Electrical Stimulation of Mesenchymal Stem Cells as a Tool for Proliferation and Differentiation in Cartilage Tissue Engineering: A Scaffold-Based Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Design
2.2. Cell Culture
2.3. Collagen Type I Hydrogel Scaffolds
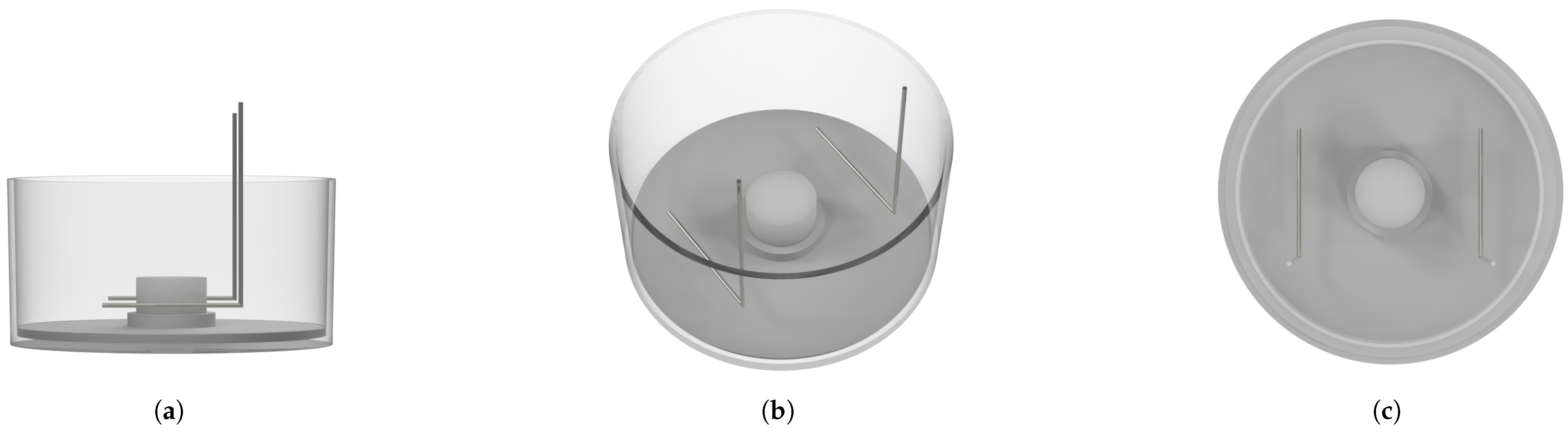
2.4. Stimulation Chamber
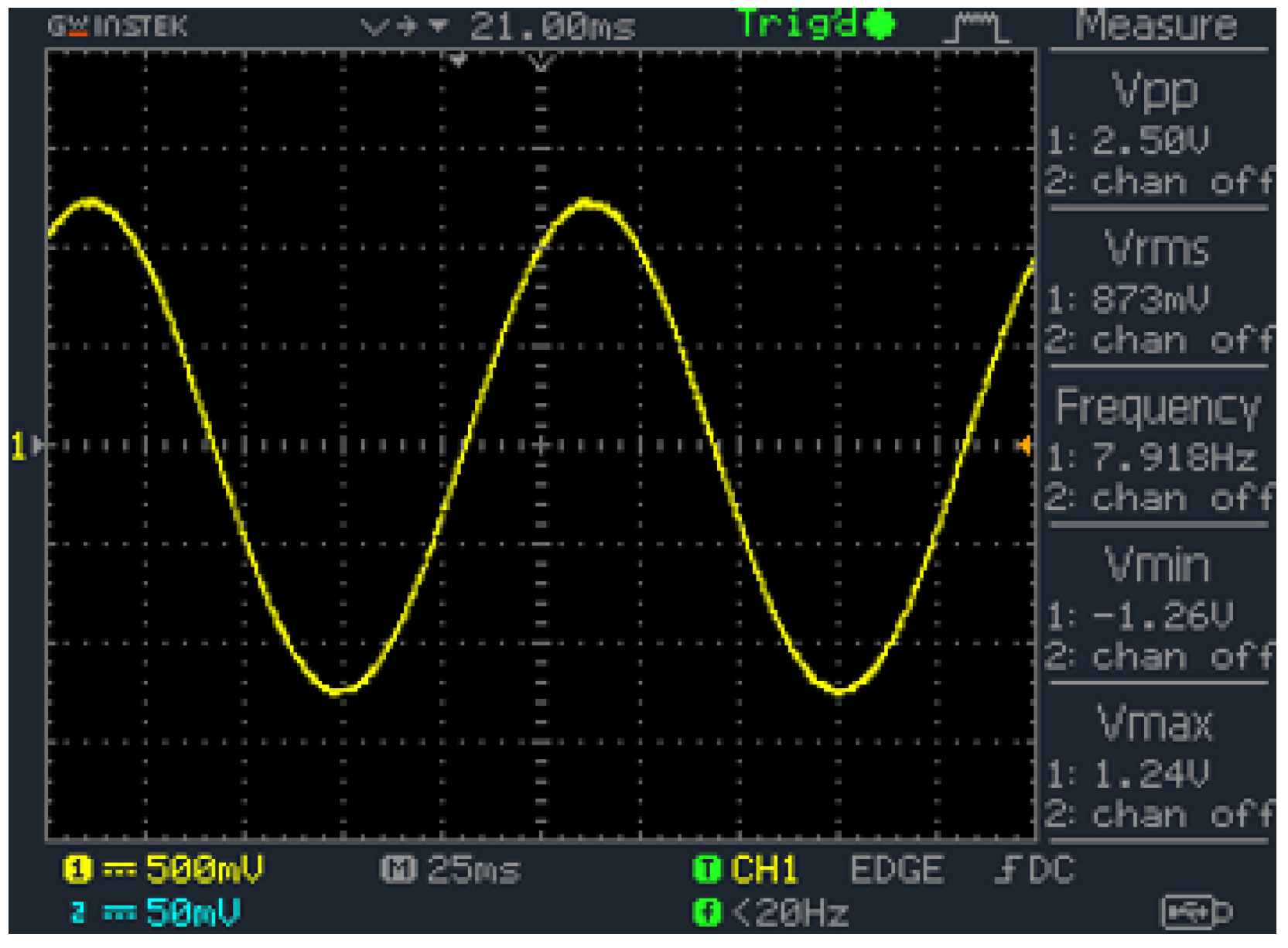
2.5. Signal Generator
2.6. Electrical Stimulation
2.7. Gene Analysis
2.8. Two-Photon Imaging
2.9. Scaffold Measurements
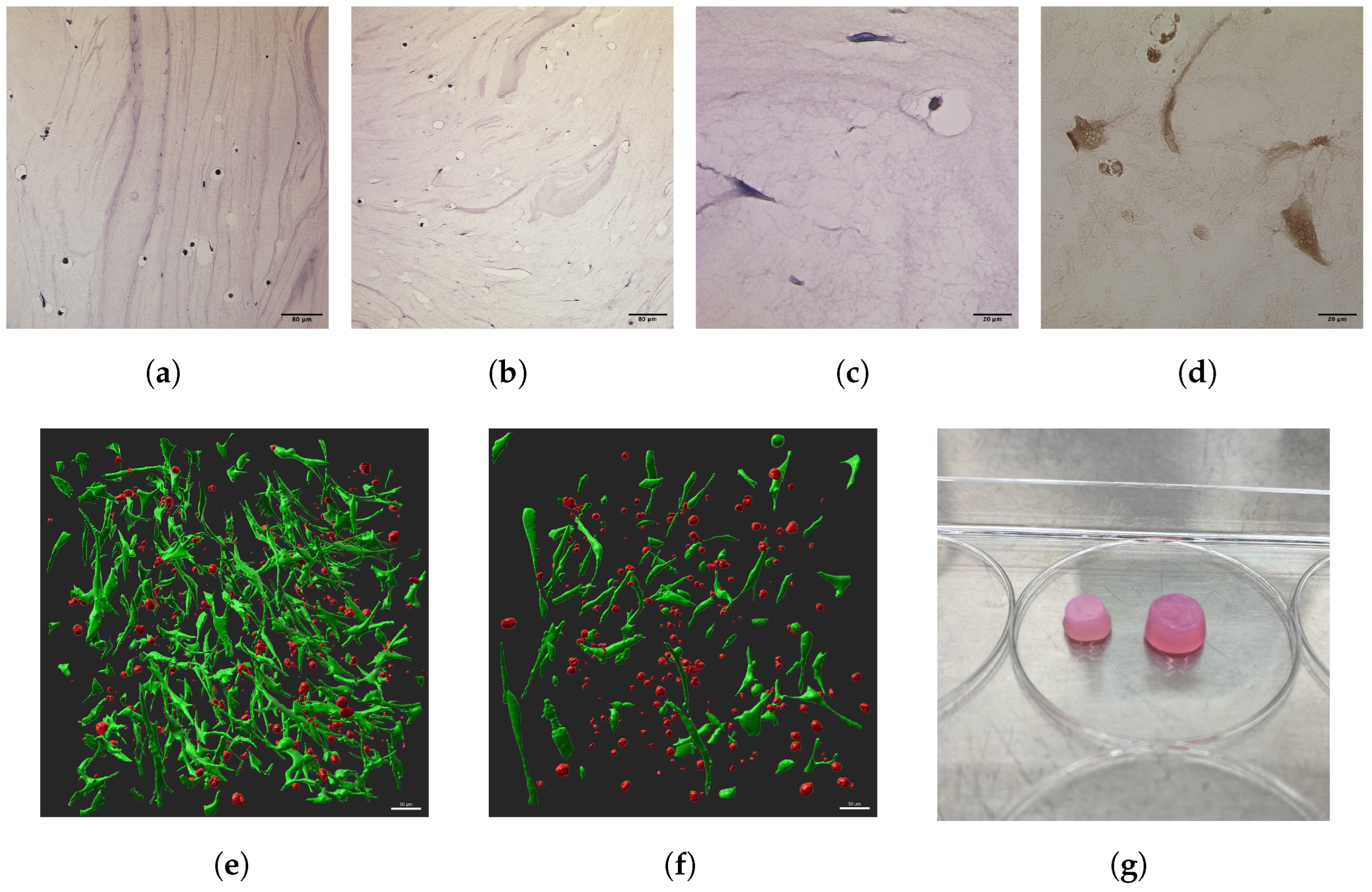
2.10. Histological Analysis
2.11. Statistical Analysis
3. Results
3.1. Morphology, Proliferation, and Viability
3.1.1. Scaffold and Cell Morphologies
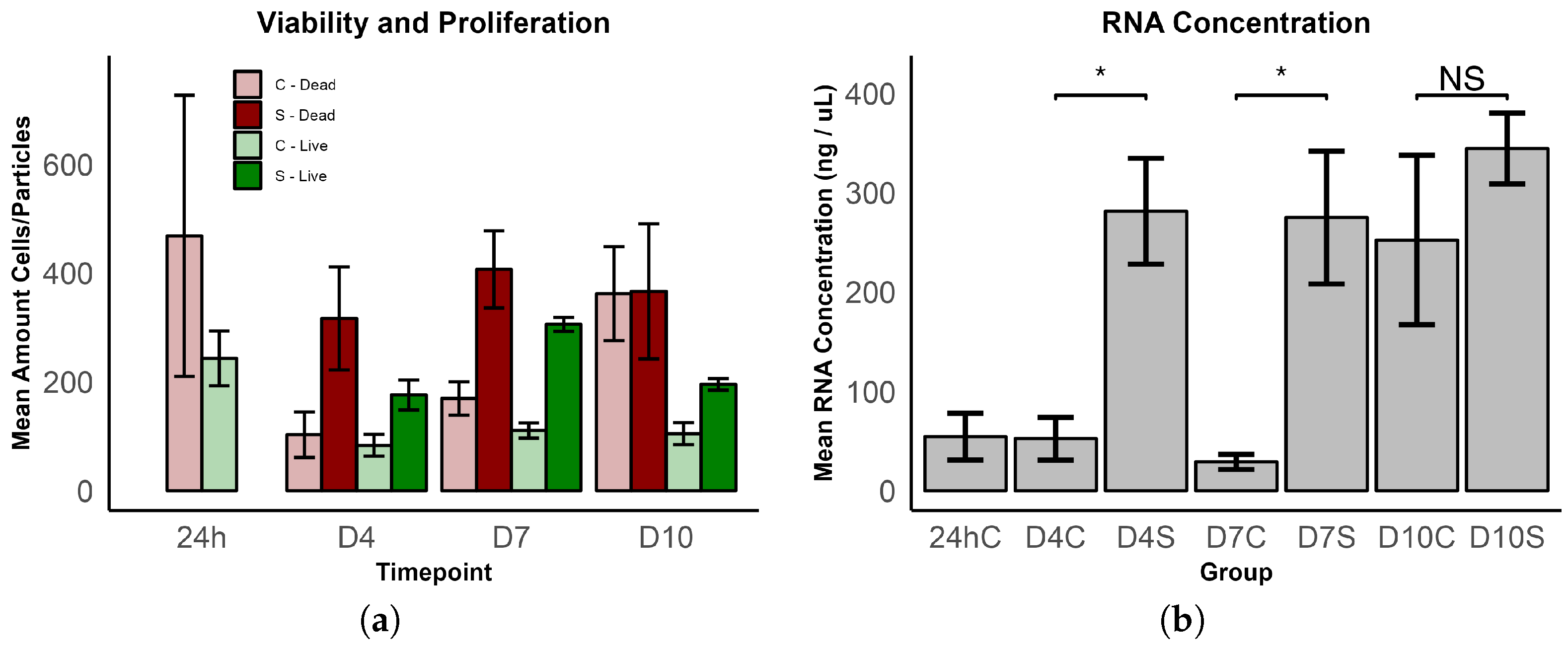
3.1.2. Growth and Viability
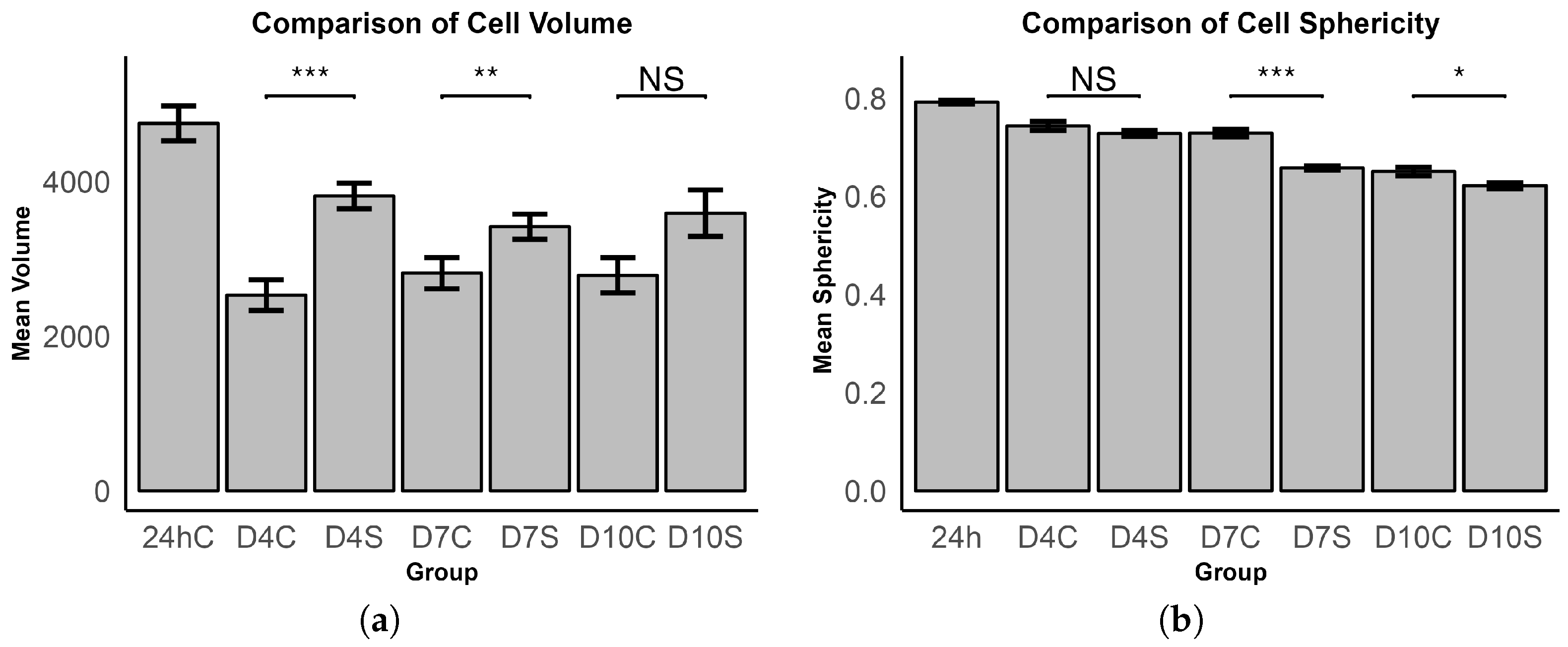
3.1.3. Cell Volume and Sphericity
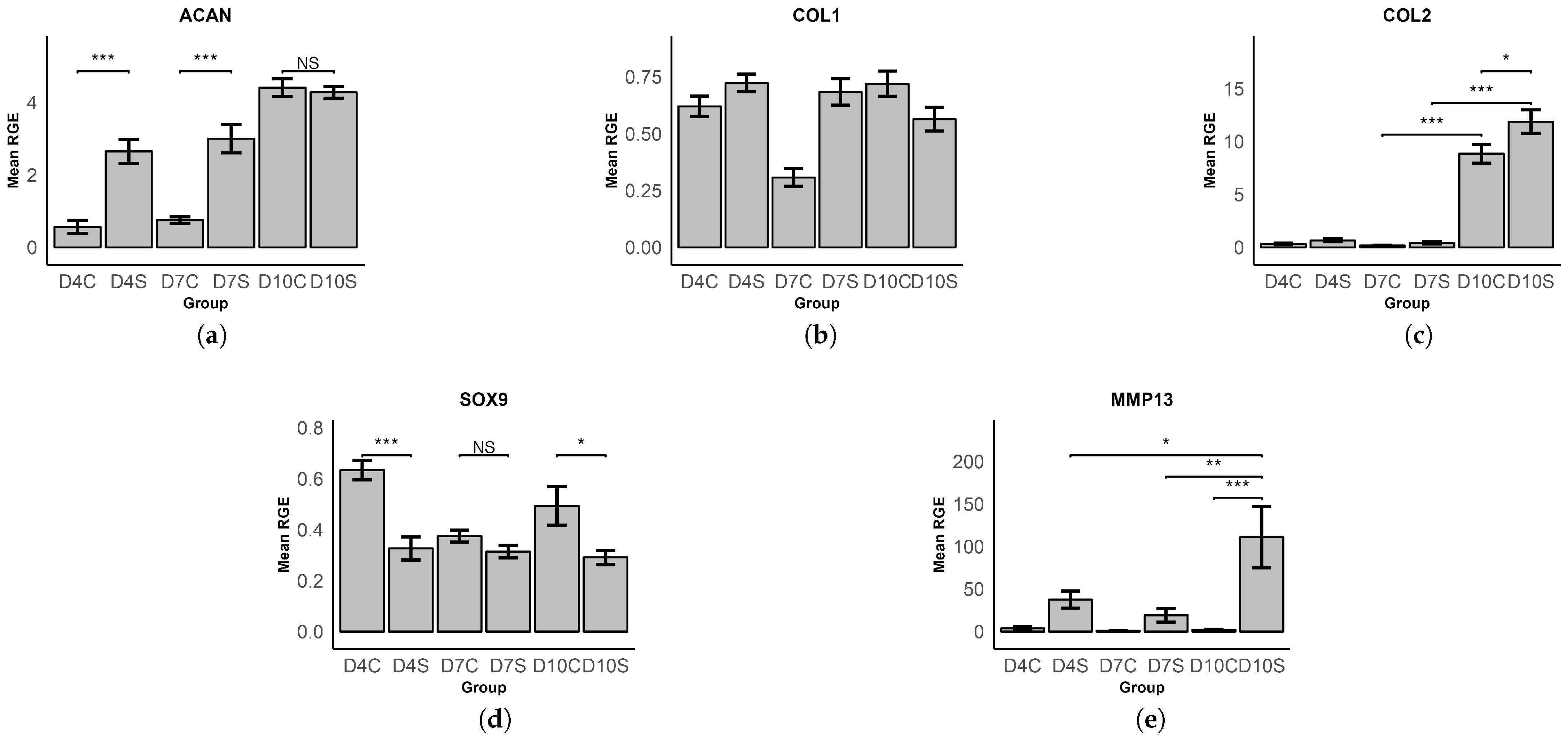
3.2. Gene Expression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Articular cartilage |
| ACAN | Aggrecan |
| AL | Activation solution |
| BMP | Bone morphogenic protein |
| B2M | 2-Microglobulin |
| CH | Collagen type I hydrogel |
| CHO | Chondrocyte |
| CD | Chondrogenic differentiation |
| CDF | Chondrogenic differentiation factors |
| COL1 | Collagen type I |
| COL2 | Collagen type II |
| ECM | Extracellular matrix |
| ES | Electrical stimulation |
| GAPDH | Glycerinaldehyde-3-phosphate dehydrogenase |
| HE | Hematoxylin and eosin |
| MSCs | Mesenchymal stem cells |
| MMP13 | Matrix-metallo-protease 13 |
| OA | Osteoarthritis |
| PPIA | Peptidylprolyl isomerase A |
| SOX9 | SRY-box transcription factor 9 |
| TGF- | Transforming growth factor- |
| VGCC | Voltage-gated calcium channel |
References
- Dillon, C.F.; Rasch, E.K.; Gu, Q.; Hirsch, R. Prevalence of knee osteoarthritis in the United States: Arthritis data from the Third National Health and Nutrition Examination Survey 1991–1994. J. Rheumatol. 2006, 33, 2271–2279. [Google Scholar]
- Felson, D.T. Epidemiology of Hip and Knee Osteoartritis 1. Epidemiol. Rev. 1988, 10, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Kovic, B.; Jin, X.; He, X.; Wang, M.; Silvestre, C. Economic and Humanistic Burden of Osteoarthritis: A Systematic Review of Large Sample Studies. Pharm. Econ. 2016, 34, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A.; Saltzman, C.; Brown, T. The impact of osteoarthritis: Implications for research. Clin. Orthop. Relat. Res. 2004, 427, S6–S15. [Google Scholar] [CrossRef] [PubMed]
