The Influence of Cold Therapy on the Physical Working Capacity at the Electromyographic Threshold for Consecutive Exercise Sessions
Abstract
1. Introduction
2. Materials and Methods
2.1. Overall Research Design
2.2. Subjects
2.3. Incremental Single-Leg Knee-Extensor Ergometry
2.4. Cold Therapy Protocol
2.5. Electrode Placements
2.6. Determining the PWCFT
2.7. Recording the EMG Signal and Processing
2.8. Statistical Analysis
3. Results
3.1. Incremental Test and PWCFT
3.2. Comparison of Surface Skin Temperature
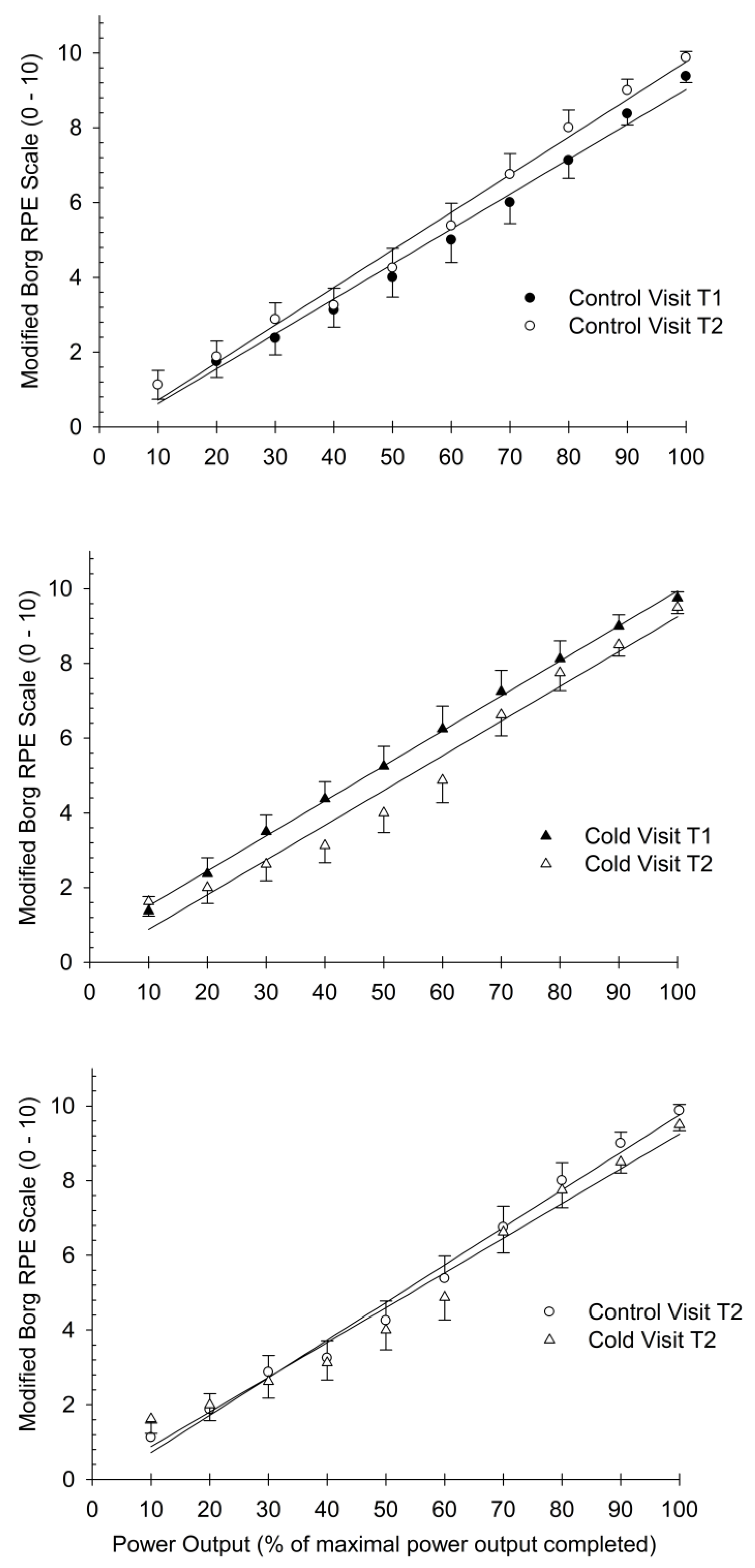
3.3. Comparison of Slopes for RPE Index
4. Discussion
4.1. Use of Cold Therapy
4.2. Utility of the Single-Leg Knee-Extensor Ergometer
4.3. Neuromuscular Response and Cold Therapy
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vaile, J.; Halson, S.; Gill, N.; Dawson, B. Effect of hydrotherapy on recovery from fatigue. Int. J. Sports Med. 2008, 29, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Vaile, J.; Halson, S.; Gill, N.; Dawson, B. Effect of hydrotherapy on the signs and symptoms of delayed onset muscle soreness. Eur. J. Appl. Physiol. 2008, 102, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E.; Fialka, V. Ice freezes pain? A review of the clinical effectiveness of analgesic cold therapy. J. Pain Symptom Manag. 1994, 9, 56–59. [Google Scholar] [CrossRef] [PubMed]
- de Souza Guerino Macedo, C.; Vicente, R.C.; Cesario, M.D.; Guirro, R.R. Cold-water immersion alters muscle recruitment and balance of basketball players during vertical jump landing. J. Sports Sci. 2016, 34, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Mawhinney, C.; Heinonen, I.; Low, D.A.; Han, C.; Jones, H.; Kalliokoski, K.K.; Kirjavainen, A.; Kemppainen, J.; DI Salvo, V.; Lolli, L.; et al. Cool-Water Immersion Reduces Postexercise Quadriceps Femoris Muscle Perfusion More than Cold-Water Immersion. Med. Sci. Sports Exerc. 2022, 54, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.C.; Machado, A.F.; Albuquerque, M.C.; Netto, L.M.; Vanderlei, F.M.; Vanderlei, L.C.; Junior, J.N.; Pastre, C.M. The effects of cold water immersion with different dosages (duration and temperature variations) on heart rate variability post-exercise recovery: A randomized controlled trial. J. Sci. Med. Sport Sports Med. Aust. 2016, 19, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Mustalampi, S.; Ylinen, J.; Kautiainen, H.; Weir, A.; Hakkinen, A. Acute effects of cold pack on mechanical properties of the quadriceps muscle in healthy subjects. Phys. Ther. Sport Off. J. Assoc. Chart. Physiother. Sports Med. 2012, 13, 265–269. [Google Scholar] [CrossRef]
