Innovations in Peripheral Nerve Regeneration
Abstract
1. Introduction
2. Peripheral Nerve Capacity of Self-Regeneration after Injury
2.1. Role of Schwann Cells in Healing Peripheral Nerve Injury
2.2. Limitations in Self-Regeneration
3. Contemporary Treatment to Peripheral Nerve Injury
3.1. Nerve Grafting and Guidance Conduits
3.2. Application in Oral and Maxillofacial Surgery
3.3. Pharmacotherapy in Treating Nerve Injury
4. Nerve Guidance Conduit
5. Pharmacotherapy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zhang, S.; Huang, M.; Zhi, J.; Wu, S.; Wang, Y.; Pei, F. Research hotspots and trends of peripheral nerve injuries based on web of science from 2017 to 2021: A bibliometric analysis. Front. Neurol. 2022, 13, 872261. [Google Scholar] [CrossRef] [PubMed]
- Marquez Neto, O.R.; Leite, M.S.; Freitas, T.; Mendelovitz, P.; Villela, E.A.; Kessler, I.M. The role of magnetic resonance imaging in the evaluation of peripheral nerves following traumatic lesion: Where do we stand? Acta Neurochir. 2017, 159, 281–290. [Google Scholar] [CrossRef]
- Flores, A.J.; Lavernia, C.J.; Owens, P.W. Anatomy and physiology of peripheral nerve injury and repair. Am. J. Orthop.-Belle-Mead 2000, 29, 167–178. [Google Scholar]
- Leung, Y.Y.; Lee, T.C.P.; Ho, S.M.Y.; Cheung, L.K. Trigeminal neurosensory deficit and patient reported outcome measures: The effect on life satisfaction and depression symptoms. PLoS ONE 2013, 8, e72891. [Google Scholar] [CrossRef]
- Lacagnina, M.J.; Willcox, K.F.; Boukelmoune, N.; Heijnen, C.J.; Grace, P.M. B Cells Promote Mechanical Allodynia after Peripheral Nerve Injury. J. Pain 2023, 24, 37. [Google Scholar] [CrossRef]
- Jaeger, S.H.; I Singer, D.; Whitenack, S.H.; Mandel, S. Nerve injury complications: Management of neurogenic pain syndromes. Hand Clin. 1986, 2, 217–234. [Google Scholar] [CrossRef]
- Koeppen, A.H. Wallerian degeneration: History and clinical significance. J. Neurol. Sci. 2004, 220, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R.; Lloyd, A.C. Schwann cells: Development and role in nerve repair. Cold Spring Harb. Perspect. Biol. 2015, 7, a020487. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. Negative regulation of myelination: Relevance for development, injury, and demyelinating disease. Glia 2008, 56, 1552–1565. [Google Scholar] [CrossRef]
- Brushart, M.; Aspalter, M.; Griffin, J.W.; Redett, R.; Hameed, H.; Zhou, C.; Wright, M.; Vyas, A.; Höke, A. Schwann cell phenotype is regulated by axon modality and central–peripheral location, and persists in vitro. Exp. Neurol. 2013, 247, 272–281. [Google Scholar] [CrossRef]
