Influence of Bone Conditions on the Accuracy of Implant Placement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Selection Criteria
2.2. Clinical Procedure
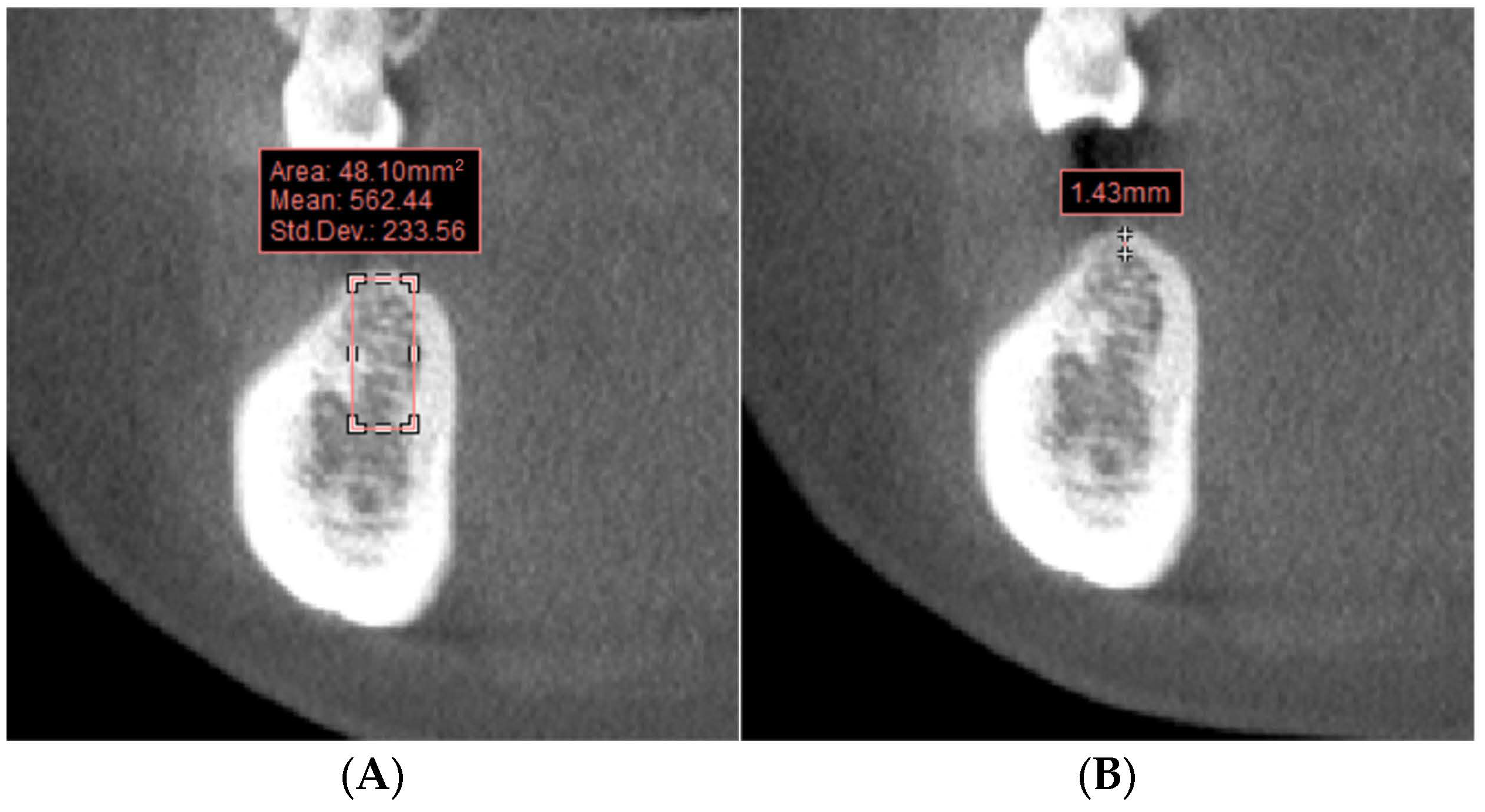
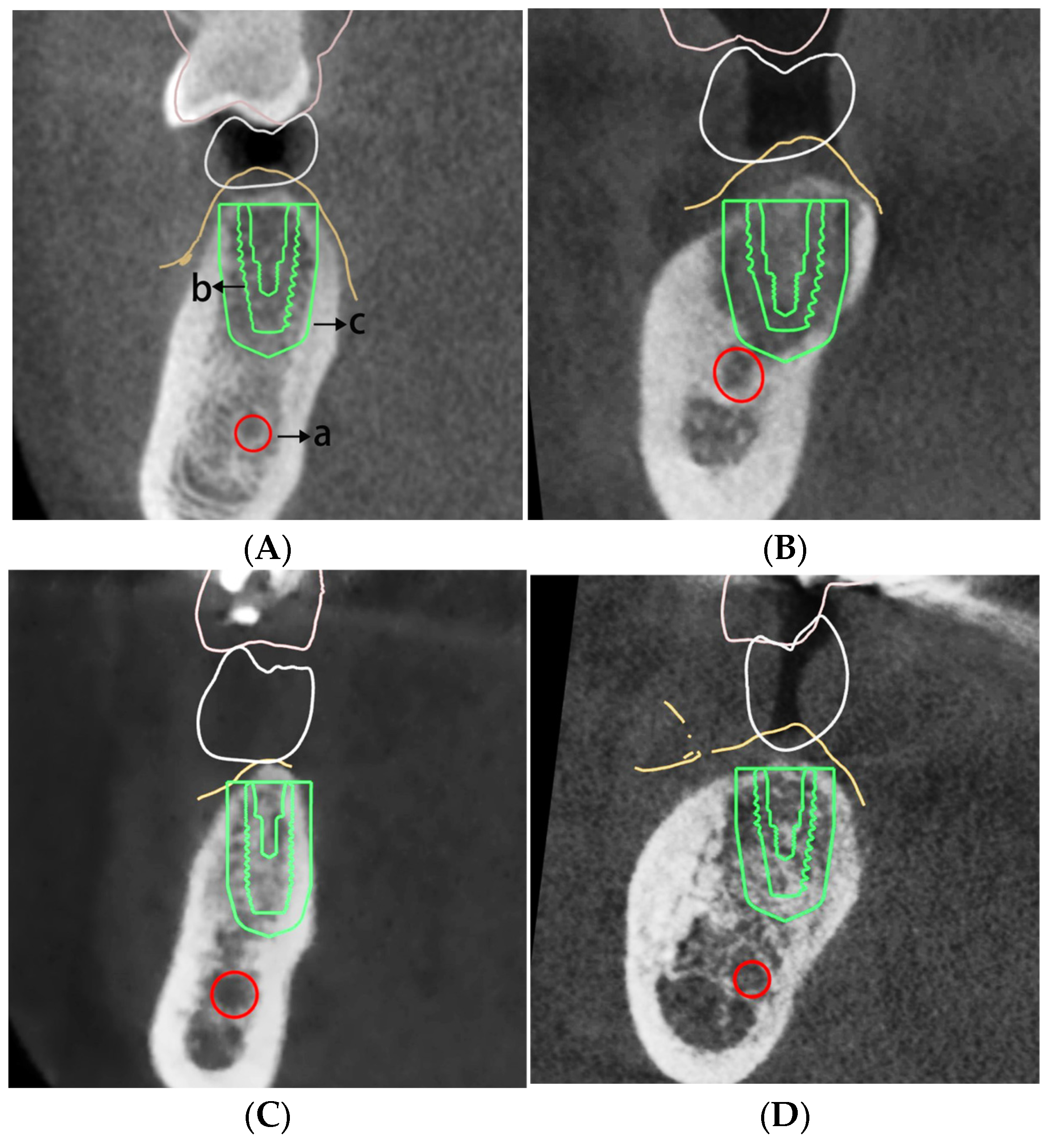
2.3. Bone Condition Measurement
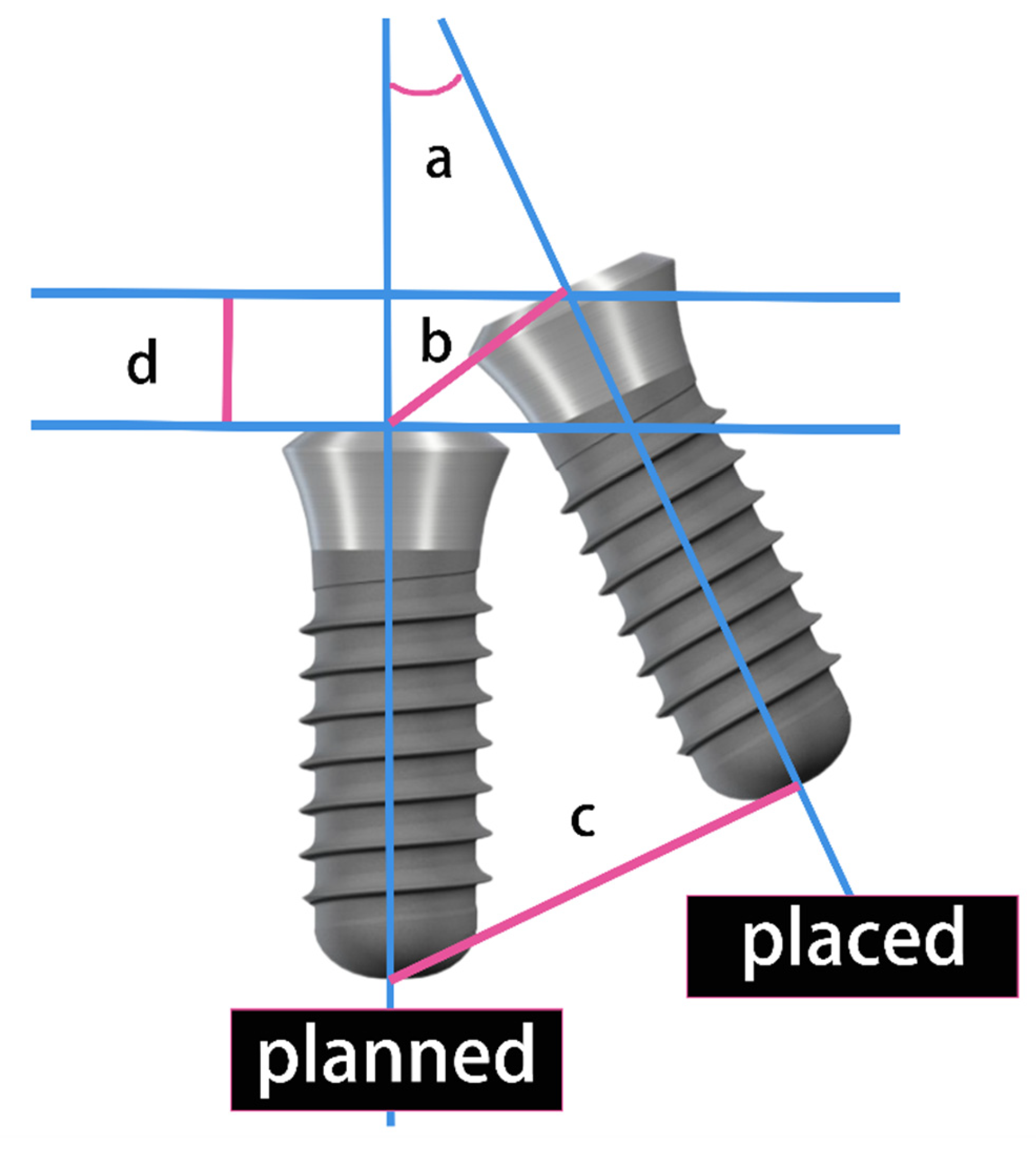
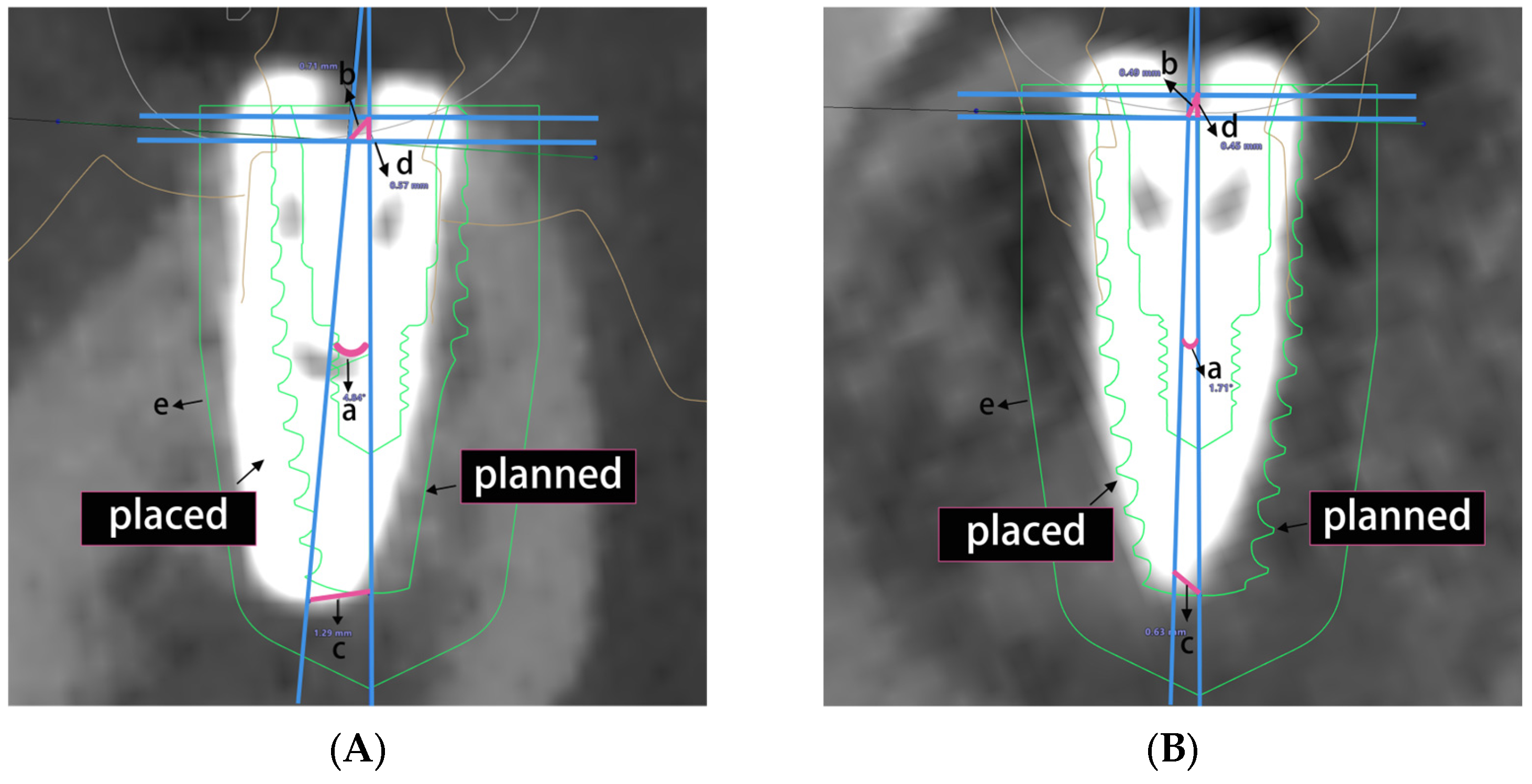
2.4. Implant Deviation Measurement
2.5. Statistical Analysis
3. Results
3.1. Overall Accuracy of Implant Placement
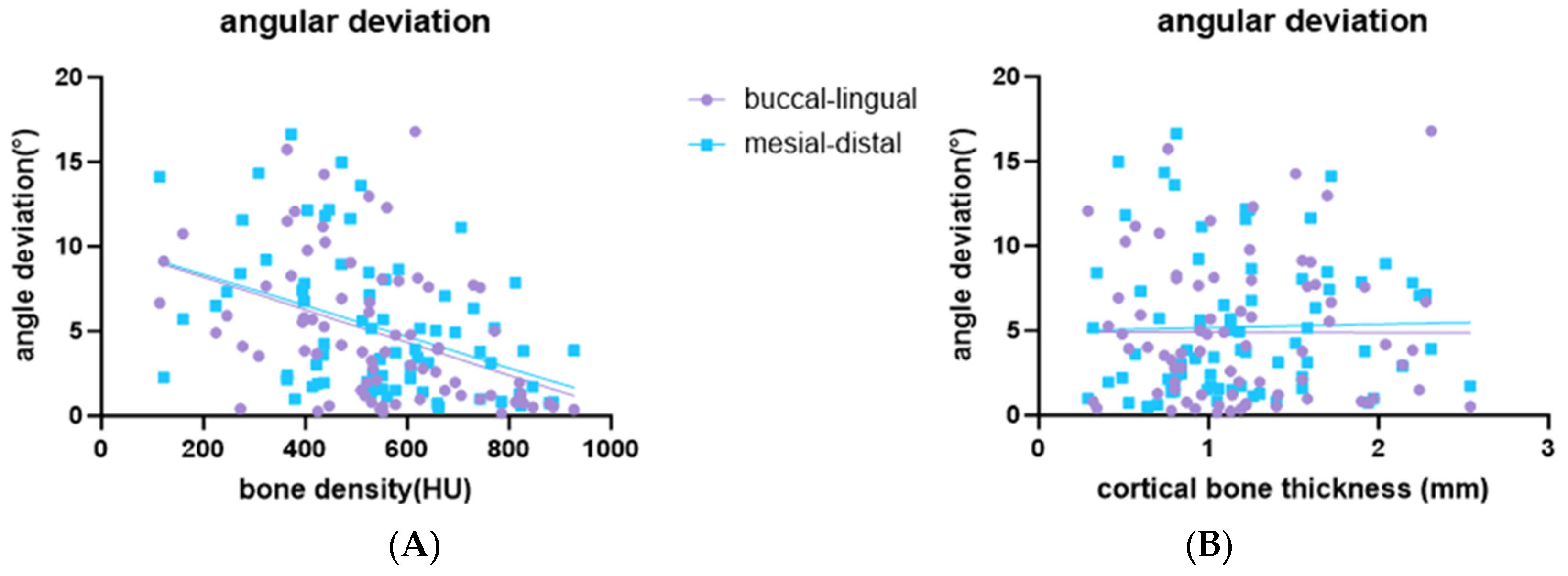
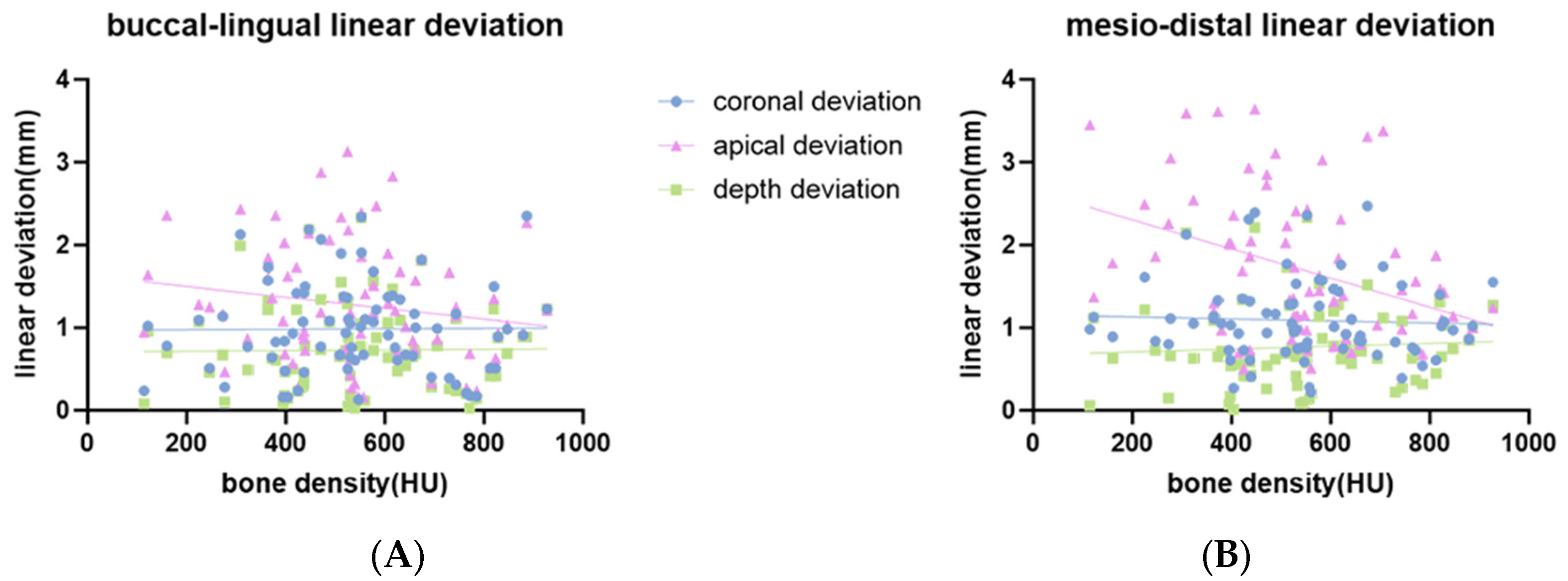
3.2. Influence of Bone Density and Cortical Bone Thickness on the Accuracy of Implant Placement
3.3. Influence of Residual Ridge Morphology on the Accuracy of Implant Placement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, Y.; Wang, J.; Ma, C.; Shen, J.; Dong, X.; Lin, D. A systematic review of the accuracy of digital surgical guides for dental implantation. Int. J. Implant Dent. 2023, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Bover-Ramos, F.; Viña-Almunia, J.; Cervera-Ballester, J.; Peñarrocha-Diago, M.; García-Mira, B. Accuracy of Implant Placement with Computer-Guided Surgery: A Systematic Review and Meta-Analysis Comparing Cadaver, Clinical, and In Vitro Studies. Int. J. Oral Maxillofac. Implants 2018, 33, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Raico Gallardo, Y.N.; da Silva-Olivio, I.R.T.; Mukai, E.; Morimoto, S.; Sesma, N.; Cordaro, L. Accuracy comparison of guided surgery for dental implants according to the tissue of support: A systematic review and meta-analysis. Clin. Oral Implants Res. 2017, 28, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, A.; Polizzi, G.; Moy, P.K. Guided surgery with tooth-supported templates for single missing teeth: A critical review. Eur. J. Oral Implantol. 2016, 9, S135–S153. [Google Scholar] [PubMed]