- Eschweiler, J.; Horn, N.; Rath, B.; Betsch, M.; Baroncini, A.; Tingart, M.; Migliorini, F. The Biomechanics of Cartilage—An Overview. Life 2021, 11, 302. [Google Scholar] [CrossRef]
- Fujii, Y.; Liu, L.; Yagasaki, L.; Inotsume, M.; Chiba, T.; Asahara, H. Cartilage Homeostasis and Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 6316. [Google Scholar] [CrossRef]
- Hashimoto, M.; Nakasa, T.; Hikata, T.; Asahara, H. Molecular network of cartilage homeostasis and osteoarthritis. Med. Res. Rev. 2008, 28, 464–481. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Di, C.; Zhang, J.; Hu, S.; Jin, H.; Tong, P. Osteoarthritis pathogenesis: A review of molecular mechanisms. Calcif. Tissue Int. 2014, 95, 495–505. [Google Scholar] [CrossRef]
- Giorgino, R.; Albano, D.; Fusco, S.; Peretti, G.M.; Mangiavini, L.; Messina, C. Knee Osteoarthritis: Epidemiology, Pathogenesis, and Mesenchymal Stem Cells: What Else Is New? An Update. Int. J. Mol. Sci. 2023, 24, 6405. [Google Scholar] [CrossRef]
- Hsueh, M.F.; Önnerfjord, P.; Bolognesi, M.P.; Easley, M.E.; Kraus, V.B. Analysis of “old” proteins unmasks dynamic gradient of cartilage turnover in human limbs. Sci. Adv. 2019, 5, eaax3203. [Google Scholar] [CrossRef]
- Bielajew, B.J.; Hu, J.C.; Athanasiou, K.A. Methodology to Quantify Collagen Subtypes and Crosslinks: Application in Minipig Cartilages. Cartilage 2021, 13, 1742S–1754S. [Google Scholar] [CrossRef] [PubMed]
- Alcaide-Ruggiero, L.; Molina-Hernández, V.; Granados, M.M.; Domínguez, J.M. Main and Minor Types of Collagens in the Articular Cartilage: The Role of Collagens in Repair Tissue Evaluation in Chondral Defects. Int. J. Mol. Sci. 2021, 22, 13329. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.Z.; Nie, M.J.; Zhao, J.Z.; Zhang, G.C.; Zhang, Q.; Wang, B. Platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: A meta-analysis. J. Orthop. Surg. Res. 2020, 15, 403. [Google Scholar] [CrossRef] [PubMed]
- Skou, S.T.; Roos, E.M. Physical therapy for patients with knee and hip osteoarthritis: Supervised, active treatment is current best practice. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 120), 112–117. [Google Scholar] [PubMed]