- Vidal, P.M.; Lemmens, E.; Dooley, D.; Hendrix, S. The role of “anti-inflammatory” cytokines in axon regeneration. Cytokine Growth Factor Rev. 2013, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Barrette, B.; Hébert, M.-A.; Filali, M.; Lafortune, K.; Vallières, N.; Gowing, G.; Julien, J.-P.; Lacroix, S. Requirement of myeloid cells for axon regeneration. J. Neurosci. 2008, 28, 9363–9376. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Kawabuchi, M. Myelin phagocytosis by macrophages and nonmacrophages during Wallerian degeneration. Microsc. Res. Tech. 2002, 57, 541–547. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef] [PubMed]
- Antoniadis, G. The Peripheral Nerve: Neuroanatomical Principles Before and After Injury. In Modern Concepts of Peripheral Nerve Repair, 1st ed.; Haastert-Talini, K.H., Assmus, G.A., Eds.; Springer: Cham, Switzerland, 2017; pp. 8–9. [Google Scholar]
- Jessen, K.R.; Mirsky, R. The success and failure of the Schwann cell response to nerve injury. Front. Cell. Neurosci. 2019, 13, 33. [Google Scholar] [CrossRef]
- Gomez-Sanchez, J.A.; Carty, L.; Iruarrizaga-Lejarreta, M.; Palomo-Irigoyen, M.; Varela-Rey, M.; Griffith, M.; Hantke, J.; Macias-Camara, N.; Azkargorta, M.; Aurrekoetxea, I.; et al. Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J. Cell Biol. 2015, 210, 153–168. [Google Scholar] [CrossRef]
- Arthur-Farraj, P.J.; Latouche, M.; Wilton, D.K.; Quintes, S.; Chabrol, E.; Banerjee, A.; Woodhoo, A.; Jenkins, B.; Rahman, M.; Turmaine, M.; et al. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 2012, 75, 633–647. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The role of c-Jun and autocrine signaling loops in the control of repair Schwann cells and regeneration. Front. Cell. Neurosci. 2022, 15, 820216. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, M.G.; Martins, R.S. Conventional Stratigies for Nerve Repair. In Modern Concepts of Peripheral Nerve Repair, 1st ed.; Haastert-Talini, K.H., Antoniadis, A.G., Eds.; Springer: Cham, Switzerland, 2017; pp. 41–43. [Google Scholar]
- Deumens, R.; Bozkurt, A.; Meek, M.F.; Marcus, M.A.; Joosten, E.A.; Weis, J.; Brook, G.A. Repairing injured peripheral nerves: Bridging the gap. Prog. Neurobiol. 2010, 92, 245–276. [Google Scholar] [CrossRef]
- Leung, Y.Y.; Cheung, L.K. Longitudinal treatment outcomes of microsurgical treatment of neurosensory deficit after lower third molar surgery: A prospective case series. PLoS ONE 2016, 11, e0150149. [Google Scholar] [CrossRef][Green Version]