- Henprasert, P.; Dawson, D.V.; El-Kerdani, T.; Song, X.; Couso-Queiruga, E.; Holloway, J.A. Comparison of the Accuracy of Implant Position Using Surgical Guides Fabricated by Additive and Subtractive Techniques. J. Prosthodont. 2020, 296, 534–541. [Google Scholar] [CrossRef]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implants 1986, 1, 11–25. [Google Scholar]
- Sugiura, T.; Yamamoto, K.; Horita, S.; Murakami, K.; Tsutsumi, S.; Kirita, T. The effects of bone density and crestal cortical bone thickness on micromotion and peri-implant bone strain distribution in an immediately loaded implant: A nonlinear finite element analysis. J. Periodontal Implant Sci. 2016, 46, 152–165. [Google Scholar] [CrossRef]
- Alkhader, M.; Hudieb, M.; Khader, Y. Predictability of bone density at posterior mandibular implant sites using cone-beam computed tomography intensity values. Eur. J. Dent. 2017, 11, 311–316. [Google Scholar] [CrossRef]
- Solanki, K.J.; Fulsundar, P.; Heganna, M.; Nair, A.; Sabane, A.V.; Sawant, H. Comparative Evaluation of the Virtual Planned Position and Actual Position of Implant in Mandibular Posterior Region Using a DIOnavi System. Int. J. Health Sci. 2022, 6, 2197–2209. [Google Scholar] [CrossRef]
- Burstein, J.; Mastin, C.; Le, B. Avoiding injury to the inferior alveolar nerve by routine use of intraoperative radiographs during implant placement. J. Oral Implantol. 2008, 34, 34–38. [Google Scholar] [CrossRef]