- Richter, D.L.; Schenck, R.C., Jr.; Wascher, D.C.; Treme, G. Knee Articular Cartilage Repair and Restoration Techniques: A Review of the Literature. Sports Health 2016, 8, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Steadman, J.R.; Rodkey, W.G.; Singleton, S.B.; Briggs, K.K. Microfracture technique forfull-thickness chondral defects: Technique and clinical results. Oper. Tech. Orthop. 1997, 7, 300–304. [Google Scholar] [CrossRef]
- Hangody, L.; Füles, P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: Ten years of experimental and clinical experience. J. Bone Jt. Surg. Am. 2003, 85-A (Suppl. 2), 25–32. [Google Scholar] [CrossRef]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Calvi, M.; Curti, M.; Ossola, C.; Duvia, M.; Angeretti, M.G.; Ronga, M.; Genovese, E.A. Knee articular cartilage injury treatment with matrix-induced autologous chondrocyte implantation (MACI): Correlation at 24 and 120 months between clinical and radiological findings using MR arthrography. Skelet. Radiol. 2021, 50, 2079–2090. [Google Scholar] [CrossRef] [PubMed]
- Redondo, M.L.; Naveen, N.B.; Liu, J.N.; Tauro, T.M.; Southworth, T.M.; Cole, B.J. Preservation of Knee Articular Cartilage. Sport. Med. Arthrosc. Rev. 2018, 26, e23–e30. [Google Scholar] [CrossRef]
- Learmonth, I.D.; Young, C.; Rorabeck, C. The operation of the century: Total hip replacement. Lancet 2007, 370, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Price, A.J.; Alvand, A.; Troelsen, A.; Katz, J.N.; Hooper, G.; Gray, A.; Carr, A.; Beard, D. Knee replacement. Lancet 2018, 392, 1672–1682. [Google Scholar] [CrossRef]
- Ke, W.; Ma, L.; Wang, B.; Song, Y.; Luo, R.; Li, G.; Liao, Z.; Shi, Y.; Wang, K.; Feng, X.; et al. N-cadherin mimetic hydrogel enhances MSC chondrogenesis through cell metabolism. Acta Biomater. 2022, 150, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Vaca-Gonzalez, J.J.; Guevara, J.M.; Moncayo, M.A.; Castro-Abril, H.; Hata, Y.; Garzon-Alvarado, D.A. Biophysical Stimuli: A Review of Electrical and Mechanical Stimulation in Hyaline Cartilage. Cartilage 2019, 10, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Q.; Wan, H.Y.; Wang, Z.X.; Jiang, N. Stimulating factors for regulation of osteogenic and chondrogenic differentiation of