- Tseng, C.Y.; Lee, J.P.; Tsai, Y.S.; Lee, S.D.; Kao, C.L.; Liu, T.C.; Lai, C.; Harris, M.B.; Kuo, C.H. Topical cooling (icing) delays recovery from eccentric exercise-induced muscle damage. J. Strength Cond. Res. Natl. Strength Cond. Assoc. 2013, 27, 1354–1361. [Google Scholar] [CrossRef]
- Sanchez-Urena, B.; Rojas-Valverde, D.; Gutierrez-Vargas, R. Effectiveness of Two Cold Water Immersion Protocols on Neuromuscular Function Recovery: A Tensiomyography Study. Front. Physiol. 2018, 9, 766. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Wijayanto, T.; Tochihara, Y. Neuromuscular function during knee extension exercise after cold water immersion. J. Physiol. Anthr. 2017, 36, 28. [Google Scholar] [CrossRef]
- Loro, W.A.; Thelen, M.D.; Rosenthal, M.D.; Stoneman, P.D.; Ross, M.D. The effects of cryotherapy on quadriceps electromyographic activity and isometric strength in patient in the early phases following knee surgery. J. Orthop. Surg. 2019, 27, 2309499019831454. [Google Scholar] [CrossRef]
- Hopkins, J.T. Knee joint effusion and cryotherapy alter lower chain kinetics and muscle activity. J. Athl. Train. 2006, 41, 177–184. [Google Scholar]
- Kinugasa, R.; Yoshida, K.; Horii, A. The effects of ice application over the vastus medialis on the activity of quadriceps femoris assessed by muscle function magnetic resonance imaging. J. Sports Med. Phys. Fit. 2005, 45, 360–364. [Google Scholar]
- Kinugasa, R.; Yoshida, K.; Watanabe, T.; Kuchiki, K.; Horii, A. Skin cooling alters the activation patterns of different heads of the quadriceps. Can. J. Appl. Physiol. Rev. Can. De Physiol. Appl. 2005, 30, 127–139. [Google Scholar] [CrossRef]
- Yona, M. Effects of cold stimulation of human skin on motor unit activity. Jpn. J. Physiol. 1997, 47, 341–348. [Google Scholar] [CrossRef]
- Feldpausch, J.E.; Blok, A.L.; Frederick, E.L.; Coburn, J.W.; Malek, M.H. The evolution of the physical work capacity at the fatigue threshold test: Past, Present, and Future. J. Strength Cond. Res. Natl. Strength Cond. Assoc. 2021, 35, 3529–3536. [Google Scholar] [CrossRef]
- Delahunty, E.T.; Bisset, L.M.; Kavanagh, J.J. Intracortical motor networks are affected in both the contralateral and ipsilateral hemisphere during single limb cold water immersion. Exp. Physiol. 2019, 104, 1296–1305. [Google Scholar] [CrossRef]
- Briscoe, M.J.; Forgach, M.S.; Trifan, E.; Malek, M.H. Validating the EMGFT from a single incremental cycling testing. Int. J. Sports Med. 2014, 35, 566–570. [Google Scholar]
- Eason, T.; Gavel, C.R.; Hawley, K.A.; Galen, S.S.; Malek, M.H. Reliability of the Log-Transformed EMG Amplitude-Power Output Relationship for Incremental Knee-Extensor Ergometry. Muscle Nerve 2015, 52, 428–434. [Google Scholar] [CrossRef]