- Radtke, C.; Kocsis, J.D. Olfactory-ensheathing cell transplantation for peripheral nerve repair: Update on recent developments. Cells Tissues Organs 2015, 200, 48–58. [Google Scholar] [CrossRef]
- Daly, W.; Yao, L.; Zeugolis, D.; Windebank, A.; Pandit, A. A biomaterials approach to peripheral nerve regeneration: Bridging the peripheral nerve gap and enhancing functional recovery. J. R. Soc. Interface 2012, 9, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Pogrel, M.; Maghen, A. The use of autogenous vein grafts for inferior alveolar and lingual nerve reconstruction. J. Oral Maxillofac. Surg. 2001, 59, 985–988. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.H.; Haycock, J.W. Next generation nerve guides: Materials, fabrication, growth factors, and cell delivery. Tissue Eng. Part B: Rev. 2012, 18, 116–128. [Google Scholar] [CrossRef]
- Safa, B.; Jain, S.; Desai, M.J.; Greenberg, J.A.; Niacaris, T.R.; Nydick, J.A.; Leversedge, F.J.; Megee, D.M.; Zoldos, J.; Rinker, B.D.; et al. Peripheral nerve repair throughout the body with processed nerve allografts: Results from a large multicenter study. Microsurgery 2020, 40, 527–537. [Google Scholar] [CrossRef]
- Ducic, I.; Yoon, J. Reconstructive options for Inferior Alveolar and Lingual Nerve Injuruies after Dental and Oral Surgery. An evidence-based review. Ann. Plast. Surg. 2019, 82, 653–660. [Google Scholar] [CrossRef]
- Baltrusch, S. The Role of Neurotropic B Vitamins in Nerve Regeneration. BioMed Res. Int. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Ang, C.D.; Alviar, M.J.M.; Dans, A.L.; Bautista-Velez, G.G.P.; Villaruz-Sulit, M.V.C.; Tan, J.J.; Co, H.U.; Bautista, M.R.M.; Roxas, A.A. Vitamin B for treating peripheral neuropathy. Cochrane Database Syst. Rev. 2008, 16, CD004573. [Google Scholar] [CrossRef]
- Altun, I.; Kurutaş, E. Vitamin B complex and vitamin B12 levels after peripheral nerve injury. Neural Regen. Res. 2016, 11, 842–845. [Google Scholar] [CrossRef]
- Modrak, M.; Talukder, M.A.H.; Gurgenashvili, K.; Noble, M.; Elfar, J.C. Peripheral nerve injury and myelination: Potential therapeutic strategies. J. Neurosci. Res. 2020, 98, 780–795. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.M.; Harman, S.M. An update on hormone therapy in postmenopausal women: Mini-review for the basic scientist. Am. J. Physiol.-Heart Circ. Physiol. 2017, 313, H1013–H1021. [Google Scholar] [CrossRef] [PubMed]
- Magnaghi, V.; Procacci, P.; Tata, A.M. Novel pharmacological approaches to Schwann cells as neuroprotective agents for peripheral nerve regeneration. Int. Rev. Neurobiol. 2009, 87, 295–315. [Google Scholar] [PubMed]