mesenchymal stem cells. World J. Stem Cells 2023, 15, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Zha, K.; Sun, Z.; Yang, Y.; Chen, M.; Gao, C.; Fu, L.; Li, H.; Sui, X.; Guo, Q.; Liu, S. Recent Developed Strategies for Enhancing Chondrogenic Differentiation of MSC: Impact on MSC-Based Therapy for Cartilage Regeneration. Stem Cells Int. 2021, 2021, 8830834. [Google Scholar] [CrossRef]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells-current trends and future prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ghazanfari, R.; Zacharaki, D.; Lim, H.C.; Scheding, S. Isolation and characterization of primary bone marrow mesenchymal stromal cells. Ann. N. Y. Acad. Sci. 2016, 1370, 109–118. [Google Scholar] [CrossRef]
- Wagner, W.; Wein, F.; Seckinger, A.; Frankhauser, M.; Wirkner, U.; Krause, U.; Blake, J.; Schwager, C.; Eckstein, V.; Ansorge, W.; et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp. Hematol. 2005, 33, 1402–1416. [Google Scholar] [CrossRef]
- Hequet, O. Hematopoietic stem and progenitor cell harvesting: Technical advances and clinical utility. J. Blood Med. 2015, 6, 55–67. [Google Scholar] [CrossRef]
- Huang, G.T.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef]
- Schuring, A.N.; Schulte, N.; Kelsch, R.; Ropke, A.; Kiesel, L.; Gotte, M. Characterization of endometrial mesenchymal stem-like cells obtained by endometrial biopsy during routine diagnostics. Fertil. Steril. 2011, 95, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Bai, X.; Ding, Y.; Lee, I.S. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering. Biomater. Res. 2019, 23, 25. [Google Scholar] [CrossRef]
- Hernandez-Bule, M.L.; Paino, C.L.; Trillo, M.A.; Ubeda, A. Electric stimulation at 448 kHz promotes proliferation of human mesenchymal stem cells. Cell Physiol. Biochem. 2014, 34, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Vaca-Gonzalez, J.J.; Clara-Trujillo, S.; Guillot-Ferriols, M.; Rodenas-Rochina, J.; Sanchis, M.J.; Ribelles, J.L.G.; Garzon-Alvarado, D.A.; Ferrer, G.G. Effect of electrical stimulation on chondrogenic differentiation of mesenchymal stem cells cultured in hyaluronic acid-Gelatin injectable hydrogels. Bioelectrochemistry 2020, 134, 107536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Neoh, K.G.; Kang, E.T. Electrical stimulation of adipose-derived mesenchymal stem cells and endothelial cells co-cultured in a conductive scaffold for potential orthopaedic applications. J. Tissue Eng. Regen. Med. 2018, 12, 878–889. [Google Scholar] [CrossRef]
