The Humanization and Maturation of an Anti-PrPc Antibody
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Gene and Peptide and Expression of Protein
2.2. Construction of Vectors
2.3. Cell Culture
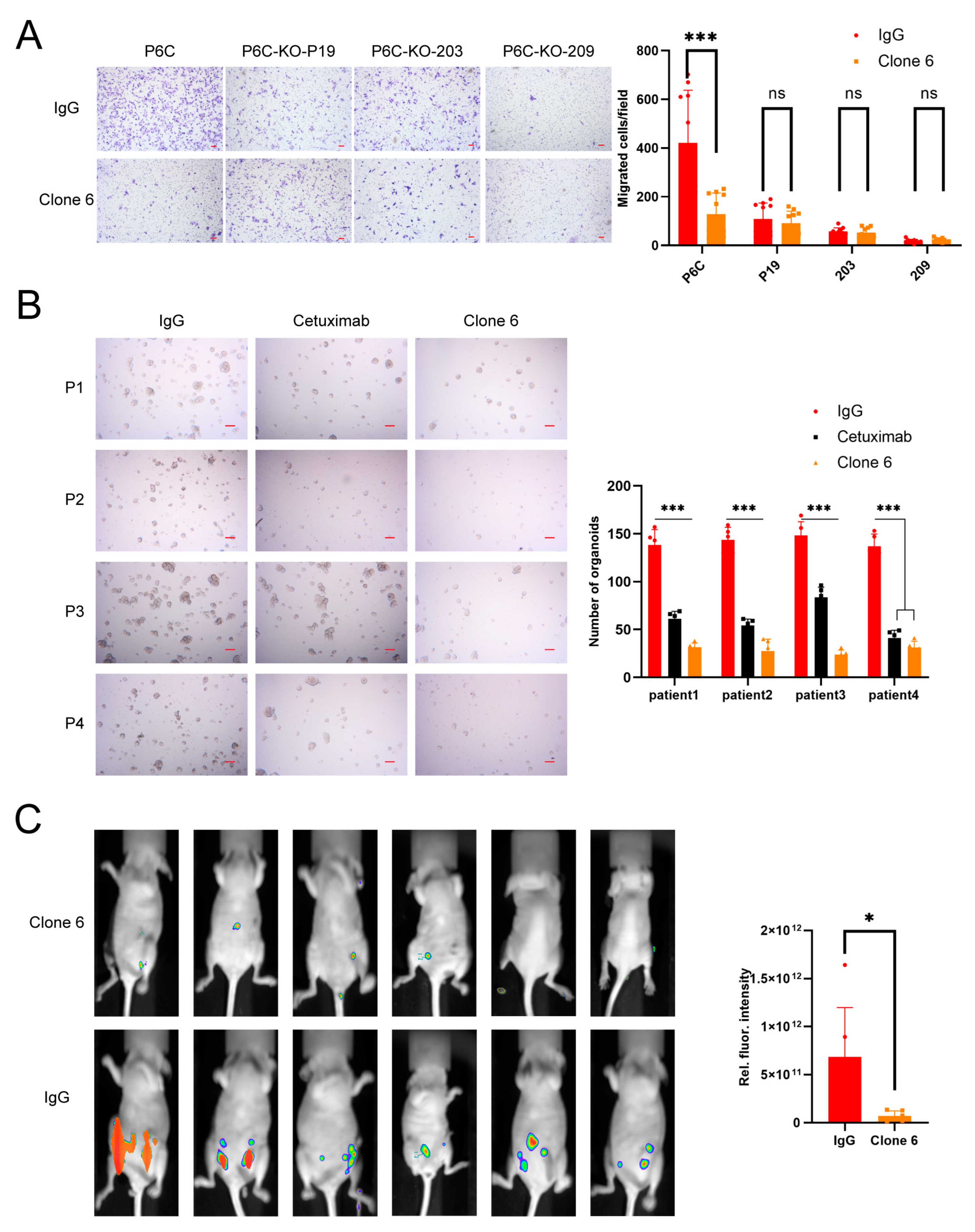
2.4. Transwell Assay
2.5. Organoid Formation
2.6. Tumorigenesis in Orthotopic Xenograft Models
2.7. Transfection and Stable Cell Line Establishment
2.8. Antibody Humanized Design
2.9. Affinity Maturation and Flow Cytometry
2.10. Antibody Affinity Measurement
2.11. Western Blotting
2.12. Immunohistochemistry
3. Results
3.1. Murine Anti-PrPc Antibody Clone 6 Can Inhibit Migration and Metastasis
3.2. Humanization and Maturation of Clone 6 Antibody
3.3. The Efficiency of the Inhibition of Tumor Cell Migration of the Matured Antibodies
3.4. The Specificity and Sensitivity of 4AA Antibody
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grimaldi, I.; Leser, F.S.; Janeiro, J.M.; da Rosa, B.G.; Campanelli, A.C.; Romão, L.; Lima, F.R.S. The multiple functions of PrP(C) in physiological, cancer, and neurodegenerative contexts. J. Mol. Med. 2022, 100, 1405–1425. [Google Scholar] [CrossRef]
- Prusiner, S.B. Novel proteinaceous infectious particles cause scrapie. Science 1982, 216, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; You, H.; Liu, F.; An, H.; Shi, Y.; Yu, Q.; Fan, D. Differentially expressed gene profiles between multidrug resistant gastric adenocarcinoma cells and their parental cells. Cancer Lett. 2002, 185, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhao, L.; Liang, J.; Liu, J.; Shi, Y.; Liu, N.; Zhang, G.; Jin, H.; Gao, J.; Xie, H.; et al. Cellular prion protein promotes invasion and metastasis of gastric cancer. FASEB J. 2006, 20, 1886–1888. [Google Scholar] [CrossRef]
- Luo, G.; Wang, W.; Wu, Q.; Lu, Y.; Su, T.; Gu, N.; Li, K.; Wang, J.; Du, R.; Zhao, X.; et al. MGr1-Antigen/37 kDa laminin receptor precursor promotes cellular prion protein induced multi-drug-resistance of gastric cancer. Oncotarget 2017, 8, 71630–71641. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Du, L.; Rao, G.; Wang, H.; Li, B.; Tian, W.; Cui, J.; He, L.; Laffin, B.; Tian, X.; Hao, C.; et al. CD44-positive cancer stem cells expressing cellular prion protein contribute to metastatic capacity in colorectal cancer. Cancer Res. 2013, 73, 2682–2694. [Google Scholar] [CrossRef] [PubMed]