- Padmanabhan, H.; Kumar, A.V.; Shivashankar, K. Incidence of neurosensory disturbance in mandibular implant surgery—A meta-analysis. J. Indian. Prosthodont. Soc. 2020, 20, 17–26. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.C.; Leles, C.R.; Normanha, L.M.; Lindh, C.; Ribeiro-Rotta, R.F. Assessments of trabecular bone density at implant sites on CT images. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 105, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Putra, R.H.; Yoda, N.; Iikubo, M.; Kataoka, Y.; Yamauchi, K.; Koyama, S.; Cooray, U.; Astuti, E.R.; Takahashi, T.; Sasaki, K. Influence of bone condition on implant placement accuracy with computer-guided surgery. Int. J. Implant. Dent. 2020, 6, 62. [Google Scholar] [CrossRef]
- Merheb, J.; Vercruyssen, M.; Coucke, W.; Quirynen, M. Relationship of implant stability and bone density derived from computerized tomography images. Clin. Implant. Dent. Relat. Res. 2018, 20, 50–57. [Google Scholar] [CrossRef]
- Ozan, O.; Orhan, K.; Turkyilmaz, I. Correlation between bone density and angular deviation of implants placed using CT-generated surgical guides. J. Craniofacial Surg. 2011, 22, 1755–1761. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, H.; Nakahara, K.; Matsunaga, S.; Usami, A.; Yoshinari, M.; Takano, N.; Ide, Y.; Abe, S. Association between the peri-implant bone structure and stress distribution around the mandibular canal: A three-dimensional finite element analysis. Dent. Mater. J. 2013, 32, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Rathee, S.; Agarwal, J.; Pachar, R.B. Measurement of Crestal Cortical Bone Thickness at Implant Site: A Cone Beam Computed Tomography Study. J. Contemp. Dent. Pract. 2017, 18, 785–789. [Google Scholar] [CrossRef]