- Hiemer, B.; Krogull, M.; Bender, T.; Ziebart, J.; Krueger, S.; Bader, R.; Jonitz-Heincke, A. Effect of electric stimulation on human chondrocytes and mesenchymal stem cells under normoxia and hypoxia. Mol. Med. Rep. 2018, 18, 2133–2141. [Google Scholar] [CrossRef]
- Mardani, M.; Roshankhah, S.; Hashemibeni, B.; Salahshoor, M.; Naghsh, E.; Esfandiari, E. Induction of chondrogenic differentiation of human adipose-derived stem cells by low frequency electric field. Adv. Biomed. Res. 2016, 5, 97. [Google Scholar]
- Kwon, H.J.; Lee, G.S.; Chun, H. Electrical stimulation drives chondrogenesis of mesenchymal stem cells in the absence of exogenous growth factors. Sci. Rep. 2016, 6, 39302. [Google Scholar] [CrossRef]
- Esfandiari, E.; Roshankhah, S.; Mardani, M.; Hashemibeni, B.; Naghsh, E.; Kazemi, M.; Salahshoor, M. The effect of high frequency electric field on enhancement of chondrogenesis in human adipose-derived stem cells. Iran. J. Basic Med. Sci. 2014, 17, 571–576. [Google Scholar]
- Sun, S.; Titushkin, I.; Cho, M. Regulation of mesenchymal stem cell adhesion and orientation in 3D collagen scaffold by electrical stimulus. Bioelectrochemistry 2006, 69, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Su, S.; Yu, Y.; Liang, S.; Ma, C.; Jiao, Y.; Xing, W.; Tian, Z.; Jiang, T.; Wang, J. Revolutionizing osteoarthritis treatment: How mesenchymal stem cells hold the key. Biomed. Pharmacother. 2024, 173, 116458. [Google Scholar] [CrossRef] [PubMed]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Electrical stimulation: A novel tool for tissue engineering. Tissue Eng. Part B Rev. 2013, 19, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yuan, T.; Xiao, Z.; Tang, P.; Xiao, Y.; Fan, Y.; Zhang, X. Hydrogels of collagen/chondroitin sulfate/hyaluronan interpenetrating polymer network for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2012, 23, 2267–2279. [Google Scholar] [CrossRef] [PubMed]
