Optimizing CT Esophagography: Ex Vivo Study on Contrast Ratios, Image Quality, and Dual-Energy Benefits
Abstract
1. Introduction
2. Materials and Methods
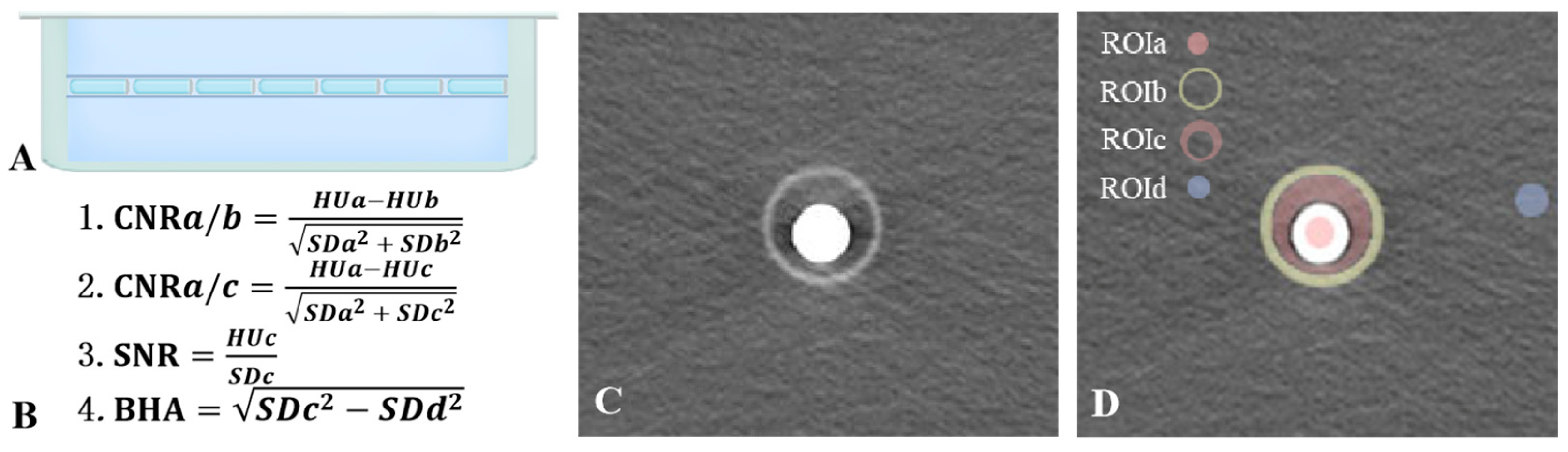
2.1. Ex Vivo Study
2.2. Image Quality Evaluation
- ROIa: The contrast agent within the Eppendorf tube, representing esophageal contrast material with high CT attenuation.
- ROIb: The wall of the hollow plastic tube, mimicking other slightly high-density tissues in the mediastinum.
- ROIc: The transition zone between the contrast agent and the surrounding plastic tube, simulating the region adjacent to the esophagus in a clinical setting.
- ROId: The background region outside the tube, representing a reference background area required for image quality analysis.
2.3. Statistical Analysis
3. Results
3.1. Image Quality Evaluation of Conventional CT Scan
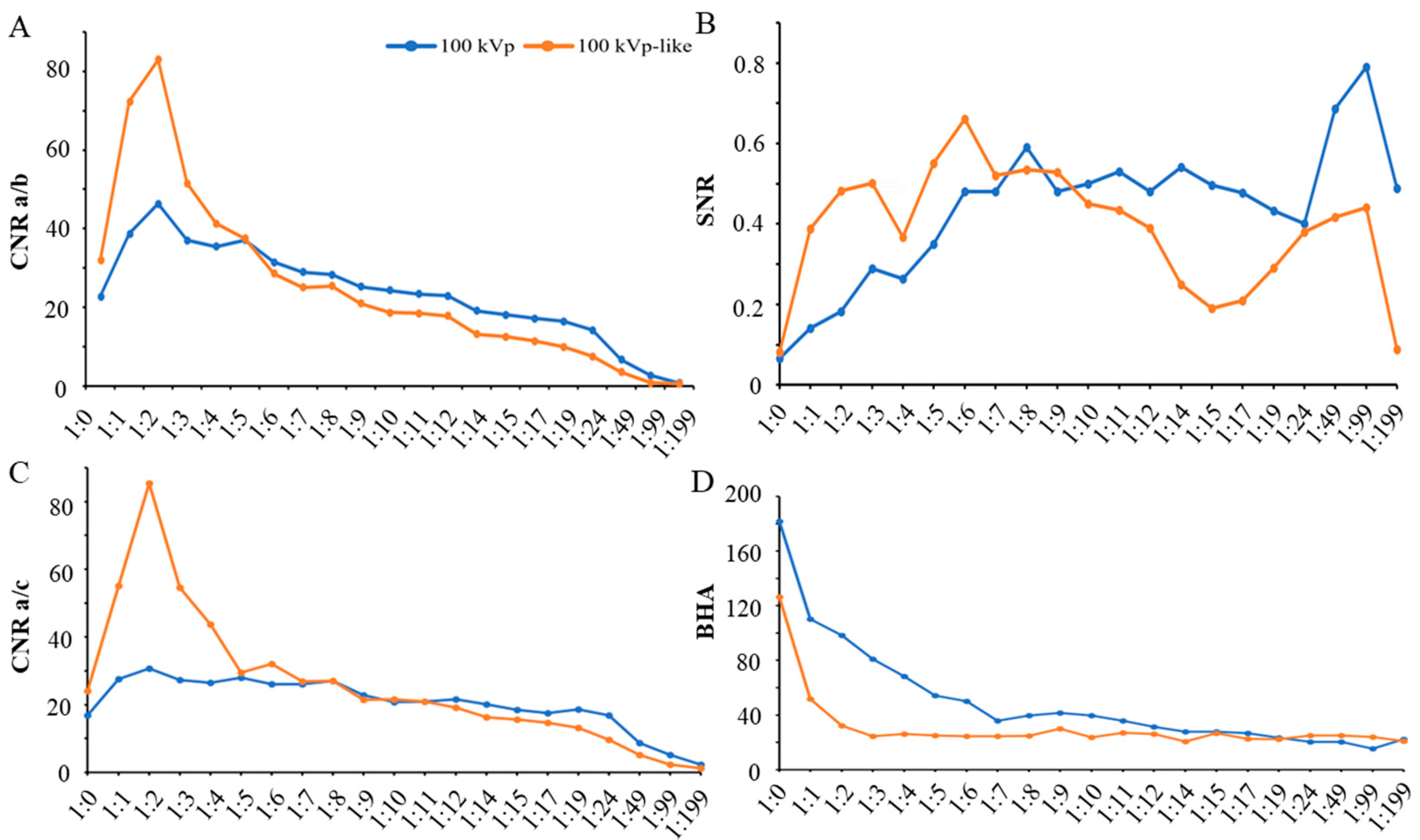
3.1.1. Intra-Reader Agreement and Objective Image Quality Analysis
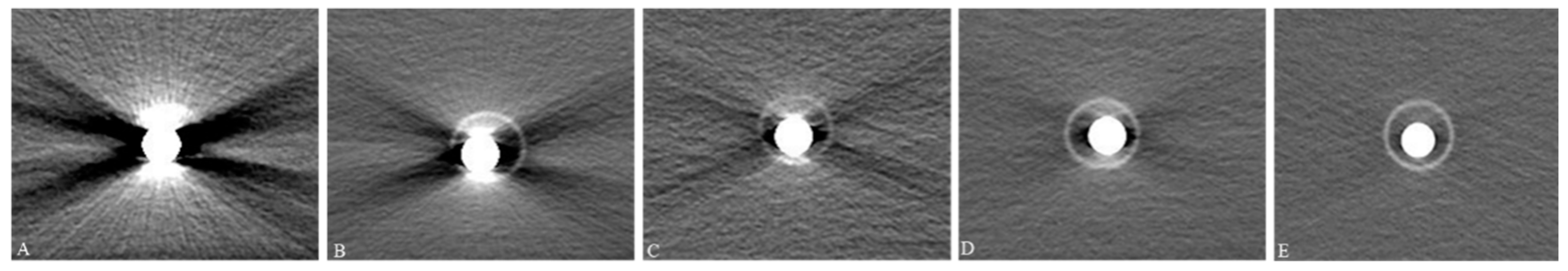
3.1.2. Subjective Image Quality Assessment and Dilution Ratios
3.2. Advantages and Limitations of Dual-Energy CT for CT Esophagography
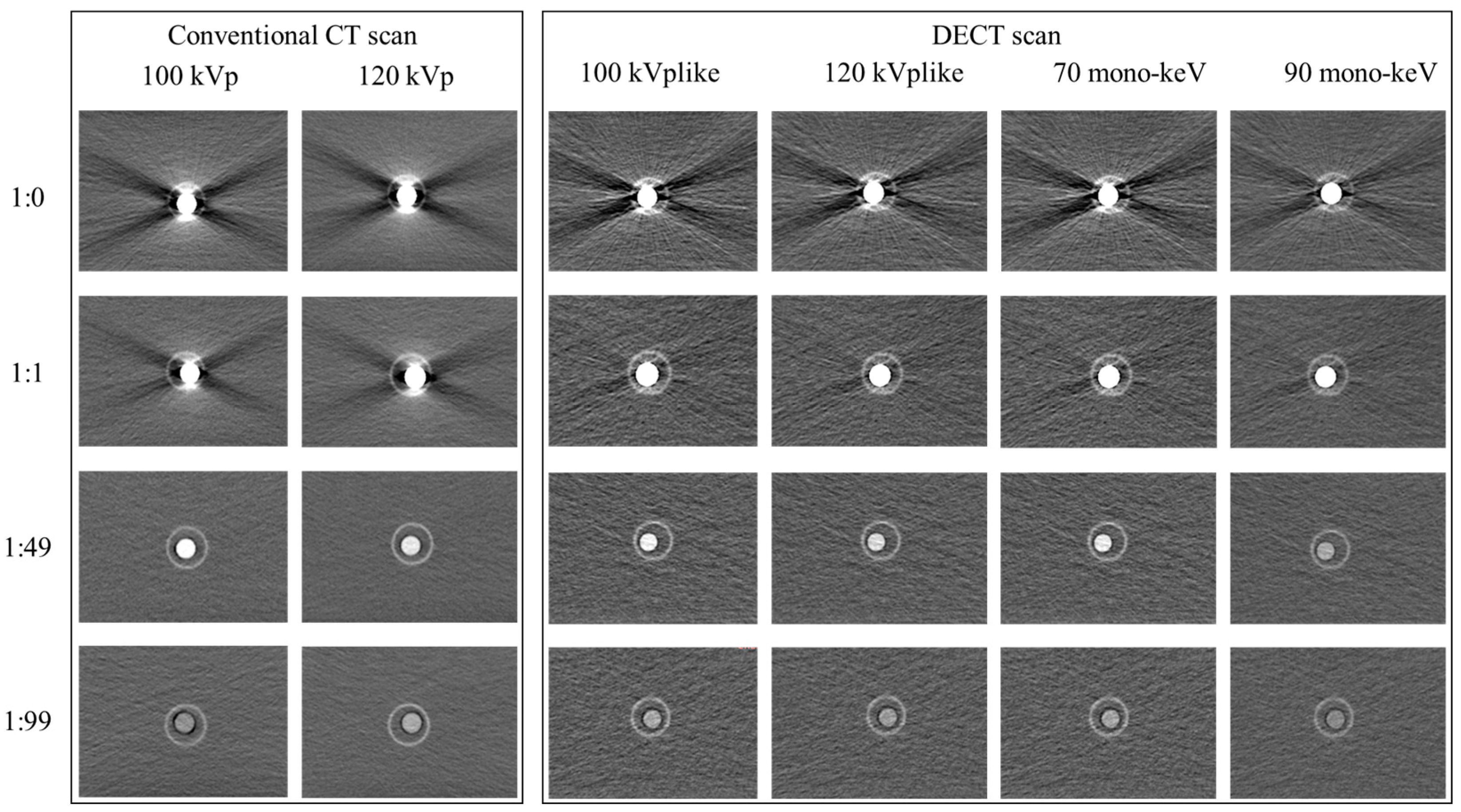
3.2.1. Additional Value of Dual-Energy CT (DECT) in Improvement of Image Quality
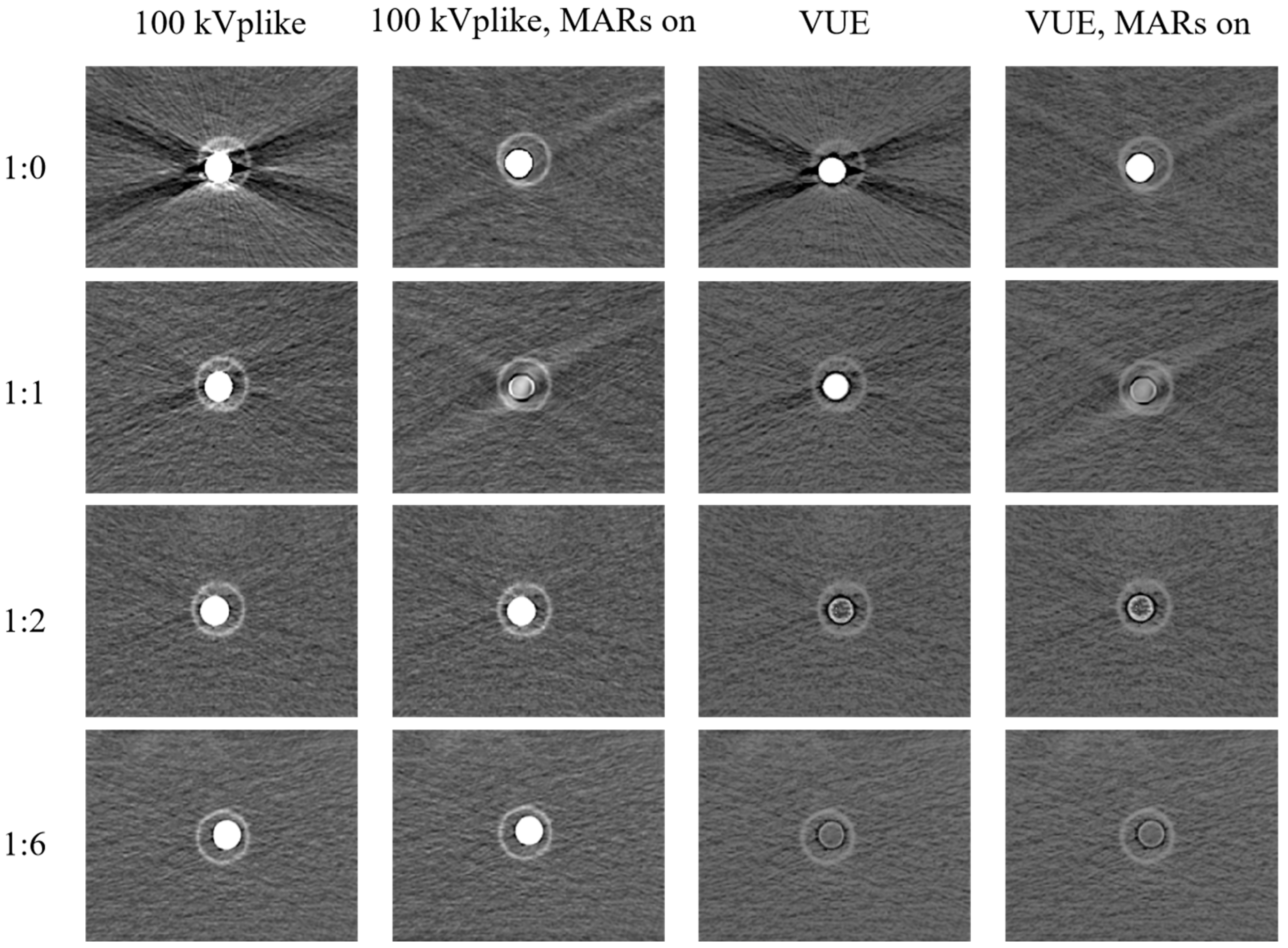
3.2.2. Unsuitability of VUE Images and MAR Reconstruction for CT Esophagography
4. Discussion
4.1. Image Quality Evaluation of CT Esophagography
4.2. Dilution Recommendations for the Preparation of Oral Contrast Agent in CT Esophagography
4.3. Additional Value of DECT in Improving Image Quality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeVivo, A.; Sheng, A.Y.; Koyfman, A.; Long, B. High risk and low prevalence diseases: Esophageal perforation. Am. J. Emerg. Med. 2022, 53, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.J.; Levenson, R.B.; Lee, K.S. Diagnostic Utility of CT and Fluoroscopic Esophagography for Suspected Esophageal Perforation in the Emergency Department. AJR Am. J. Roentgenol. 2020, 215, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Sdralis, E.K.; Petousis, S.; Rashid, F.; Lorenzi, B.; Charalabopoulos, A. Epidemiology, diagnosis, and management of esophageal perforations: Systematic review. Dis. Esophagus 