- Abuhajar, E.; Salim, N.A.; Sallam, M.; Jarab, F.; Satterthwaite, J.D. The impact of surgical guide design and bone quality on heat generation during pilot implant site preparation: An in vitro study. BMC Oral Health 2023, 23, 273. [Google Scholar] [CrossRef]
- Chatvaratthana, K.; Thaworanunta, S.; Seriwatanachai, D.; Wongsirichat, N. Correlation between the thickness of the crestal and buccolingual cortical bone at varying depths and implant stability quotients. PLoS ONE 2017, 12, e0190293. [Google Scholar] [CrossRef]
- Tözüm, M.D.; Ataman-Duruel, E.T.; Duruel, O.; Nares, S.; Tözüm, T.F. Association between ridge morphology and complexity of implant placement planning in the posterior mandible. J. Prosthet. Dent. 2022, 128, 361–367. [Google Scholar] [CrossRef]
- Watanabe, H.; Mohammad Abdul, M.; Kurabayashi, T.; Aoki, H. Mandible size and morphology determined with CT on a premise of dental implant operation. Surg. Radiol. Anat. 2010, 32, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, G.O.; Khoynezhad, S.; Yansane, A.I.; Taylor, J.; Buser, D.; Friedland, B. Influence of the Posterior Mandible Ridge Morphology on Virtual Implant Planning. Int. J. Oral Maxillofac. Implants 2017, 32, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Juodzbalys, G.; Kubilius, M. Clinical and radiological classification of the jawbone anatomy in endosseous dental implant treatment. J. Oral Maxillofac. Res. 2013, 4, e2. [Google Scholar] [CrossRef]
- Benavides, E.; Rios, H.F.; Ganz, S.D.; An, C.H.; Resnik, R.; Reardon, G.T.; Feldman, S.J.; Mah, J.K.; Hatcher, D.; Kim, M.J.; et al. Use of cone beam computed tomography in implant dentistry: The International Congress of Oral Implantologists consensus report. Implant Dent. 2012, 21, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Tahmaseb, A.; Wu, V.; Wismeijer, D.; Coucke, W.; Evans, C. The accuracy of static computer-aided implant surgery: A systematic review and meta-analysis. Clin. Oral Implants Res. 2018, 29, 416–435. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.C.; Huang, H.L.; Shen, Y.W.; Cai, J.Y.; Fuh, L.J.; Hsu, J.T. Variations in crestal cortical bone thickness at dental implant sites in different regions of the jawbone. Clin. Implant. Dent. Relat. Res. 2017, 19, 440–446. [Google Scholar] [CrossRef]
- Dahiya, K.; Kumar, N.; Bajaj, P.; Sharma, A.; Sikka, R.; Dahiya, S. Qualitative Assessment of Reliability of Cone-beam Computed Tomography in evaluating Bone Density at Posterior Mandibular Implant Site. J. Contemp. Dent. Pract. 2018, 19, 426–430. [Google Scholar] [CrossRef]
- Schneider, D.; Marquardt, P.; Zwahlen, M.; Jung, R.E. A systematic review on the accuracy and the clinical outcome of computer-guided template-based implant dentistry. Clin. Oral Implants Res. 2009, 20, 73–86. [Google Scholar] [CrossRef]
- Xu, L.W.; You, J.; Zhang, J.X.; Liu, Y.F.; Peng, W. Impact of Surgical Template on the Accuracy of Implant Placement. J. Prosthodont. 2016, 25, 641–646. [Google Scholar] [CrossRef]
- Biguetti, C.C.; Lakkasetter Chandrashekar, B.; Simionato, G.B.; Momesso, N.R.; Duarte, M.A.H.; Rodrigues, D.C.; Matsumoto, M.A. Influence of age and gender on alveolar bone healing post tooth extraction in 129 Sv mice: A microtomographic, histological, and biochemical characterization. Clin. Oral Investig. 2023, 27, 4605–4616. [Google Scholar] [CrossRef]
- Santos, L.; Elliott-Sale, K.J.; Sale, C. Exercise and bone health across the lifespan. Biogerontology 2017, 18, 931–946. [Google Scholar] [CrossRef] [PubMed]