- Maiese, K. Regeneration in the nervous system with erythropoietin. Front. Biosci. Landmark Ed. 2016, 21, 561. [Google Scholar] [CrossRef] [PubMed]
- Sargin, D.; Friedrichs, H.; El-Kordi, A.; Ehrenreich, H. Erythropoietin as neuroprotective and neuroregenerative treatment strategy: Comprehensive overview of 12 years of preclinical and clinical research. Best Pract. Res. Clin. Anaesthesiol. 2010, 24, 573–594. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.T.; Weiner, B.K.; Tasciotti, E.; Mathis, K.B. Does the combination of erythropoietin and tapered oral corticosteroids improve recovery following iatrogenic nerve injury? Injury 2016, 47, 1819–1823. [Google Scholar] [CrossRef] [PubMed]
- Hemani, S.; Lane, O.; Agarwal, S.; Yu, S.P.; Woodbury, A. Systematic review of erythropoietin (EPO) for neuroprotection in human studies. Neurochem. Res. 2021, 46, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Miloro, M. Microneurosurgery. In Peterson’s Principles of Oral and Maxillofacial Surgery, 4th ed.; Miloro, M., Ghali, G.E., Larson, P.E., Waite, P., Eds.; Springer: Cham, Switzerland, 2022; pp. 1313–1344. [Google Scholar]
- Muheremu, A.; Ao, Q. Past, present, and future of nerve conduits in the treatment of peripheral nerve injury. BioMed Res. Int. 2015, 237507. [Google Scholar] [CrossRef] [PubMed]
- Chrząszcz, P.; Derbisz, K.; Suszyński, K.; Miodoński, J.; Trybulski, R.; Lewin-Kowalik, J.; Marcol, W. Application of peripheral nerve conduits in clinical practice: A literature review. Neurol. I Neurochir. Pol. 2018, 52, 427–435. [Google Scholar] [CrossRef]
- Li, R.; Liu, Z.; Pan, Y.; Chen, L.; Zhang, Z.; Lu, L. Peripheral nerve injuries treatment: A systematic review. Cell Biochem. Biophys. 2014, 68, 449–454. [Google Scholar] [CrossRef]
- Lundborg, G.; Gelberman, R.H.; Longo, F.M.; Powell, H.C.; Varon, S. In vivo regeneration of cut nerves encased in silicone tubes: Growth across a six-millimeter gap. J. Neuropathol. Exp. Neurol. 1982, 41, 412–422. [Google Scholar] [CrossRef]
- Gao, S.; Chen, X.; Lu, B.; Meng, K.; Zhang, K.-Q.; Zhao, H. Recent advances on nerve guide conduits based on textile methods. Smart Mater. Med. 2023, 4, 368–383. [Google Scholar] [CrossRef]
- Johnson, B.N.; Lancaster, K.Z.; Zhen, G.; He, J.; Gupta, M.K.; Kong, Y.L.; Engel, E.A.; Krick, K.D.; Ju, A.; Meng, F.; et al. 3D printed anatomical nerve regeneration pathways. Adv. Funct. Mater. 2015, 25, 6205–6217. [Google Scholar] [CrossRef]
- Krishna, D.V.; Sankar, M.R. Engineered approach coupled with machine learning in biofabrication of patient-specific nerve guide conduits-Review. Bioprinting 2023, 30, e00264. [Google Scholar]