- Galen, S.S.; Malek, M.H. A single electromyographic testing point is valid to monitor neuromuscular fatigue during continuous exercise. J. Strength Cond. Res. Natl. Strength Cond. Assoc. 2014, 28, 2754–2759. [Google Scholar] [CrossRef]
- Ferris, J.R.; Tomlinson, M.A.; Ward, T.N.; Pepin, M.E.; Malek, M.H. Reduced Electromyographic Fatigue Threshold after Performing a Cognitive Fatiguing Task. J. Strength Cond. Res. Natl. Strength Cond. Assoc. 2021, 35, 267–274. [Google Scholar] [CrossRef]
- Elhaj, H.M.; Imam, O.; Page, B.W.; Vitale, J.M.; Malek, M.H. Perceived consumption of a high dose caffeine drink delays neuromuscular fatigue. J. Strength Cond. Res./Natl. Strength Cond. Assoc. 2022, 36, 1185–1190. [Google Scholar] [CrossRef]
- Richardson, R.S.; Frank, L.R.; Haseler, L.J. Dynamic knee-extensor and cycle exercise: Functional MRI of muscular activity. Int. J. Sports Med. 1998, 19, 182–187. [Google Scholar] [CrossRef]
- Khan, F.L.; Lawal, J.M.; Kapture, D.O.; Swingle, J.D.; Malek, M.H. Revisiting the single visit protocol for determining the electromyographic fatigue threshold. J. Strength Cond. Res. Natl. Strength Cond. Assoc. 2017, 31, 3503–3507. [Google Scholar] [CrossRef]
- Malek, M.H.; Coburn, J.W.; Marelich, W.D. Advanced Statistics for Kinesiology and Exercise Science: A Practical Guide to ANOVA and Regression Analyses; Routledge: Abingdon, UK; New York, NY, USA, 2018; p. 152. [Google Scholar]
- Nadler, S.F.; Weingand, K.; Kruse, R.J. The physiologic basis and clinical application of cryotherapy and thermotherapy for the pain practitioner. Pain Physician 2004, 7, 395–399. [Google Scholar] [CrossRef]
- Gregson, W.; Black, M.A.; Jones, H.; Milson, J.; Morton, J.; Dawson, B.; Atkinson, G.; Green, D.J. Influence of cold water immersion on limb and cutaneous blood flow at rest. Am. J. Sports Med. 2011, 39, 1316–1323. [Google Scholar] [CrossRef]
- Ho, S.S.; Coel, M.N.; Kagawa, R.; Richardson, A.B. The effects of ice on blood flow and bone metabolism in knees. Am. J. Sports Med. 1994, 22, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Merrick, M.A.; McBrier, N.M. Progression of secondary injury after musculoskeletal trauma-a window of opportunity? J. Sport Rehabil. 2010, 19, 380–388. [Google Scholar] [CrossRef][Green Version]
- Liao, X.; Xu, X. The effect of cold therapy combined with ERAS in the postoperative care of patients undergoing total knee arthroplasty. Am. J. Transl. Res. 2022, 14, 3154–3163. [Google Scholar] [PubMed]
- Pournot, H.; Bieuzen, F.; Louis, J.; Mounier, R.; Fillard, J.R.; Barbiche, E.; Hausswirth, C. Time-course of changes in inflammatory response after whole-body cryotherapy multi exposures following severe exercise. PLoS ONE 2011, 6, e22748. [Google Scholar] [CrossRef]