- Antonacopoulou, A.G.; Palli, M.; Marousi, S.; Dimitrakopoulos, F.I.; Kyriakopoulou, U.; Tsamandas, A.C.; Scopa, C.D.; Papavassiliou, A.G.; Kalofonos, H.P. Prion protein expression and the M129V polymorphism of the PRNP gene in patients with colorectal cancer. Mol. Carcinog. 2010, 49, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yu, S.; Nakamura, F.; Yin, S.; Xu, J.; Petrolla, A.A.; Singh, N.; Tartakoff, A.; Abbott, D.W.; Xin, W.; et al. Binding of pro-prion to filamin A disrupts cytoskeleton and correlates with poor prognosis in pancreatic cancer. J. Clin. Investig. 2009, 119, 2725–2736. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, S.; Huang, D.; Cui, M.; Hu, H.; Zhang, L.; Wang, W.; Parameswaran, N.; Jackson, M.; Osborne, B.; et al. Cellular Prion Protein Mediates Pancreatic Cancer Cell Survival and Invasion through Association with and Enhanced Signaling of Notch1. Am. J. Pathol. 2016, 186, 2945–2956. [Google Scholar] [CrossRef][Green Version]
- Bianchini, M.; Giambelluca, M.A.; Scavuzzo, M.C.; Di Franco, G.; Guadagni, S.; Palmeri, M.; Furbetta, N.; Gianardi, D.; Funel, N.; Pollina, L.E.; et al. The occurrence of prion protein in surgically resected pancreatic adenocarcinoma. Pancreatology 2020, 20, 1218–1225. [Google Scholar] [CrossRef]
- Meslin, F.; Conforti, R.; Mazouni, C.; Morel, N.; Tomasic, G.; Drusch, F.; Yacoub, M.; Sabourin, J.C.; Grassi, J.; Delaloge, S.; et al. Efficacy of adjuvant chemotherapy according to Prion protein expression in patients with estrogen receptor-negative breast cancer. Ann. Oncol. 2007, 18, 1793–1798. [Google Scholar] [CrossRef] [PubMed]
- Wiegmans, A.P.; Saunus, J.M.; Ham, S.; Lobb, R.; Kutasovic, J.R.; Dalley, A.J.; Miranda, M.; Atkinson, C.; Foliaki, S.T.; Ferguson, K.; et al. Secreted cellular prion protein binds doxorubicin and correlates with anthracycline resistance in breast cancer. JCI Insight 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Cho, Y.A.; Kim, E.; Choe, J.Y.; Park, J.W.; Lee, J.; Lee, J.W.; Moon, S.H.; Kim, Y.S.; Kim, S.E.; et al. Cellular Prion Protein Is Closely Associated with Early Recurrence and Poor Survival in Patients with Hepatocellular Carcinoma. Diagnostics 2022, 12, 1635. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, M.; Zheng, Y.; Hu, Z.; Liang, J.; Bi, G.; Bian, Y.; Sui, Q.; Zhan, C.; Lin, M.; et al. Identification and analysis of a prognostic ferroptosis and iron-metabolism signature for esophageal squamous cell carcinoma. J. Cancer 2022, 13, 1611–1622. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.H.; Santos, T.G.; Rodrigues, B.R.; Queiroz-Hazarbassanov, N.; Cunha, I.W.; Wasilewska-Sampaio, A.P.; Costa-Silva, B.; Marchi, F.A.; Bleggi-Torres, L.F.; Sanematsu, P.I.; et al. Disruption of prion protein-HOP engagement impairs glioblastoma growth and cognitive decline and improves overall survival. Oncogene 2015, 34, 3305–3314. [Google Scholar] [CrossRef]