- Hui, T.; Wang, C.; Yu, L.; Zhou, C.; Qiu, M. Phosphorene hydrogel conduits as “neurotrophin reservoirs” for promoting regeneration of peripheral nerves. J. Mater. Chem. B 2023, 11, 3808–3815. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, W.; Wu, R.; Guan, T.; Li, Z.; Tu, Q.; Liu, Y.; Gu, X.; Liu, M. Promising application of a novel biomaterial, light chain of silk fibroin combined with NT3, in repairment of rat sciatic nerve defect injury. Int. J. Biol. Macromol. 2023, 240, 124447. [Google Scholar] [CrossRef]
- Ikegami, Y.; Shafiq, M.; Aishima, S.; Ijima, H. Heparin/Growth Factors-Immobilized Aligned Electrospun Nanofibers Promote Nerve Regeneration in Polycaprolactone/Gelatin-Based Nerve Guidance Conduits. Adv. Fiber Mater. 2023, 5, 554–573. [Google Scholar] [CrossRef]
- Dong, Q.; Yang, X.; Liang, X.; Liu, J.; Wang, B.; Zhao, Y.; Huselstein, C.; Feng, X.; Tong, Z.; Chen, Y. Composite Hydrogel Conduit Incorporated with Platelet-Rich Plasma Improved the Regenerative Microenvironment for Peripheral Nerve Repair. ACS Appl. Mater. Interfaces 2023, 15, 24120–24133. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Javed, R.; Ao, Q. Xenogeneic decellularized extracellular matrix-based biomaterials for peripheral nerve repair and regeneration. Curr. Neuropharmacol. 2021, 19, 2152. [Google Scholar] [CrossRef]
- Kellaway, S.C.; Roberton, V.; Jones, J.N.; Loczenski, R.; Phillips, J.B.; White, L.J. Engineered neural tissue made using hydrogels derived from decellularised tissues for the regeneration of peripheral nerves. Acta Biomater. 2023, 157, 124–136. [Google Scholar] [CrossRef]
- Mao, X.; Li, T.; Cheng, J.; Tao, M.; Li, Z.; Ma, Y.; Javed, R.; Bao, J.; Liang, F.; Guo, W.; et al. Nerve ECM and PLA-PCL based electrospun bilayer nerve conduit for nerve regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1103435. [Google Scholar] [CrossRef]
- Huang, Z.; Kankowski, S.; Ertekin, E.; Almog, M.; Nevo, Z.; Rochkind, S.; Haastert-Talini, K. Modified hyaluronic acid-laminin-hydrogel as luminal filler for clinically approved hollow nerve guides in a rat critical defect size model. Int. J. Mol. Sci. 2021, 22, 6554. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Hibbitts, A.J.; Kočí, Z.; Kneafsey, S.; Matsiko, A.; Žilić, L.; Dervan, A.; Hinton, P.; Chen, G.; Cavanagh, B.; Dowling, J.K.; et al. Multi-factorial nerve guidance conduit engineering improves outcomes in inflammation, angiogenesis and large defect nerve repair. Matrix Biol. 2022, 106, 34–57. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Gu, G.; Cong, M.; Du, M.; Wang, W.; Shen, M.; Zhang, Q.; Shi, H.; Gu, X.; Ding, F. Repair of peripheral nerve defects by nerve grafts incorporated with extracellular vesicles from skin-derived precursor Schwann cells. Acta Biomater. 2021, 134, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Shiekh, P.A.; Qayoom, I.; Srivastava, E.; Kumar, A. Evaluation of polymeric aligned NGCs and exosomes in nerve injury models in diabetic peripheral neuropathy condition. Eur. Polym. J. 2021, 146, 110256. [Google Scholar] [CrossRef]
- Tang, H.; Li, J.; Wang, H.; Ren, J.; Ding, H.; Shang, J.; Wang, M.; Wei, Z.; Feng, S. Human umbilical cord mesenchymal stem cell-derived exosomes loaded into a composite conduit promote functional recovery after peripheral nerve injury in rats. Neural Regen. Res. 2023, 19, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, Y.; Xu, Y.; Jiang, W.; Shao, Y.; Xing, J.; Chen, Y.; Han, Y. Biomimetic nerve guidance conduit containing engineered exosomes of adipose-derived stem cells promotes peripheral nerve regeneration. Stem Cell Res. Ther. 2021, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.; David, G.; Jacobs, D.; Kuczwara, P.; Woessner, A.E.; Kim, J.-W.; Quinn, K.P.; Song, Y. Neuro-regenerative behavior of adipose-derived stem cells in aligned collagen I hydrogels. Mater. Today Bio 2023, 22, 100762. [Google Scholar] [CrossRef] [PubMed]
- Namini, M.S.; Ebrahimi-Barough, S.; Ai, J.; Jahromi, H.K.; Mikaeiliagah, E.; Azami, M.; Bahrami, N.; Lotfibakhshaiesh, N.; Saremi, J.; Shirian, S. Tissue-Engineered Core–Shell Silk-Fibroin/Poly-l-Lactic Acid Nerve Guidance Conduit Containing Encapsulated Exosomes of Human Endometrial Stem Cells Promotes Peripheral Nerve Regeneration. ACS Biomater. Sci. Eng. 2023, 9, 3496–3511. [Google Scholar] [CrossRef]
- Souza, N.M.; Gonçalves, M.F.; Ferreira, L.F.R.; Bilal, M.; Iqbal, H.M.N.; Soriano, R.N. Revisiting the role of biologically active natural and synthetic compounds as an intervention to treat injured nerves. Mol. Neurobiol. 2021, 58, 4980–4998. [Google Scholar] [CrossRef]
- Manto, K.M.; Govindappa, P.K.; Parisi, D.; Karuman, Z.; Martinazzi, B.; Hegarty, J.P.; Talukder, M.A.H.; Elfar, J.C. (4-Aminopyridine)–PLGA–PEG as a Novel Thermosensitive and Locally Injectable Treatment for Acute Peripheral Nerve Injury. ACS Appl. Bio Mater. 2021, 4, 4140–4151. [Google Scholar] [CrossRef] [PubMed]
- Talukder, M.A.H.; Lee, J.I.; Hegarty, J.P.; Gurjar, A.A.; O’Brien, M.; Karuman, Z.; Wandling, G.D.; Govindappa, P.K.; Elfar, J.C. Obligatory role of Schwann cell-specific erythropoietin receptors in erythropoietin-induced functional recovery and neurogenic muscle atrophy after nerve injury. Muscle Nerve 2021, 63, 268–272. [Google Scholar] [CrossRef]