- Aribau-Gumà, C.; Jorba-García, A.; Sánchez-Torres, A.; Sànchez-Garcés, M.À. Alveolar ridge preservation: An overview of systematic reviews. Int. J. Oral Maxillofac. Surg. 2022, 51, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Ohiomoba, H.; Sonis, A.; Yansane, A.; Friedland, B. Quantitative evaluation of maxillary alveolar cortical bone thickness and density using computed tomography imaging. Am. J. Orthod. Dentofacial Orthop. 2017, 151, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Geng, N.; Ren, J.; Zhang, C.; Zhou, T.; Feng, C.; Chen, S. Immediate implant placement in the posterior mandibular region was assisted by dynamic real-time navigation: A retrospective study. BMC Oral Health 2024, 24, 208. [Google Scholar] [CrossRef]


| Implant System | Implant Dimension (Diameter × Length) | Total Implants |
|---|---|---|
| Alpha-Bio Tec, ICE (Warsaw, Poland) | 4.65 mm × 11.5 mm | 1 |
| DENTSPLY Implants, OsseoSpeed™ TX (York, PA, USA) | 4.0 mm × 11.0 mm | 2 |
| Nobel Biocare, NobelActive®, RP (Gothenburg, Sweden) | 4.3 mm × 10.0 mm | 1 |
| Nobel Replace®, Conical Connection (Gothenburg, Sweden) | 4.3 mm × 10.0 mm | 4 |
| 4.3 mm × 11.5 mm | 1 | |
| 5.0 mm × 8.0 mm | 1 | |
| 5.0 mm × 10.0 mm | 2 | |
| 5.0 mm × 11.5 mm | 1 | |
| Straumann BLT, Roxolid®, Loxim® (Basel, Switzerland) | 3.3 mm × 12.0 mm | 1 |
| 4.1 mm × 10.0 mm | 12 | |
| 4.1 mm × 12.0 mm | 1 | |
| 4.8 mm × 8.0 mm | 12 | |
| 4.8 mm × 10.0 mm | 29 | |
| 4.8 mm × 12.0 mm | 2 | |
| Straumann BL, Roxolid®, Loxim® (Basel, Switzerland) | 4.8 mm × 8.0 mm | 2 |
| 4.8 mm × 10.0 mm | 3 |
| Angular Deviation (°) | Coronal Deviation (mm) | Apical Deviation (mm) | Depth Deviation (mm) | |
|---|---|---|---|---|
| Buccal-lingual orientation | 4.90 ± 4.15 | 0.99 ± 0.55 | 1.27 ± 0.71 | 0.73 ± 0.53 |