- Lin, S.C.; Lin, C.H.; Shih, N.C.; Liu, H.L.; Wang, W.C.; Lin, K.Y.; Liu, Z.Y.; Tseng, Y.J.; Chang, H.K.; Lin, Y.C.; et al. Cellular prion protein transcriptionally regulated by NFIL3 enhances lung cancer cell lamellipodium formation and migration through JNK signaling. Oncogene 2020, 39, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.; Sin, M.J.; Kim, M.J.; Kim, H.J.; Kim, Y.S.; Choi, E.K.; Kim, M.Y. Involvement of Cellular Prion Protein in Invasion and Metastasis of Lung Cancer by Inducing Treg Cell Development. Biomolecules 2021, 11, 285. [Google Scholar] [CrossRef]
- Go, G.; Lee, S.H. The Cellular Prion Protein: A Promising Therapeutic Target for Cancer. Int. J. Mol. Sci. 2020, 21, 9208. [Google Scholar] [CrossRef]
- Ding, M.; Chen, Y.; Lang, Y.; Cui, L. The Role of Cellular Prion Protein in Cancer Biology: A Potential Therapeutic Target. Front Oncol. 2021, 11, 742949. [Google Scholar] [CrossRef]
- Mouillet-Richard, S.; Ghazi, A.; Laurent-Puig, P. The Cellular Prion Protein and the Hallmarks of Cancer. Cancers 2021, 13, 5032. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Okazaki, I.M.; Eto, T.; Kinoshita, K.; Muramatsu, M.; Nagaoka, H.; Honjo, T. AID enzyme-induced hypermutation in an actively transcribed gene in fibroblasts. Science 2002, 296, 2033–2036. [Google Scholar] [CrossRef]
- Luo, R.; Zhao, Y.; Fan, Y.; An, L.; Jiang, T.; Ma, S.; Hang, H. High efficiency CHO cell display-based antibody maturation. Sci. Rep. 2020, 10, 8102. [Google Scholar] [CrossRef]
- Yang, S.Y.; Fugmann, S.D.; Schatz, D.G. Control of gene conversion and somatic hypermutation by immunoglobulin promoter and enhancer sequences. J. Exp. Med. 2006, 203, 2919–2928. [Google Scholar] [CrossRef] [PubMed]
- Larijani, M.; Martin, A. The biochemistry of activation-induced deaminase and its physiological functions. Semin. Immunol. 2012, 24, 255–263. [Google Scholar] [CrossRef]
- Tang, Z.; Ma, J.; Zhang, W.; Gong, C.; He, J.; Wang, Y.; Yu, G.; Yuan, C.; Wang, X.; Sun, Y.; et al. The Role of Prion Protein Expression in Predicting Gastric Cancer Prognosis. J. Cancer 2016, 7, 984–990. [Google Scholar] [CrossRef][Green Version]
- de Wit, M.; Jimenez, C.R.; Carvalho, B.; Belien, J.A.; Delis-van Diemen, P.M.; Mongera, S.; Piersma, S.R.; Vikas, M.; Navani, S.; Pontén, F.; et al. Cell surface proteomics identifies glucose transporter type 1 and prion protein as candidate biomarkers for colorectal adenoma-to-carcinoma progression. Gut 2012, 61, 855–864. [Google Scholar] [CrossRef]
- Zanusso, G.; Liu, D.; Ferrari, S.; Hegyi, I.; Yin, X.; Aguzzi, A.; Hornemann, S.; Liemann, S.; Glockshuber, R.; Manson, J.C.; et al. Prion protein expression in different species: Analysis with a panel of new mAbs. Proc. Natl. Acad. Sci. USA 1998, 95, 8812–8816. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Pan, Y.L.; Ning, X.X.; Sun, L.J.; Lan, M.; Hong, L.; Du, J.P.; Liu, N.; Liu, C.J.; Qiao, T.D.; et al. Overexpression of PrPC and its antiapoptosis function in gastric cancer. Tumour Biol. 2006, 27, 84–91. [Google Scholar] [CrossRef]