- Lee, J.I.; Hur, J.M.; You, J.; Lee, D.H. Functional recovery with histomorphometric analysis of nerves and muscles after combination treatment with erythropoietin and dexamethasone in acute peripheral nerve injury. PLoS ONE 2020, 15, e0238208. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, N.; Fattahian, H.; Jahandideh, A.; Akbarein, H. The effects of dexamethasone and erythropoietin on mice sciatic nerve crush injury: Histopathologic and functional outcomes. Arch. Vet. Sci. 2023, 28, 3. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Z.; Zhang, X. Carbamylated erythropoietin: A prospective drug candidate for neuroprotection. Biochem. Insights 2015, 8, 25–29. [Google Scholar]
- Diao, M.; Qu, Y.; Liu, H.; Ma, Y.; Lin, X. Effect of carbamylated erythropoietin on neuronal apoptosis in fetal rats during intrauterine hypoxic-ischemic encephalopathy. Biol. Res. 2019, 52, 28. [Google Scholar] [CrossRef]
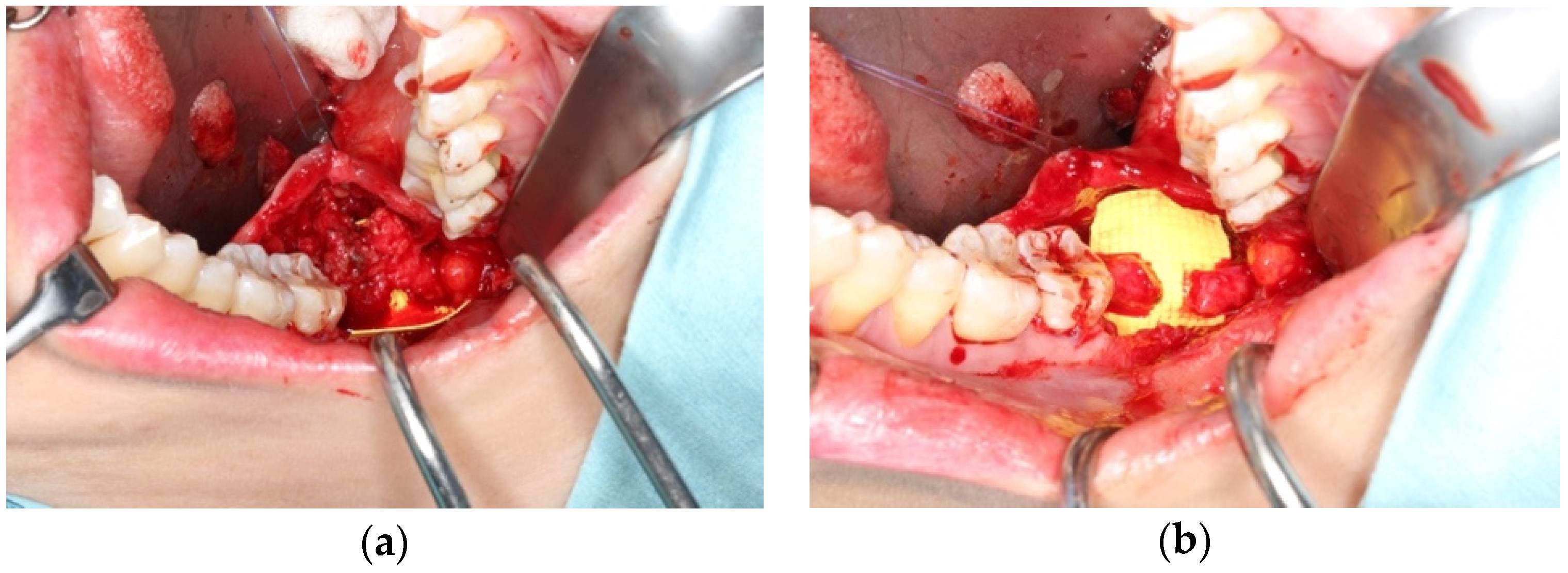
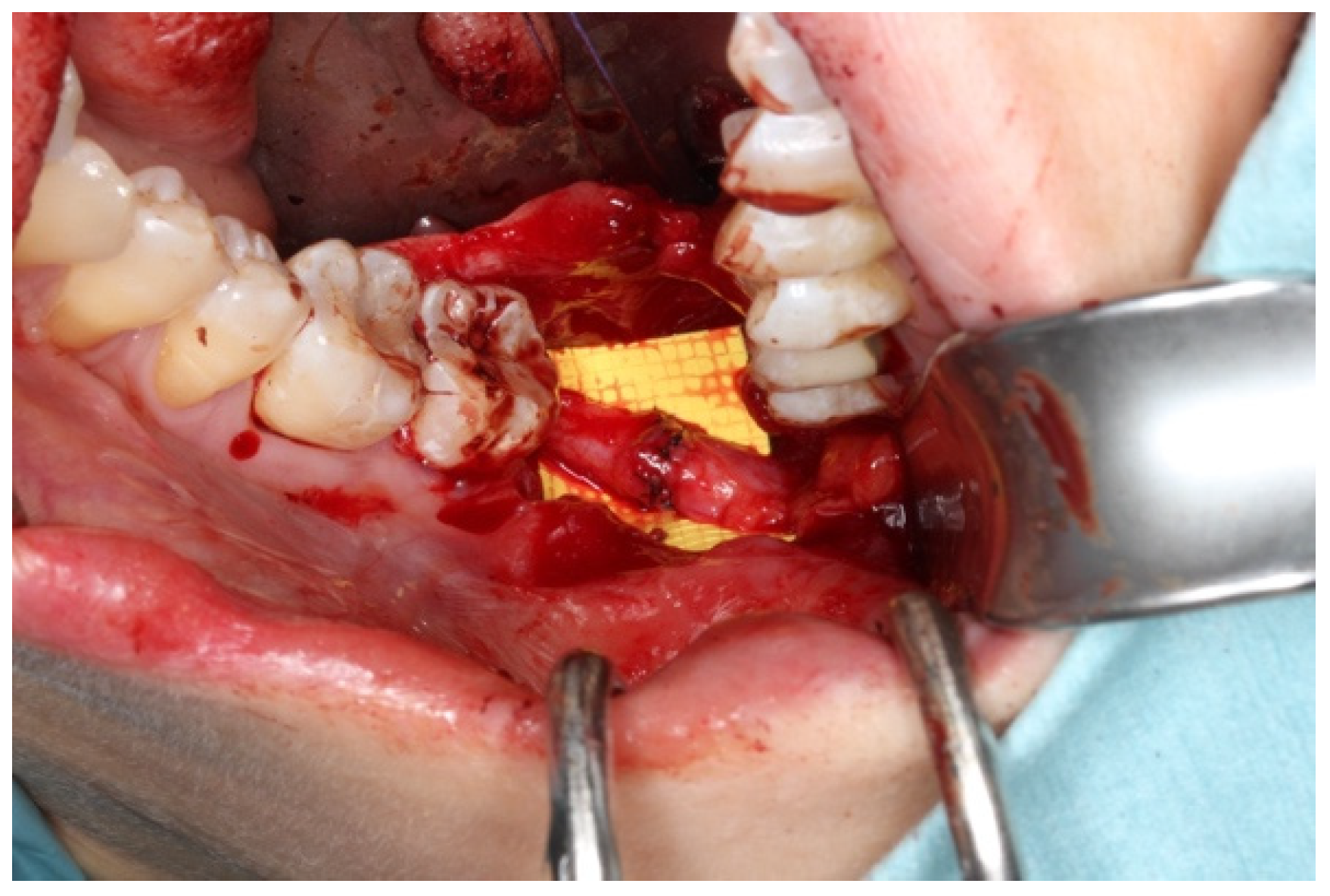

| Brand Name | Company | Material |
|---|---|---|
| NeuraGen®, NeurawrapTM | Integra Life Science Corp, Princeton, NJ, USA | Type I collagen |
| NeuroMatrixTM, NeuroflexTM, NeuroMendTM | Stryker, Kalamazoo, MI, USA | Type I collagen |
| Neurolac® | Polyganics, Groningen, The Netherlands | PDLLA/CL |
| Neurotube® | Synovis, Birmingham, AL, USA | PGA |
| SalubridgeTM, SalutunnelTM | Salumedica, Atlanta, GA, USA | Polyvinyl alcohol hydrogel |
| Surgrisis®, AxoguardTM | AxoGenInc, Alachua, FL, USA | Porcrine small intestinal submucosa |
| Avance® | AxogenInc, Alachua, FL, USA | Decellularized human nerve allograft |
| Reaxon®Direct | Kerimedical, Genève, Switzerland | Chitosan |
| RevolNerv® | Orthomed, Saint-Jeannet, France | Collagen Type I and III from porcine skin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lam, T.C.; Leung, Y.Y. Innovations in Peripheral Nerve Regeneration. Bioengineering 2024, 11, 444. https://doi.org/10.3390/bioengineering11050444
Lam TC, Leung YY. Innovations in Peripheral Nerve Regeneration. Bioengineering. 2024; 11(5):444. https://doi.org/10.3390/bioengineering11050444
Chicago/Turabian StyleLam, Ting Chak, and Yiu Yan Leung. 2024. "Innovations in Peripheral Nerve Regeneration" Bioengineering 11, no. 5: 444. https://doi.org/10.3390/bioengineering11050444
APA StyleLam, T. C., & Leung, Y. Y. (2024). Innovations in Peripheral Nerve Regeneration. Bioengineering, 11(5), 444. https://doi.org/10.3390/bioengineering11050444