- Vega, S.L.; Kwon, M.Y.; Burdick, J.A. Recent advances in hydrogels for cartilage tissue engineering. Eur. Cell Mater. 2017, 33, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, P.; Lai, J.H.; Yang, F.; Bhutani, N. 3D Hydrogel Scaffolds for Articular Chondrocyte Culture and Cartilage Generation. J. Vis. Exp. 2015, 104, e53085. [Google Scholar]
- Dymond, A.M.; Kaechele, L.E.; Jurist, J.M.; Crandall, P.H. Brain tissue reaction to some chronically implanted metals. J. Neurosurg. 1970, 33, 574–580. [Google Scholar] [CrossRef]
- Stensaas, S.S.; Stensaas, L.J. Histopathological evaluation of materials implanted in the cerebral cortex. Acta Neuropathol. 1978, 41, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Merrill, D.R.; Bikson, M.; Jefferys, J.G. Electrical stimulation of excitable tissue: Design of efficacious and safe protocols. J. Neurosci. Methods 2005, 141, 171–198. [Google Scholar] [CrossRef]
- Snutch, T.P.; Peloquin, J.; Mathews, E.; McRory, J.E. Molecular Properties of Voltage-Gated Calcium Channels; Springer: Boston, MA, USA, 2005; pp. 61–94. [Google Scholar]
- Vaiciuleviciute, R.; Uzieliene, I.; Bernotas, P.; Novickij, V.; Alaburda, A.; Bernotiene, E. Electrical Stimulation in Cartilage Tissue Engineering. Bioengineering 2023, 10, 454. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, J.; Meng, X.; Wang, F. Effects of Electrical Stimulation on Articular Cartilage Regeneration with a Focus on Piezoelectric Biomaterials for Articular Cartilage Tissue Repair and Engineering. Int. J. Mol. Sci. 2023, 24, 1836. [Google Scholar] [CrossRef]
- Vaca-González, J.J.; Guevara, J.M.; Vega, J.F.; Garzón-Alvarado, D.A. An In Vitro Chondrocyte Electrical Stimulation Framework: A Methodology to Calculate Electric Fields and Modulate Proliferation, Cell Death and Glycosaminoglycan Synthesis. Cell. Mol. Bioeng. 2016, 9, 116–126. [Google Scholar] [CrossRef]
- Parate, D.; Franco-Obregón, A.; Fröhlich, J.; Beyer, C.; Abbas, A.A.; Kamarul, T.; Hui, J.H.P.; Yang, Z. Enhancement of mesenchymal stem cell chondrogenesis with short-term low intensity pulsed electromagnetic fields. Sci. Rep. 2017, 7, 9421. [Google Scholar] [CrossRef]
- Uzieliene, I.; Bernotas, P.; Mobasheri, A.; Bernotiene, E. The Role of Physical Stimuli on Calcium Channels in Chondrogenic Differentiation of Mesenchymal Stem Cells. Int. J. Mol. Sci. 2018, 19, 2998. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Ohmiya, Y.; Honma, K.I.; Honma, S.; Nagai, T.; Saito, K.; Yasuda, K. Synchronized ATP oscillations have a critical role in prechondrogenic condensation during chondrogenesis. Cell Death Dis. 2012, 3, e278. [Google Scholar] [CrossRef] [PubMed]
