Scholar] [CrossRef] [PubMed]
- Gray, H.A.; Guan, S.; Thomeer, L.T.; Schache, A.G.; de Steiger, R.; Pandy, M.G. Three-dimensional motion of the knee-joint complex during normal walking revealed by mobile biplane x-ray imaging. J. Orthop. Res. 2019, 37, 615–630. [Google Scholar] [CrossRef]
- Postolka, B.; Schütz, P.; Fucentese, S.F.; Freeman, M.A.R.; Pinskerova, V.; List, R.; Taylor, W.R. Tibio-femoral kinematics of the healthy knee joint throughout complete cycles of gait activities. J. Biomech. 2020, 110, 109915. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.A.R.; Pinskerova, V. The movement of the knee studied by magnetic resonance imaging. Clin. Orthop. Relat. Res. 2003, 410, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Steinbrück, A.; Schröder, C.; Woiczinski, M.; Fottner, A.; Pinskerova, V.; Müller, P.E.; Jansson, V. Femorotibial kinematics and load patterns after total knee arthroplasty: An in vitro comparison of posterior-stabilized versus medial-stabilized design. Clin. Biomech. (Bristol, Avon) 2016, 33, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Brendle, S.A.; Krueger, S.; Fehrenbacher, J.; Grifka, J.; Müller, P.E.; Mihalko, W.M.; Richter, B.; Grupp, T.M. Kinematic Patterns of Different Loading Profiles Before and After Total Knee Arthroplasty: A Cadaveric Study. Bioengineering 2024, 11, 1064. [Google Scholar] [CrossRef]
- Hinarejos, P.; Leal-Blanquet, J.; Fraile-Suari, A.; Sánchez-Soler, J.; Torres-Claramunt, R.; Monllau, J.C. Increased posterior translation but similar clinical outcomes using ultracongruent instead of posterior stabilized total knee arthroplasties in a prospective randomized trial. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 3041–3048. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Lee, S.M.; Seong, S.C.; Lee, S.; Jang, J.; Lee, M.C. Different intraoperative kinematics with comparable clinical outcomes of ultracongruent and posterior stabilized mobile-bearing total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 3036–3043. [Google Scholar] [CrossRef]
- Massin, P.; Boyer, P.; Sabourin, M. Less femorotibial rotation and AP translation in deep-dished total knee arthroplasty. An intraoperative kinematic study using navigation. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1714–1719. [Google Scholar] [CrossRef]
- Postolka, B.; Taylor, W.R.; List, R.; Fucentese, S.F.; Koch, P.P.; Schütz, P. ISB clinical biomechanics award winner 2021: Tibio-femoral kinematics of natural versus replaced knees—A comparison using dynamic videofluoroscopy. Clin. Biomech. (Bristol, Avon) 2022, 96, 105667. [Google Scholar] [CrossRef]
- Koster, L.A.; Kaptein, B.L.; van der Linden-van der Zwaag, E.H.M.J.; Nelissen, R.G.H.H. Knee kinematics are not different between asymmetrical and symmetrical tibial baseplates in total knee arthroplasty: A fluoroscopic analysis of step-up and lunge motions. Knee Surg. Sports Traumatol. Arthrosc. 2024, 32, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Dupraz, I.; Thorwächter, C.; Grupp, T.M.; Hammerschmid, F.; Woiczinski, M.; Jansson, V.; Müller, P.E.; Steinbrück, A. Impact of femoro-tibial size combinations and TKA design on kinematics. Arch. Orthop. Trauma Surg. 2022, 142, 1197–1212. [Google Scholar] [CrossRef] [PubMed]
- Gray, H.A.; Guan, S.; Young, T.J.; Dowsey, M.M.; Choong, P.F.; Pandy, M.G. Comparison of posterior-stabilized, cruciate-retaining, and medial-stabilized knee implant motion during gait. J. Orthop. Res. 2020, 38, 1753–1768. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.N.; Gill, D.R.; McAuliffe, M.J.; McDougall, C.; Stoney, J.D.; Vertullo, C.J.; Wall, C.J.; Corfield, S.; Page, R.; Cuthbert, A.R.; et al. Hip, Knee and Shoulder Arthroplasty: 2023 Annual Report; Australian Orthopaedic Association National Joint Replacement Registry, AOA: Sydney, Australia, 2023. [Google Scholar] [CrossRef]
- Krackow, K.A. Instability in total knee arthroplasty: Loose as a goose. J. Arthroplast. 2003, 18, 45–47. [Google Scholar] [CrossRef]