- Bleakley, C.; McDonough, S.; Gardner, E.; Baxter, G.D.; Hopkins, J.T.; Davison, G.W. Cold-water immersion (cryotherapy) for preventing and treating muscle soreness after exercise. Cochrane Database Syst. Rev. 2012, 2012, CD008262. [Google Scholar] [CrossRef]
- Mortensen, S.P.; Dawson, E.A.; Yoshiga, C.C.; Dalsgaard, M.K.; Damsgaard, R.; Secher, N.H.; Gonzalez-Alonso, J. Limitations to systemic and locomotor limb muscle oxygen delivery and uptake during maximal exercise in humans. J. Physiol. 2005, 566, 273–285. [Google Scholar] [CrossRef]
- Richardson, R.S. Oxygen transport: Air to muscle cell. Med. Sci. Sports Exerc. 1998, 30, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.S.; Knight, D.R.; Poole, D.C.; Kurdak, S.S.; Hogan, M.C.; Grassi, B.; Wagner, P.D. Determinants of maximal exercise VO2 during single leg knee-extensor exercise in humans. Am. J. Physiol. 1995, 268, H1453–H1461. [Google Scholar] [CrossRef] [PubMed]
- Rud, B.; Hallén, J. Is the balance between skeletal muscular metabolic capacity and oxygen supply capacity the same in endurance trained and untrained subjects? Eur. J. Appl. Physiol. 2009, 105, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Noble, E.B.; Pilarski, J.M.; Vora, H.K.; Zuniga, J.M.; Malek, M.H. Log-Transformed Electromyography Amplitude-Power Output Relationship: Single-Leg Knee-Extensor Versus Single-Leg Cycle Ergometry. J. Strength Cond. Res. Natl. Strength Cond. Assoc. 2019, 33, 1311–1319. [Google Scholar] [CrossRef]
- Bremer, N.; Peoples, G.; Hasler, B.; Litzenburg, R.; Johnson, A.; Malek, M.H. Repeated Incremental Workbouts Separated by 1 h Increase the Electromyographic Fatigue Threshold. J. Strength Cond. Res./Natl. Strength Cond. Assoc. 2021, 35, 1397–1402. [Google Scholar] [CrossRef]
- Rupp, K.A.; Selkow, N.M.; Parente, W.R.; Ingersoll, C.D.; Weltman, A.L.; Saliba, S.A. The effect of cold water immersion on 48-h performance testing in collegiate soccer players. J. Strength Cond. Res. Natl. Strength Cond. Assoc. 2012, 26, 2043–2050. [Google Scholar] [CrossRef]
- Peiffer, J.J.; Abbiss, C.R.; Nosaka, K.; Peake, J.M.; Laursen, P.B. Effect of cold water immersion after exercise in the heat on muscle function, body temperatures, and vessel diameter. J. Sci. Med. Sport Sports Med. Aust. 2009, 12, 91–96. [Google Scholar] [CrossRef]
- Sims, S.T.; Heather, A.K. Myths and Methodologies: Reducing scientific design ambiguity in studies comparing sexes and/or menstrual cycle phases. Exp. Physiol. 2018, 103, 1309–1317. [Google Scholar] [CrossRef]