- Matta, C.; Zákány, R.; Mobasheri, A. Voltage-Dependent Calcium Channels in Chondrocytes: Roles in Health and Disease. Curr. Rheumatol. Rep. 2015, 17, 43. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J. TGF-β but not BMP signaling induces prechondrogenic condensation through ATP oscillations during chondrogenesis. Biochem. Biophys. Res. Commun. 2012, 424, 793–800. [Google Scholar] [CrossRef]
- Ciobanu, F.; Golzio, M.; Kovacs, E.; Teissie, J. Control by Low Levels of Calcium of Mammalian Cell Membrane Electropermeabilization. J. Membr. Biol. 2018, 251, 221–228. [Google Scholar] [CrossRef]
- Kwon, H.J. Extracellular ATP signaling via P2X(4) receptor and cAMP/PKA signaling mediate ATP oscillations essential for prechondrogenic condensation. J. Endocrinol. 2012, 214, 337–348. [Google Scholar] [CrossRef]
- Chen, S.; Fu, P.; Cong, R.; Wu, H.; Pei, M. Strategies to minimize hypertrophy in cartilage engineering and regeneration. Genes Dis. 2015, 2, 76–95. [Google Scholar] [CrossRef]
- Martin, S.J.; Henry, C.M. Distinguishing between apoptosis, necrosis, necroptosis and other cell death modalities. Methods 2013, 61, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Moulisová, V.; Poveda-Reyes, S.; Sanmartín-Masiá, E.; Quintanilla-Sierra, L.; Salmerón-Sánchez, M.; Gallego Ferrer, G. Hybrid Protein–Glycosaminoglycan Hydrogels Promote Chondrogenic Stem Cell Differentiation. ACS Omega 2017, 2, 7609–7620. [Google Scholar] [CrossRef] [PubMed]
- Hameed, P.; Manivasagam, G. An overview of bio-actuation in collagen hydrogels: A mechanobiological phenomenon. Biophys. Rev. 2021, 13, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Pelttari, K.; Winter, A.; Steck, E.; Goetzke, K.; Hennig, T.; Ochs, B.G.; Aigner, T.; Richter, W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006, 54, 3254–3266. [Google Scholar] [CrossRef]
- Jonitz, A.; Lochner, K.; Tischer, T.; Hansmann, D.; Bader, R. TGF-β1 and IGF-1 influence the re-differentiation capacity of human chondrocytes in 3D pellet cultures in relation to different oxygen concentrations. Int. J. Mol. Med. 2012, 30, 666–672. [Google Scholar] [CrossRef]