- Sharkey, P.F.; Lichstein, P.M.; Shen, C.; Tokarski, A.T.; Parvizi, J. Why are total knee arthroplasties failing today--has anything changed after 10 years? J. Arthroplast. 2014, 29, 1774–1778. [Google Scholar] [CrossRef]
- Wilson, C.J.; Theodoulou, A.; Damarell, R.A.; Krishnan, J. Knee instability as the primary cause of failure following Total Knee Arthroplasty (TKA): A systematic review on the patient, surgical and implant characteristics of revised TKA patients. Knee 2017, 24, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.W.; Kim, M.S.; Koh, I.J.; Sohn, S.; Kim, C.; In, Y. Comparison of Anterior-Stabilized and Posterior-Stabilized Total Knee Arthroplasty in the Same Patients: A Prospective Randomized Study. J. Arthroplast. 2019, 34, 1682–1689. [Google Scholar] [CrossRef]
- Wautier, D.; Thienpont, E. Changes in anteroposterior stability and proprioception after different types of knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 1792–1800. [Google Scholar] [CrossRef]
- Scott, D.F.; Hellie, A.A. Mid-Flexion, Anteroposterior Stability of Total Knee Replacement Implanted with Kinematic Alignment: A Randomized, Quantitative Radiographic Laxity Study with Posterior-Stabilized and Medial-Stabilized Implants. J. Bone Jt. Surg. Am. 2023, 105, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.W.; Jacobs, H.; Shumborski, S.; Talbot, S.; Redgment, A.; Brighton, R.; Walter, W.L. Sagittal Stability and Implant Design Affect Patient Reported Outcomes After Total Knee Arthroplasty. J. Arthroplast. 2020, 35, 747–751. [Google Scholar] [CrossRef] [PubMed]
- De Groot, J.D.; Brokelman, R.B.G.; Lammers, P.G.; van Stralen, G.M.J.; Kooijman, C.M.; Hokwerda, S.T. Performance of medial pivot, posterior stabilized and rotating platform total knee arthroplasty based on anteroposterior stability and patient-reported outcome measures; a multicentre double-blinded randomized controlled trial of 210 knees. Arch. Orthop. Trauma Surg. 2024, 144, 2327–2335. [Google Scholar] [CrossRef] [PubMed]
- Edelstein, A.I.; Bhatt, S.; Wright-Chisem, J.; Sullivan, R.; Beal, M.; Manning, D.W. The Effect of Implant Design on Sagittal Plane Stability: A Randomized Trial of Medial- versus Posterior-Stabilized Total Knee Arthroplasty. J. Knee Surg. 2020, 33, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Lützner, J.; Firmbach, F.-P.; Lützner, C.; Dexel, J.; Kirschner, S. Similar stability and range of motion between cruciate-retaining and cruciate-substituting ultracongruent insert total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 1638–1643. [Google Scholar] [CrossRef]
- Kour, R.Y.N.; Guan, S.; Dowsey, M.M.; Choong, P.F.; Pandy, M.G. Kinematic function of knee implant designs across a range of daily activities. J. Orthop. Res. 2023, 41, 1217–1227. [Google Scholar] [CrossRef]
- Brendle, S.A.; Krueger, S.; Grifka, J.; Müller, P.E.; Grupp, T.M. A New Methodology for the Accurate Measurement of Tibiofemoral Kinematics in Human Cadaveric Knees: An Evaluation of the Anterior–Posterior Laxity Pre- and Post-Cruciate Ligament Resection. Life 2024, 14, 877. [Google Scholar] [CrossRef] [PubMed]
- Willing, R.; Moslemian, A.; Yamomo, G.; Wood, T.; Howard, J.; Lanting, B. Condylar-Stabilized TKR May Not Fully Compensate for PCL-Deficiency: An In Vitro Cadaver Study. J. Orthop. Res. 2019, 37, 2172–2181. [Google Scholar] [CrossRef] [PubMed]
- Borque, K.A.; Gold, J.E.; Incavo, S.J.; Patel, R.M.; Ismaily, S.E.; Noble, P.C. Anteroposterior Knee Stability During Stair Descent. J. Arthroplast. 2015, 30, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.S.; Borukhov, I.; LiArno, S. Obtaining anatomic motion and laxity characteristics in a total knee design. Knee 2022, 35, 133–141. [Google Scholar] [CrossRef]
- Grood, E.S.; Suntay, W.J. A joint coordinate system for the clinical description of three-dimensional motions: Application to the knee. J. Biomech. Eng. 1983, 105, 136–144. [Google Scholar] [CrossRef]
- Daniel, D.M.; Malcom, L.L.; Losse, G.; Stone, M.L.; Sachs, R.; Burks, R. Instrumented measurement of anterior laxity of the knee. J. Bone Jt. Surg. Am. 1985, 67, 720–726. [Google Scholar] [CrossRef]