| Mesio-distal orientation | 5.21 ± 4.12 | 1.09 ± 0.48 | 1.70 ± 0.85 | 0.77 ± 0.49 |
| Buccal-Lingual Orientation | Mesio-Distal Orientation | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Angular Deviation | Coronal Deviation | Apical Deviation | Depth Deviation | Angular Deviation | Coronal Deviation | Apical Deviation | Depth Deviation | ||
| Bone density | β | −0.01 *** | 0.355 | −0.658 | 0.038 | −0.009 *** | −0.117 | −1.759 *** | 0.173 |
| 95% CI | [−0.014, −0.004] | [−0.68, 0.735] | [−1.554, 0.238] | [−0.634, 0.709] | [−0.014, 0.004] | [−0.734, 0.5] | [−2.756, −0.761] | [−0.448, 0.793] | |
| Cortical bone thickness | β | −0.026 | −0.098 | 0.06 | 0.014 | 0.202 | −0.001 | 0.042 | −0.008 |
| 95% CI | [−1.845, 1.792] | [−0.341, 0.144] | [−0.253, 0.372] | [−0.216, 0.246] | [−1.605, 2.008] | [−0.213, 0.211] | [−0.328, 0.413] | [−0.222, 0.206] | |
| r2 | 0.19 | 0.01 | 0.04 | 0.00 | 0.19 | 0.00 | 0.16 | 0.01 | |
| Overall p-value | <0.001 | 0.69 | 0.26 | 0.99 | 0.001 | 0.93 | 0.002 | 0.84 | |
| *** p < 0.001 | |||||||||
| Residual Ridge Morphology | n | Rank Mean | H | p | Conover–Iman Post hoc | Bonferroni Correction | ||
|---|---|---|---|---|---|---|---|---|
| Buccal-lingual orientation | angular deviation | A | 21 | 31.05 | 6.013 | 0.111 | ||
| B | 20 | 47.45 | ||||||
| C | 17 | 36.88 | ||||||
| D | 17 | 36.59 | ||||||
| coronal deviation | A | 21 | 26.1 | 9.785 | 0.02 | A < B ** A < C ** A < D * | A < B *** A < C ** A < D ** | |
| B | 20 | 46.18 | ||||||
| C | 17 | 42.38 | ||||||
| D | 17 | 38.71 | ||||||
| apical deviation | A | 21 | 24.67 | 11.105 | 0.011 | A < B ** A < C ** A < D ** | A < B *** A < C *** A < D *** | |
| B | 20 | 44.85 | ||||||
| C | 17 | 42.44 | ||||||