2017, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kaman, L.; Iqbal, J.; Kundil, B.; Kochhar, R. Management of Esophageal Perforation in Adults. Gastroenterol. Res. 2010, 3, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.D.; Carucci, L.R.; Bartel, T.B.; Cash, B.D.; Chang, K.J.; Feig, B.W.; Fowler, K.J.; Garcia, E.M.; Kambadakone, A.R.; Lambert, D.L.; et al. ACR Appropriateness Criteria(®) Dysphagia. J. Am. Coll. Radiol. 2019, 16, S104–S115. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Norton-Gregory, A.A.; Kulkarni, N.M.; O’Connor, S.D.; Budovec, J.J.; Zorn, A.P.; Desouches, S.L. CT Esophagography for Evaluation of Esophageal Perforation. Radiogr. A Rev. Publ. Radiol. Soc. N. Am. Inc 2021, 41, 447–461. [Google Scholar] [CrossRef]
- Fadoo, F.; Ruiz, D.E.; Dawn, S.K.; Webb, W.R.; Gotway, M.B. Helical CT esophagography for the evaluation of suspected esophageal perforation or rupture. Am. J. Roentgenol. 2004, 182, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Chirica, M.; Kelly, M.D.; Siboni, S.; Aiolfi, A.; Riva, C.G.; Asti, E.; Ferrari, D.; Leppäniemi, A.; Ten Broek, R.P.G.; Brichon, P.Y.; et al. Esophageal emergencies: WSES guidelines. World J. Emerg. Surg. 2019, 14, 26. [Google Scholar] [CrossRef]
- Tonolini, M.; Bianco, R. Spontaneous esophageal perforation (Boerhaave syndrome): Diagnosis with CT-esophagography. J. Emergencies Trauma Shock 2013, 6, 58–60. [Google Scholar] [CrossRef]
- Suarez-Poveda, T.; Morales-Uribe, C.H.; Sanabria, A.; Llano-Sánchez, A.; Valencia-Delgado, A.M.; Rivera-Velázquez, L.F.; Bedoya-Ospina, J.F. Diagnostic performance of CT esophagography in patients with suspected esophageal rupture. Emerg. Radiol. 2014, 21, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Hogan, B.A.; Winter, D.C.; Broe, D.; Broe, P.; Lee, M.J. Prospective trial comparing contrast swallow, computed tomography and endoscopy to identify anastomotic leak following oesophagogastric surgery. Surg. Endosc. 2008, 22, 767–771. [Google Scholar] [CrossRef]
- Upponi, S.; Ganeshan, A.; D’Costa, H.; Betts, M.; Maynard, N.; Bungay, H.; Slater, A. Radiological detection of post-oesophagectomy anastomotic leak—A comparison between multidetector CT and fluoroscopy. Br. J. Radiol. 2008, 81, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Strauss, C.; Mal, F.; Perniceni, T.; Bouzar, N.; Lenoir, S.; Gayet, B.; Palau, R. Computed tomography versus water-soluble contrast swallow in the detection of intrathoracic anastomotic leak complicating esophagogastrectomy (Ivor Lewis): A prospective study in 97 patients. Ann. Surg. 2010, 251, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Palacio, D.; Marom, E.M.; Correa, A.; Betancourt-Cuellar, S.L.; Hofstetter, W.L. Diagnosing conduit leak after esophagectomy for esophageal cancer by computed tomography leak protocol and standard esophagram: Is old school still the best? Clin. Imaging 2018, 51, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Conradie, W.J.; Gebremariam, F.A. Can computed tomography esophagography reliably diagnose traumatic penetrating upper digestive tract injuries? Clin. Imaging 2015, 39, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Lantos, J.E.; Levine, M.S.; Rubesin, S.E.; Lau, C.T.; Torigian, D.A. Comparison between esophagography and chest computed tomography for evaluation of leaks after esophagectomy and gastric pull-through. J. Thorac. Imaging 2013, 28, 121–128. [Google Scholar] [CrossRef]
- Little, B.P.; Mendoza, D.P.; Fox, A.; Wu, C.C.; Ackman, J.B.; Shepard, J.A.; Muniappan, A.; Digumarthy, S.R. Direct and indirect CT imaging features of esophago-airway fistula in adults. J. Thorac. Dis. 2020, 12, 3157–3166. [Google Scholar] [CrossRef] [PubMed]
- Masarapu, V.; Xia, E.; Son, H. Esophageal emergencies: Another important cause of acute chest pain. Insights Imaging 2020, 11, 109. [Google Scholar] [CrossRef]
- Evans, B.A.; Craig, W.Y.; Cinelli, C.M.; Siegel, S.G. CT esophagogram in the emergency setting: Typical findings and suggested workflow. Emerg. Radiol. 2024, 31, 33–44. [Google Scholar] [CrossRef]
- Saksobhavivat, N.; Shanmuganathan, K.; Boscak, A.R.; Sliker, C.W.; Stein, D.M.; Bodanapally, U.K.; Archer-Arroyo, K.; Miller, L.A.; Fleiter, T.R.; Alexander, M.T.; et al. Diagnostic accuracy of triple-contrast multi-detector computed tomography for detection of penetrating gastrointestinal injury: A prospective study. Eur. Radiol. 2016, 26, 4107–4120. [Google Scholar] [CrossRef]
- Terrazas, M.; Marjon, L.; Geter, M.; Schwartz, J.; Thompson, W. Esophagography and chest CT for detection of perforated esophagus: What factors influence accuracy? Abdom. Radiol. 2020, 45, 2980–2988. [Google Scholar] [CrossRef]
- Szucs-Farkas, Z.; Verdun, F.R.; von Allmen, G.; Mini, R.L.; Vock, P. Effect of X-ray tube parameters, iodine concentration, and patient size on image quality in pulmonary computed tomography angiography: A chest-phantom-study. Investig. Radiol. 2008, 43, 374–381. [Google Scholar] [CrossRef]
- Andreini, D.; Pontone, G.; Mushtaq, S.; Bartorelli, A.L.; Conte, E.; Bertella, E.; Baggiano, A.; Annoni, A.; Formenti, A.; Ballerini, G.; et al. Coronary stent evaluation with coronary computed tomographic angiography: Comparison between low-osmolar, high-iodine concentration iomeprol-400 and iso-osmolar, lower-iodine concentration iodixanol-320. J. Cardiovasc. Comput. Tomogr. 2014, 8, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Dunet, V.; Bernasconi, M.; Hajdu, S.D.; Meuli, R.A.; Daniel, R.T.; Zerlauth, J.B. Impact of metal artifact reduction software on image quality of gemstone spectral imaging dual-energy cerebral CT angiography after intracranial aneurysm clipping. Neuroradiology 2017, 59, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.Z.; Miao, F.; Li, J.Y.; Dong, H.P.; Shen, Y.; Chen, K.M. High-definition CT Gemstone spectral imaging of the brain: Initial results of selecting optimal monochromatic image for beam-hardening artifacts and image noise reduction. J. Comput. Assist. Tomogr. 2011, 35, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Im, A.L.; Lee, Y.H.; Bang, D.H.; Yoon, K.H.; Park, S.H. Dual energy CT in patients with acute abdomen; is it possible for virtual non-enhanced images to replace true non-enhanced images? Emerg. Radiol. 2013, 20, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Wang, Q.; Hu, X.; Li, Z.; Hu, D.; Shen, Y. Utility of CT in detecting and monitoring subphrenic jujube pits: A retrospective cross-sectional study of clinical cases and ex vivo experiments. Quant. Imaging Med. Surg. 2022, 12, 5114–5128. [Google Scholar] [CrossRef] [PubMed]
- Sawall, S.; Klein, L.; Amato, C.; Wehrse, E.; Dorn, S.; Maier, J.; Heinze, S.; Schlemmer, H.P.; Ziener, C.H.; Uhrig, M.; et al. Iodine contrast-to-noise ratio improvement at unit dose and contrast media volume reduction in whole-body photon-counting CT. Eur. J. Radiol. 2020, 126, 108909. [Google Scholar] [CrossRef]
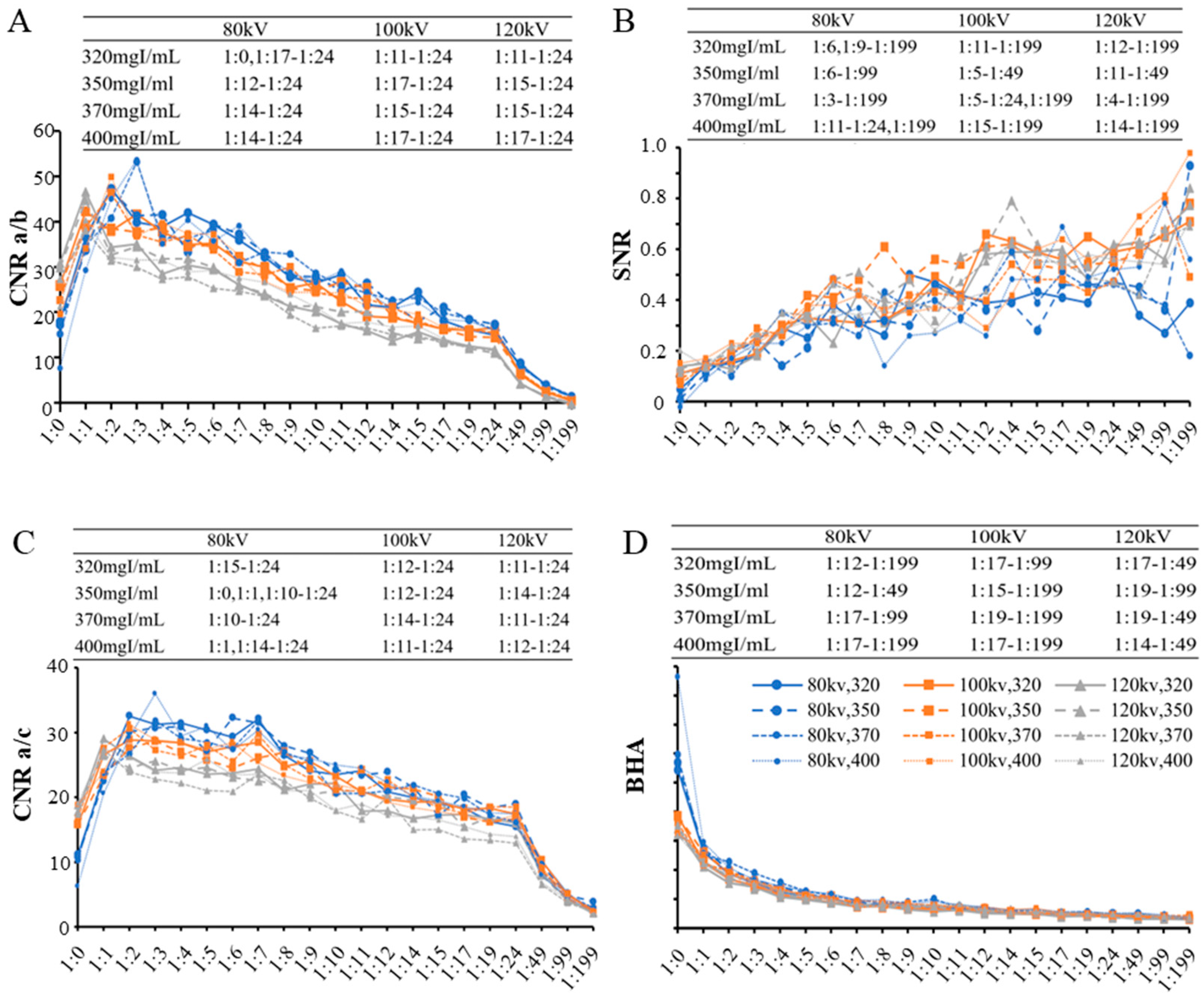
| Concentration Specifications | 80 kVp | 100 kVp | 120 kVp |
|---|---|---|---|
| 320 mgI/mL | 1:15~1:24 | 1:12~1:24 | 1:12~1:24 |
| 350 mgI/mL | 1:12~1:24 | 1:15~1:24 | 1:15~1:24 |
| 370 mgI/mL | 1:14~1:24 | 1:15~1:24 | 1:15~1:24 |
| 400 mgI/mL | 1:14~1:24 | 1:17~1:24 | 1:14~1:24 |
| Concentration Specifications | 80 kVp | 100 kVp | 120 kVp |
|---|---|---|---|
| 320 mgI/mL | 1:4~1:49 | 1:4~1:49 | 1:3~1:24 |
| 350 mgI/mL | 1:6~1:49 | 1:4~1:49 | 1:3~1:24 |
| 370 mgI/mL | 1:6~1:49 | 1:4~1:49 | 1:3~1:24 |
| 400 mgI/mL | 1:6~1:49 | 1:4~1:49 | 1:3~1:24 |
| Concentration Specifications | 80 kVp | 100 kVp | 120 kVp | Dual-Energy Scan 80/140 (Fast Switching) |
|---|---|---|---|---|
| 320 mgI/mL | 1:6~1:19 | 1:6~1:19 | 1:5~1:24 | 1:1~1:24 |
| 350 mgI/mL | 1:10~1:19 | 1:8~1:19 | 1:6~1:19 | 1:1~1:24 |
| 370 mgI/mL | 1:10~1:24 | 1:8~1:19 | 1:6~1:19 | 1:1~1:24 |
| 400 mgI/mL | 1:11~1:24 | 1:8~1:19 | 1:6~1:19 | 1:1~1:24 |
| Scanning Voltage | Concentration Specifications 1 | Volume 2 |
|---|---|---|
| 80 kV | 320 mgI/mL 320 mgI/mL, 370 mgI/mL 400 mgI/mL | 5~14 mL 5~9 mL 5~8 mL |
| 100 kV | 320 mgI/mL 350 mgI/mL, 370 mgI/mL, 400 mgI/mL | 5~14 mL 5~11 mL |
| 120 kV | 320 mgI/mL 350 mgI/mL, 370 mgI/mL, 400 mgI/mL | 5~16 mL 5~14 mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, L.; Chen, X.; Jiang, Y.; Wang, Y.; Hu, X.; Hu, D.; Li, Z.; Shen, Y. Optimizing CT Esophagography: Ex Vivo Study on Contrast Ratios, Image Quality, and Dual-Energy Benefits. Bioengineering 2024, 11, 1300. https://doi.org/10.3390/bioengineering11121300
Hao L, Chen X, Jiang Y, Wang Y, Hu X, Hu D, Li Z, Shen Y. Optimizing CT Esophagography: Ex Vivo Study on Contrast Ratios, Image Quality, and Dual-Energy Benefits. Bioengineering. 2024; 11(12):1300. https://doi.org/10.3390/bioengineering11121300
Chicago/Turabian StyleHao, Luwen, Xin Chen, Yuchen Jiang, Yufan Wang, Xuemei Hu, Daoyu Hu, Zhen Li, and Yaqi Shen. 2024. "Optimizing CT Esophagography: Ex Vivo Study on Contrast Ratios, Image Quality, and Dual-Energy Benefits" Bioengineering 11, no. 12: 1300. https://doi.org/10.3390/bioengineering11121300
APA StyleHao, L., Chen, X., Jiang, Y., Wang, Y., Hu, X., Hu, D., Li, Z., & Shen, Y. (2024). Optimizing CT Esophagography: Ex Vivo Study on Contrast Ratios, Image Quality, and Dual-Energy Benefits. Bioengineering, 11(12), 1300. https://doi.org/10.3390/bioengineering11121300