- Bull, A.M.J.; Kessler, O.; Alam, M.; Amis, A.A. Changes in knee kinematics reflect the articular geometry after arthroplasty. Clin. Orthop. Relat. Res. 2008, 466, 2491–2499. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reynolds, R.J.; Walker, P.S.; Buza, J. Mechanisms of anterior-posterior stability of the knee joint under load-bearing. J. Biomech. 2017, 57, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.S.; Arno, S.; Borukhoy, I.; Bell, C.P. Characterising knee motion and laxity in a testing machine for application to total knee evaluation. J. Biomech. 2015, 48, 3551–3558. [Google Scholar] [CrossRef] [PubMed]
- King, A.P.; Eckersley, R.J. Inferential Statistics III: Nonparametric Hypothesis Testing. In Statistics for Biomedical Engineers and Scientists; Elsevier: Amsterdam, The Netherlands, 2019; pp. 119–145. ISBN 9780081029398. [Google Scholar]
- Willing, R.; Walker, P.S. Measuring the sensitivity of total knee replacement kinematics and laxity to soft tissue imbalances. J. Biomech. 2018, 77, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Shalhoub, S.; Moschetti, W.E.; Dabuzhsky, L.; Jevsevar, D.S.; Keggi, J.M.; Plaskos, C. Laxity Profiles in the Native and Replaced Knee-Application to Robotic-Assisted Gap-Balancing Total Knee Arthroplasty. J. Arthroplast. 2018, 33, 3043–3048. [Google Scholar] [CrossRef] [PubMed]
- Minoda, Y.; Nakagawa, S.; Sugama, R.; Ikawa, T.; Noguchi, T.; Hirakawa, M. Midflexion Laxity After Implantation Was Influenced by the Joint Gap Balance Before Implantation in TKA. J. Arthroplast. 2015, 30, 762–765. [Google Scholar] [CrossRef] [PubMed]
- Arnout, N.; Vanlommel, L.; Vanlommel, J.; Luyckx, J.P.; Labey, L.; Innocenti, B.; Victor, J.; Bellemans, J. Post-cam mechanics and tibiofemoral kinematics: A dynamic in vitro analysis of eight posterior-stabilized total knee designs. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 3343–3353. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.R.; Schütz, P.; Bergmann, G.; List, R.; Postolka, B.; Hitz, M.; Dymke, J.; Damm, P.; Duda, G.; Gerber, H.; et al. A comprehensive assessment of the musculoskeletal system: The CAMS-Knee data set. J. Biomech. 2017, 65, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Hull, M.L. Errors in using fixed flexion facet centers to determine tibiofemoral kinematics increase fourfold for multi-radius femoral component designs with early versus late decreases in the radius of curvature. Knee 2022, 35, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Dandridge, O.; Garner, A.; Jeffers, J.R.T.; Amis, A.A.; Cobb, J.P.; van Arkel, R.J. Validity of repeated-measures analyses of in vitro arthroplasty kinematics and kinetics. J. Biomech. 2021, 129, 110669. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brendle, S.A.; Krueger, S.; Grifka, J.; Müller, P.E.; Mihalko, W.M.; Richter, B.; Grupp, T.M. Constraint of Different Knee Implant Designs Under Anterior–Posterior Shear Forces and Internal–External Rotation Moments in Human Cadaveric Knees. Bioengineering 2025, 12, 87. https://doi.org/10.3390/bioengineering12010087
Brendle SA, Krueger S, Grifka J, Müller PE, Mihalko WM, Richter B, Grupp TM. Constraint of Different Knee Implant Designs Under Anterior–Posterior Shear Forces and Internal–External Rotation Moments in Human Cadaveric Knees. Bioengineering. 2025; 12(1):87. https://doi.org/10.3390/bioengineering12010087
Chicago/Turabian StyleBrendle, Saskia A., Sven Krueger, Joachim Grifka, Peter E. Müller, William M. Mihalko, Berna Richter, and Thomas M. Grupp. 2025. "Constraint of Different Knee Implant Designs Under Anterior–Posterior Shear Forces and Internal–External Rotation Moments in Human Cadaveric Knees" Bioengineering 12, no. 1: 87. https://doi.org/10.3390/bioengineering12010087
APA StyleBrendle, S. A., Krueger, S., Grifka, J., Müller, P. E., Mihalko, W. M., Richter, B., & Grupp, T. M. (2025). Constraint of Different Knee Implant Designs Under Anterior–Posterior Shear Forces and Internal–External Rotation Moments in Human Cadaveric Knees. Bioengineering, 12(1), 87. https://doi.org/10.3390/bioengineering12010087