- Zhou, L.; Shang, Y.; Liu, C.; Li, J.; Hu, H.; Liang, C.; Han, Y.; Zhang, W.; Liang, J.; Wu, K. Overexpression of PrPc, combined with MGr1-Ag/37LRP, is predictive of poor prognosis in gastric cancer. Int. J. Cancer 2014, 135, 2329–2337. [Google Scholar] [CrossRef]
- McEwan, J.F.; Windsor, M.L.; Cullis-Hill, S.D. Antibodies to prion protein inhibit human colon cancer cell growth. Tumour Biol. 2009, 30, 141–147. [Google Scholar] [CrossRef]
- Esiri, M.M.; Carter, J.; Ironside, J.W. Prion protein immunoreactivity in brain samples from an unselected autopsy population: Findings in 200 consecutive cases. Neuropathol. Appl. Neurobiol. 2000, 26, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, S.; Miao, Z.; Liu, Y.; Liu, X.; Xiao, Z.X.; Cao, Y. AbRSA: A robust tool for antibody numbering. Protein Sci. 2019, 28, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Eswar, N.; Webb, B.; Marti-Renom, M.A.; Madhusudhan, M.S.; Eramian, D.; Shen, M.Y.; Pieper, U.; Sali, A. Comparative protein structure modeling using Modeller. Curr. Protoc. Bioinform. 2006, 16, 172–177. [Google Scholar] [CrossRef]
- Webb, B.; Sali, A. Protein Structure Modeling with MODELLER. Methods Mol. Biol. 2021, 2199, 239–255. [Google Scholar] [CrossRef]
- Zong, F.; Long, C.; Hu, W.; Chen, S.; Dai, W.; Xiao, Z.X.; Cao, Y. Abalign: A comprehensive multiple sequence alignment platform for B-cell receptor immune repertoires. Nucleic Acids Res. 2023, 51, W17–W24. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, N.; Zhao, Y.; Hang, H. Coupling recombinase-mediated cassette exchange with somatic hypermutation for antibody affinity maturation in CHO cells. Biotechnol. Bioeng. 2016, 113, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.H.; Liu, H.M.; Li, B.W.; Hao, J.J.; Yang, Y.L.; Wang, M.R.; Wang, X.H.; Wang, J.; Jin, H.J.; Du, L.; et al. Establishment of a human colorectal cancer cell line P6C with stem cell properties and resistance to chemotherapeutic drugs. Acta Pharmacol. Sin. 2013, 34, 793–804. [Google Scholar] [CrossRef]
- Liu, T.; Zwingman, T.; Li, R.; Pan, T.; Wong, B.S.; Petersen, R.B.; Gambetti, P.; Herrup, K.; Sy, M.S. Differential expression of cellular prion protein in mouse brain as detected with multiple anti-PrP monoclonal antibodies. Brain Res. 2001, 896, 118–129. [Google Scholar] [CrossRef]
- Ferrer, I.; Puig, B.; Blanco, R.; Martí, E. Prion protein deposition and abnormal synaptic protein expression in the cerebellum in Creutzfeldt-Jakob disease. Neuroscience 2000, 97, 715–726. [Google Scholar] [CrossRef]