- Schaumberg, M.A.; Jenkins, D.G.; Janse de Jonge, X.A.K.; Emmerton, L.M.; Skinner, T.L. Three-step method for menstrual and oral contraceptive cycle verification. J. Sci. Med. Sport Sports Med. Aust. 2017, 20, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, J.L.; Deaner, R.O. Men and women differ in their interest and willingness to participate in exercise and sports science research. Scand. J. Med. Sci. Sports 2023, 33, 1850–1865. [Google Scholar] [CrossRef] [PubMed]
- Harlan, K.G.; Merucci, R.B.; Weaver, J.J.; Windle, T.C.; Malek, M.H. Preexhaustion exercise differentially influences neuromuscular fatigue based on habitual physical activity history. J. Strength Cond. Res. Natl. Strength Cond. Assoc. 2021, 35, 739–745. [Google Scholar] [CrossRef] [PubMed]


| Control Visit | Cold Therapy Visit | |||
|---|---|---|---|---|
| Physiological Outcomes | Trial 1 | Trial 2 | Trial 1 | Trial 2 |
| Maximal power output (W) | 60 ± 7 | 63 ± 7 | 64 ± 7 | 67 ± 7 |
| PWCFT (W) | 33 ± 4 | 33 ± 4 | 34 ± 4 | 32 ± 4 |
| PWCFT (% maximal PO) | 58 ± 5 | 54 ± 5 | 53 ± 5 | 48 ± 5 |
| Heart rate at end-exercise (bpm) | 144 ± 12 | 144 ± 14 | 147 ± 12 | 147 ± 14 |
| Heart rate at end-exercise (% predicted) | 73 ± 6 | 73 ± 7 | 75 ± 6 | 75 ± 7 |
| Modified RPE at end-exercise (0–10 scale) | 9 ± 0 | 10 ± 0 * | 10 ± 0 | 10 ± 0 |
| Control Visit | Cold Therapy Visit | |||
|---|---|---|---|---|
| Surface Muscle Temperature (°C) | Trial 1 | Trial 2 | Trial 1 | Trial 2 |
| Prior to start of the exercise | 30.3 ± 0.4 | 32.5 ± 0.6 * | 29.5 ± 0.4 * | 26.1 ± 0.6 *† |
| Immediately at end-exercise | 32.3 ± 0.5 | 32.6 ± 0.7 | 31.8 ± 0.5 | 30.5 ± 0.7 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maasri, R.E.; Jarvie, J.R.; Karski, J.S.; Smith, L.J.; Malek, M.H. The Influence of Cold Therapy on the Physical Working Capacity at the Electromyographic Threshold for Consecutive Exercise Sessions. Bioengineering 2024, 11, 292. https://doi.org/10.3390/bioengineering11030292
Maasri RE, Jarvie JR, Karski JS, Smith LJ, Malek MH. The Influence of Cold Therapy on the Physical Working Capacity at the Electromyographic Threshold for Consecutive Exercise Sessions. Bioengineering. 2024; 11(3):292. https://doi.org/10.3390/bioengineering11030292
Chicago/Turabian StyleMaasri, Rami E., Jonathan R. Jarvie, Jacob S. Karski, Logan J. Smith, and Moh H. Malek. 2024. "The Influence of Cold Therapy on the Physical Working Capacity at the Electromyographic Threshold for Consecutive Exercise Sessions" Bioengineering 11, no. 3: 292. https://doi.org/10.3390/bioengineering11030292
APA StyleMaasri, R. E., Jarvie, J. R., Karski, J. S., Smith, L. J., & Malek, M. H. (2024). The Influence of Cold Therapy on the Physical Working Capacity at the Electromyographic Threshold for Consecutive Exercise Sessions. Bioengineering, 11(3), 292. https://doi.org/10.3390/bioengineering11030292