| Gene | Forward | Reverse |
|---|---|---|
| GAPDH | ACAACTTTGGTATCGTGGAAGG | GCCATCACGCCACAGTTTC |
| B2M | TGAGTATGCCTGCCGTGTGA | GCGGCATCTTCAAACCTCCAT |
| PPIA | CTTGGGCCGCGTCTCCTTT | TCCTTTCTCTCCAGTGCTCAGA |
| ACAN | TGCTATGGAGACAAGGATGAG | GATGAGGGGTCGGGGTA |
| COL1 | TCTAGACATGTTCAGCTTTGTGGAC | TCTGTACGCAGGTGATTGGTG |
| COL2 | TCCTCTGCGACGACATAATC | CAGTGGCGAGGTCAGTT |
| SOX9 | AAGACGCTGGGCAAGCTCTG | GTAATCCGGGTGGTCCTTCTTG |
| MMP13 | ATACTACCATCCTACAAATCTCGC | GCCAGTCACCTCTAAGCCG |
| Timepoint | Control | Stimulated |
|---|---|---|
| Day 7 | 175.9 | 114.6 |
| Day 10 | 177.1 | 59.5 |
| Group | Avg. Living Cells | Avg. Dead Particles | Ratio |
|---|---|---|---|
| 24 h | 243.7 | 468.7 | 0.520 |
| D4C | 83.7 | 103.0 | 0.812 |
| D4S | 176.0 | 317.0 | 0.555 |
| D7C | 110.7 | 169.7 | 0.652 |
| D7S | 306.0 | 407.0 | 0.752 |
| D10C | 105.0 | 362.3 | 0.290 |
| D10S | 195.7 | 366.7 | 0.534 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehmenkötter, N.; Greven, J.; Hildebrand, F.; Kobbe, P.; Eschweiler, J. Electrical Stimulation of Mesenchymal Stem Cells as a Tool for Proliferation and Differentiation in Cartilage Tissue Engineering: A Scaffold-Based Approach. Bioengineering 2024, 11, 527. https://doi.org/10.3390/bioengineering11060527
Lehmenkötter N, Greven J, Hildebrand F, Kobbe P, Eschweiler J. Electrical Stimulation of Mesenchymal Stem Cells as a Tool for Proliferation and Differentiation in Cartilage Tissue Engineering: A Scaffold-Based Approach. Bioengineering. 2024; 11(6):527. https://doi.org/10.3390/bioengineering11060527
Chicago/Turabian StyleLehmenkötter, Nicolas, Johannes Greven, Frank Hildebrand, Philipp Kobbe, and Jörg Eschweiler. 2024. "Electrical Stimulation of Mesenchymal Stem Cells as a Tool for Proliferation and Differentiation in Cartilage Tissue Engineering: A Scaffold-Based Approach" Bioengineering 11, no. 6: 527. https://doi.org/10.3390/bioengineering11060527
APA StyleLehmenkötter, N., Greven, J., Hildebrand, F., Kobbe, P., & Eschweiler, J. (2024). Electrical Stimulation of Mesenchymal Stem Cells as a Tool for Proliferation and Differentiation in Cartilage Tissue Engineering: A Scaffold-Based Approach. Bioengineering, 11(6), 527. https://doi.org/10.3390/bioengineering11060527