- Valente, K.N.; Levy, N.E.; Lee, K.H.; Lenhoff, A.M. Applications of proteomic methods for CHO host cell protein characterization in biopharmaceutical manufacturing. Curr. Opin. Biotechnol. 2018, 53, 144–150. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, Y.G.; Lee, G.M. CHO cells in biotechnology for production of recombinant proteins: Current state and further potential. Appl. Microbiol. Biotechnol. 2012, 93, 917–930. [Google Scholar] [CrossRef] [PubMed]




| Affinity to Different Forms of PrPc KD (M) | |||
|---|---|---|---|
| Antibody | PrPc-35aa | PrPc-Fc | 293T-PrPc |
| Clone 6 | 3.14 × 10−10 | 2.09 × 10−8 | N.D. |
| HAb 6 | 2.92 × 10−9 | 1.24 × 10−8 | N.D. |
| A16D-A97V | 1.13 × 10−10 | 2.06 × 10−9 | 1.01 × 10−9 |
| 4AA | 2.81 × 10−10 | 2.03 × 10−10 | 9.27 × 10−11 |
| Mutations | Count |
|---|---|
| A40T | 27 |
| Q39K plus S76I | 10 |
| W insertion between W104 and G105 | 4 |
| Q39K | 2 |
| S76I | 2 |
| A40T plus W insertion between W104 and G105 | 2 |
| APGK insertion between Q39 and A40 | 2 |
| Wild type | 2 |
| A40T plus S76I | 1 |
| C96S | 1 |
| S76N | 1 |
| L4V | 1 |
| A40T F59L | 1 |
| S76N plus S58N | 1 |
| Antibody Mutants | KD (M) | Kon (1/Ms) | koff (1/s) |
|---|---|---|---|
| Parent clone (A16D-A97V) | 2.06 × 10−9 | 1.17 × 104 | 2.42 × 10−5 |
| 4AA | 2.03 × 10−10 | 1.12 × 104 | 2.27 × 10−6 |
| A40T | 1.33 × 10−9 | 1.11 × 104 | 1.48 × 10−5 |
| A40T plus S76I | 2.47 × 10−9 | 9.09 × 103 | 2.25 × 10−5 |
| Q39K | 3.14 × 10−9 | 1.08 × 104 | 3.40 × 10−5 |
| Q39K plus S76I | 3.55 × 10−9 | 1.05 × 104 | 3.72 × 10−5 |
| S76I | 4.86 × 10−9 | 1.29 × 104 | 6.27 × 10−5 |
| S76N plus S58N | 6.91 × 10−9 | 1.20 × 104 | 8.29 × 10−5 |
| S76N | 7.76 × 10−9 | 1.18 × 104 | 9.18 × 10−5 |
| W insertion between W104 and G105 | 3.17 × 10−8 | 4.73 × 103 | 1.50 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Ran, F.; Du, L.; Wang, X.; Liu, L.; Liu, J.; Chen, Q.; Cao, Y.; Bi, L.; Hang, H. The Humanization and Maturation of an Anti-PrPc Antibody. Bioengineering 2024, 11, 242. https://doi.org/10.3390/bioengineering11030242
Zhang C, Ran F, Du L, Wang X, Liu L, Liu J, Chen Q, Cao Y, Bi L, Hang H. The Humanization and Maturation of an Anti-PrPc Antibody. Bioengineering. 2024; 11(3):242. https://doi.org/10.3390/bioengineering11030242
Chicago/Turabian StyleZhang, Cheng, Fanlei Ran, Lei Du, Xiaohui Wang, Lei Liu, Jinming Liu, Quan Chen, Yang Cao, Lijun Bi, and Haiying Hang. 2024. "The Humanization and Maturation of an Anti-PrPc Antibody" Bioengineering 11, no. 3: 242. https://doi.org/10.3390/bioengineering11030242
APA StyleZhang, C., Ran, F., Du, L., Wang, X., Liu, L., Liu, J., Chen, Q., Cao, Y., Bi, L., & Hang, H. (2024). The Humanization and Maturation of an Anti-PrPc Antibody. Bioengineering, 11(3), 242. https://doi.org/10.3390/bioengineering11030242