| D | 17 | 41.97 | ||||||
| depth deviation | A | 21 | 27.38 | 9.854 | 0.02 | A < B ** A < C ** | A < B *** A < C ** | |
| B | 20 | 46.43 | ||||||
| C | 17 | 44.47 | ||||||
| D | 17 | 34.74 | ||||||
| Mesio-distal orientation | angular deviation | A | 21 | 28.98 | 5.962 | 0.116 | ||
| B | 20 | 44.05 | ||||||
| C | 17 | 42.74 | ||||||
| D | 17 | 37.29 | ||||||
| coronal deviation | A | 21 | 23.71 | 12.656 | 0.005 | A < B *** A < C ** A < D ** | A < B *** A < C *** A < D *** | |
| B | 20 | 44.88 | ||||||
| C | 17 | 43.12 | ||||||
| D | 17 | 42.44 | ||||||
| apical deviation | A | 21 | 22.14 | 16.043 | 0.001 | A < B *** A < C *** A < D *** | A < B *** A < C *** A < D *** | |
| B | 20 | 44.18 | ||||||
| C | 17 | 47.06 | ||||||
| D | 17 | 41.26 | ||||||
| depth deviation | A | 21 | 32.79 | 1.7 | 0.637 | |||
| B | 20 | 40.67 | ||||||
| C | 17 | 39.47 | ||||||
| D | 17 | 39.82 | ||||||
| * p < 0.05, ** p < 0.01, *** p < 0.001 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Z.; Shen, Y.; Qi, S.; Cao, L.; Fan, X.; Lu, C.; Wang, J. Influence of Bone Conditions on the Accuracy of Implant Placement. Bioengineering 2024, 11, 1161. https://doi.org/10.3390/bioengineering11111161
Gong Z, Shen Y, Qi S, Cao L, Fan X, Lu C, Wang J. Influence of Bone Conditions on the Accuracy of Implant Placement. Bioengineering. 2024; 11(11):1161. https://doi.org/10.3390/bioengineering11111161
Chicago/Turabian StyleGong, Zhicheng, Yuyin Shen, Shengcai Qi, Lai Cao, Xinyi Fan, Chunhui Lu, and Jue Wang. 2024. "Influence of Bone Conditions on the Accuracy of Implant Placement" Bioengineering 11, no. 11: 1161. https://doi.org/10.3390/bioengineering11111161
APA StyleGong, Z., Shen, Y., Qi, S., Cao, L., Fan, X., Lu, C., & Wang, J. (2024). