An Observational Study on the Prediction of Range of Motion in Soldiers Diagnosed with Patellar Tendinopathy Using Ultrasound Shear Wave Elastography
Abstract
1. Introduction
2. Material and Methods
2.1. Patients
2.2. Exclusion Criteria
2.3. Conservative Treatment Protocol
2.4. Clinical Assessment
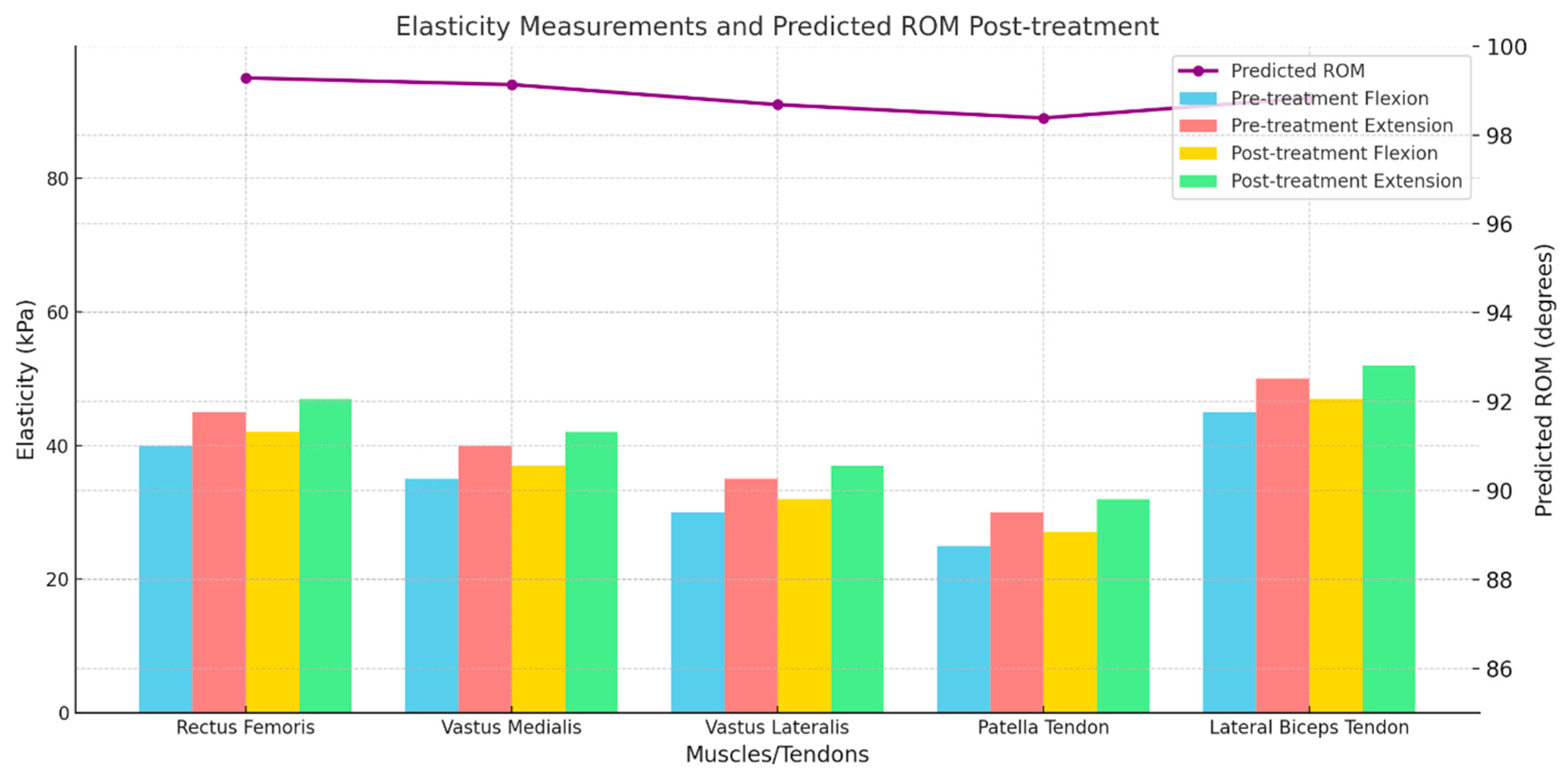
2.5. Elasticity Measurement by Shear Wave Elastography (SWE)
2.6. ROM Prediction
2.7. Statistical Methods
3. Results
3.1. Patient Characteristics
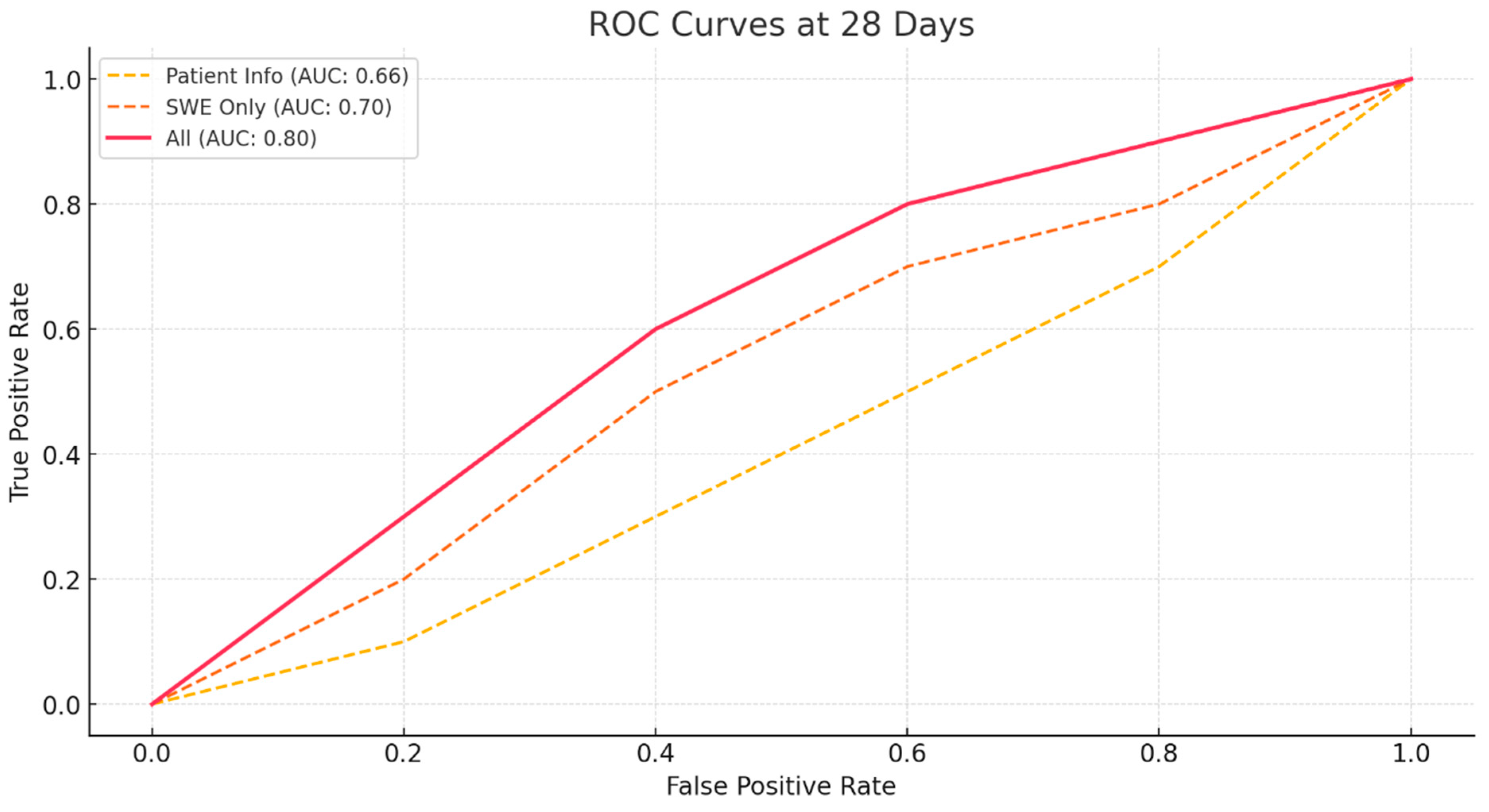
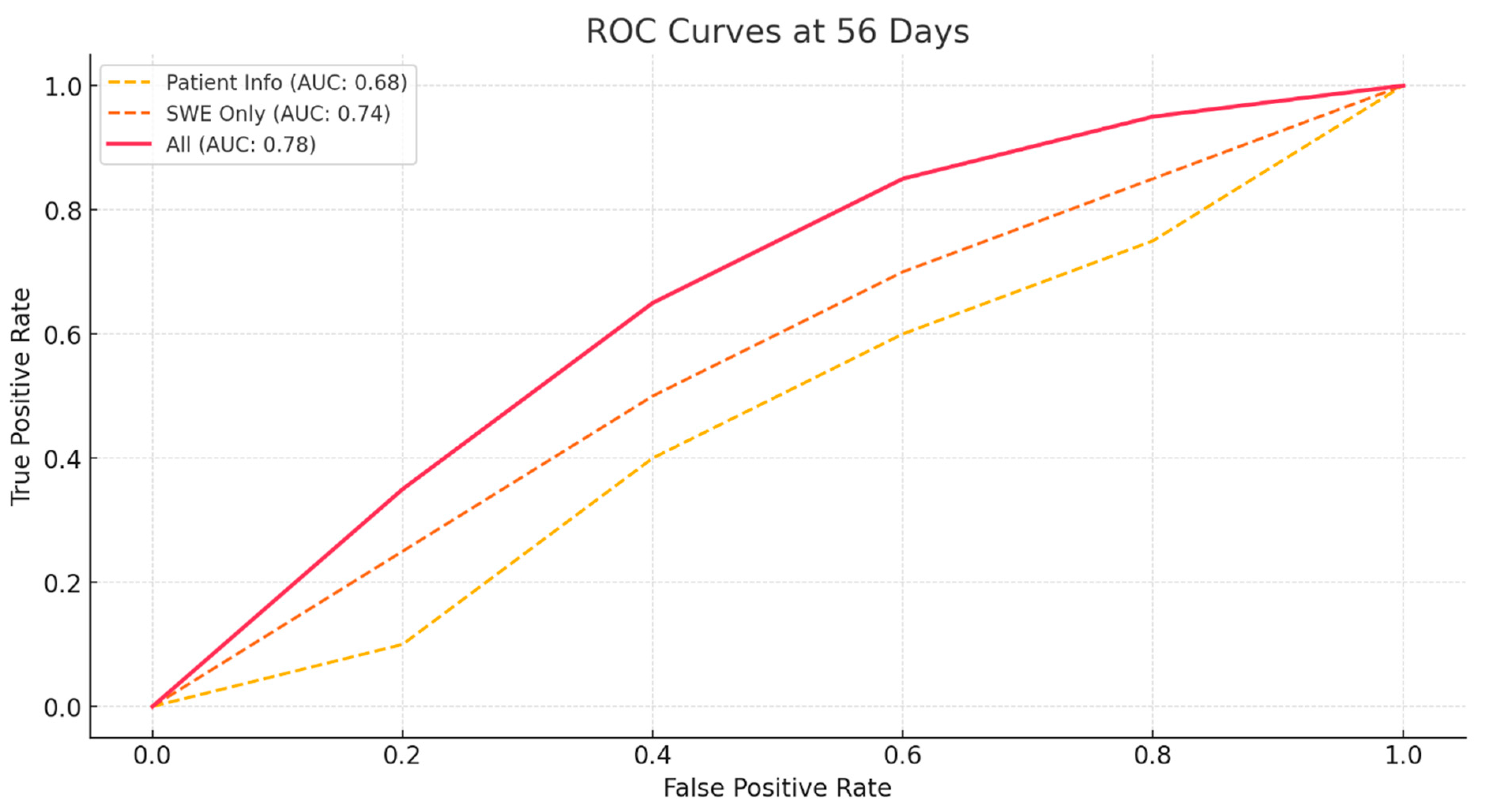
3.2. Prediction of ROM
- A model using only the patient’s basic information and preoperative ROM.
- A model using only the SWE values pre-treatment and 1-week post-treatment.
- A model combining all values from both the patient’s basic information, preoperative ROM, and SWE values.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PT | Patella Tendinopathy |
| SWE | Shear Wave Elastography |
| ROM | Range of motion |
| RMI | Reliability of the measurement |
| LR | Logistic regression |
| ROC | Receiver operating characteristic |
| AUC | Area Under ROC Curve |
References
- Alessandro, S.; Ilaria, F.; Chandra, B.; Barbero, M.; Falla, D.; Corrado, C.; Raciti, M.V.; Tarantino, F.; Lorenzo, P. Shear wave and strain sonoelastography for the evaluation of the Achilles tendon during isometric contractions. Insights Imaging 2021, 12, 26. [Google Scholar]
- Taljanovic, M.S.; Gimber, L.H.; Becker, G.W.; Latt, L.D.; Klauser, A.S.; Melville, D.M.; Gao, L.; Witte, R.S. Shear-wave elastography: Basic physics and musculoskeletal applications. Radiographics 2017, 37, 855–870. [Google Scholar] [CrossRef] [PubMed]
- Sarvazyan, A.P.; Rudenko, O.V.; Swanson, S.D.; Fowlkes, J.B.; Emelianov, S.Y. Shear wave elasticity imaging: A new ultrasonic technology of medical diagnostics. Ultrasound Med. Biol. 1998, 24, 1419–1435. [Google Scholar] [CrossRef] [PubMed]
- Prado-Costa, R.; Rebelo, J.; Monteiro-Barroso, J.; Preto, A.S. Ultrasound elastography: Compression elastography and shear-wave elastography in the assessment of tendon injury. Insights Imaging 2018, 9, 791–814. [Google Scholar] [CrossRef]
- Ooi, C.-C.; Malliaras, P.; Schneider, M.; Connell, D.A. “Soft, hard, or just right?” Applications and limitations of axial-strain sonoelastography and shear-wave elastography in the assessment of tendon injuries. Skelet. Radiol. 2014, 43, 1–12. [Google Scholar] [CrossRef]
- Flatres, A.; Aarab, Y.; Nougaret, S.; Garnier, F.; Larcher, R.; Amalric, M.; Klouche, K.; Etienne, P.; Subra, G.; Jaber, S. Real-time shear wave ultrasound elastography: A new tool for the evaluation of diaphragm and limb muscle stiffness in critically ill patients. Crit. Care 2020, 24, 34. [Google Scholar] [CrossRef]
- Arda, K.; Ciledag, N.; Aktas, E.; Arıbas, B.K.; Köse, K. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. Am. J. Roentgenol. 2011, 197, 532–536. [Google Scholar] [CrossRef]
- Ryu, J.; Jeong, W.K. Current status of musculoskeletal application of shear wave elastography. Ultrasonography 2017, 36, 185. [Google Scholar] [CrossRef]
- Creze, M.; Nordez, A.; Soubeyrand, M.; Rocher, L.; Maître, X.; Bellin, M.-F. Shear wave sonoelastography of skeletal muscle: Basic principles, biomechanical concepts, clinical applications, and future perspectives. Skelet. Radiol. 2018, 47, 457–471. [Google Scholar] [CrossRef]
- Ličen, U.; Opara, M.; Kozinc, Ž. The Agreement and Correlation Between Shear-Wave Elastography, Myotonometry, and Passive Joint Stiffness Measurements: A Brief Review. SN Compr. Clin. Med. 2024, 6, 27. [Google Scholar] [CrossRef]
- Theodorou, A.; Komnos, G.; Hantes, M. Patellar tendinopathy: An overview of prevalence, risk factors, screening, diagnosis, treatment and prevention. Arch. Orthop. Trauma Surg. 2023, 143, 6695–6705. [Google Scholar] [CrossRef] [PubMed]
- Nutarelli, S.; Lodi, C.M.T.D.; Cook, J.L.; Deabate, L.; Filardo, G. Epidemiology of patellar tendinopathy in athletes and the general population: A systematic review and meta-analysis. Orthop. J. Sports Med. 2023, 11, 23259671231173659. [Google Scholar] [CrossRef]
- De Vries, A.J.; Koolhaas, W.; Zwerver, J.; Diercks, R.L.; Nieuwenhuis, K.; Van Der Worp, H.; Brouwer, S.; Van Den Akker-Scheek, I. The impact of patellar tendinopathy on sports and work performance in active athletes. Res. Sports Med. 2017, 25, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Blazina, M.E.; Kerlan, R.K.; Jobe, F.W.; Carter, V.S.; Carlson, G.J. Jumper’s knee. Orthop. Clin. N. Am. 1973, 4, 665–678. [Google Scholar] [CrossRef]
- Challoumas, D.; Pedret, C.; Biddle, M.; Ng, N.Y.B.; Kirwan, P.; Cooper, B.; Nicholas, P.; Wilson, S.; Clifford, C.; Millar, N.L. Management of patellar tendinopathy: A systematic review and network meta-analysis of randomised studies. BMJ Open Sport Exerc. Med. 2021, 7, e001110. [Google Scholar] [CrossRef]
- Cocco, G.; Ricci, V.; Corvino, A.; Abate, M.; Vaccaro, A.; Bernabei, C.; Cantisani, V.; Vallone, G.; Caiazzo, C.; Caulo, M. Musculoskeletal disorders in padel: From biomechanics to sonography. J. Ultrasound 2024, 27, 335–354. [Google Scholar] [CrossRef]
- Kettunen, J.A.; Kvist, M.; Alanen, E.; Kujala, U.M. Long-term prognosis for Jumper’s knee in male athletes: Prospective follow-up study. Am. J. Sports Med. 2002, 30, 689–692. [Google Scholar] [CrossRef]
- Drakes, S.M. Patellar Tendinopathy: Diagnosis and Management. Curr. Phys. Med. Rehabil. Rep. 2023, 11, 344–351. [Google Scholar] [CrossRef]
- Muaidi, Q.I. Rehabilitation of patellar tendinopathy. J. Musculoskelet. Neuronal Interact. 2020, 20, 535. [Google Scholar]
- Meiburger, K.M.; Acharya, U.R.; Molinari, F. Automated localization and segmentation techniques for B-mode ultrasound images: A review. Comput. Biol. Med. 2018, 92, 210–235. [Google Scholar] [CrossRef]
- Dan, M.; Parr, W.; Broe, D.; Cross, M.; Walsh, W.R. Biomechanics of the knee extensor mechanism and its relationship to patella tendinopathy: A review. J. Orthop. Res. 2018, 36, 3105–3112. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Merchan, E.C. The treatment of patellar tendinopathy. J. Orthop. Traumatol. 2013, 14, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Leggett, R.W.; Williams, L.R. A reliability index for models. Ecol. Model. 1981, 13, 303–312. [Google Scholar] [CrossRef]
- Boateng, E.Y.; Abaye, D.A. A review of the logistic regression model with emphasis on medical research. J. Data Anal. Inf. Process. 2019, 7, 190. [Google Scholar] [CrossRef]
- Jayasekara, D.; Sooriyarachchi, M. A simulation based study for comparing tests associated with receiver operating characteristic (ROC) curves. Commun. Stat.-Simul. Comput. 2014, 43, 2444–2467. [Google Scholar] [CrossRef]
- Krakowski, P.; Karpiński, R.; Maciejewski, R.; Jonak, J. Evaluation of the diagnostic accuracy of MRI in detection of knee cartilage lesions using Receiver Operating Characteristic curves. J. Phys. Conf. Ser. 2021, 1736, 012028. [Google Scholar] [CrossRef]
- Gatz, M.; Betsch, M.; Bode, D.; Schweda, S.; Dirrichs, T.; Migliorini, F.; Tingart, M.; Quack, V. Intra individual comparison of unilateral Achilles tendinopathy using B-mode, power Doppler, ultrasound tissue characterization and shear wave elastography. J. Sports Med. Phys. Fit. 2020, 60, 1462–1469. [Google Scholar] [CrossRef]
- Aicale, R.; Oliviero, A.; Maffulli, N. Management of Achilles and patellar tendinopathy: What we know, what we can do. J. Foot Ankle Res. 2020, 13, 59. [Google Scholar] [CrossRef]
- Ng, J.W.G.; Price, K.; Deepak, S. Knee pain in children. Paediatr. Child Health 2019, 29, 521–527. [Google Scholar] [CrossRef]
- Visuri, T.; Pihlajamäki, H.; Mattila, V.; Kiuru, M. Elongated patellae at the final stage of Osgood–Schlatter disease: A radiographic study. Knee 2007, 14, 198–203. [Google Scholar] [CrossRef]
| Total | Group A (ROM > 120) | Group B (ROM < 120) | |
|---|---|---|---|
| Case (number) | 95 | 61 | 34 |
| Mean age (years) | 24.62 ± 3.49 | 24.88 ± 3.55 | 25.14 ± 4.02 |
| Sex (Male/female) | 86/9 | 56/5 | 30/4 |
| Average ROM | 122.79 ± 12.90 | 123.69 ± 12.11 | 121.17 ± 13.88 |
| Model | Accuracy (28 Days) | Sensitivity (28 Days) | Specificity (28 Days) | AUC (28 Days) | Accuracy (56 Days) | Sensitivity (56 Days) | Specificity (56 Days) | AUC (56 Days) |
|---|---|---|---|---|---|---|---|---|
| Patient Basic Info | 0.58 | 0.42 | 0.72 | 0.66 | 0.62 | 0.82 | 0.26 | 0.68 |
| SWE Only | 0.65625 | 0.56 | 0.74 | 0.7 | 0.68 | 0.84 | 0.41 | 0.74 |
| All | 0.76 | 0.72 | 0.79 | 0.8 | 0.79 | 0.92 | 0.56 | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-W.; Lee, D.-H.; Seo, Y.-C. An Observational Study on the Prediction of Range of Motion in Soldiers Diagnosed with Patellar Tendinopathy Using Ultrasound Shear Wave Elastography. Bioengineering 2024, 11, 1263. https://doi.org/10.3390/bioengineering11121263
Kim M-W, Lee D-H, Seo Y-C. An Observational Study on the Prediction of Range of Motion in Soldiers Diagnosed with Patellar Tendinopathy Using Ultrasound Shear Wave Elastography. Bioengineering. 2024; 11(12):1263. https://doi.org/10.3390/bioengineering11121263
Chicago/Turabian StyleKim, Min-Woo, Dong-Ha Lee, and Young-Chae Seo. 2024. "An Observational Study on the Prediction of Range of Motion in Soldiers Diagnosed with Patellar Tendinopathy Using Ultrasound Shear Wave Elastography" Bioengineering 11, no. 12: 1263. https://doi.org/10.3390/bioengineering11121263
APA StyleKim, M.-W., Lee, D.-H., & Seo, Y.-C. (2024). An Observational Study on the Prediction of Range of Motion in Soldiers Diagnosed with Patellar Tendinopathy Using Ultrasound Shear Wave Elastography. Bioengineering, 11(12), 1263. https://doi.org/10.3390/bioengineering11121263






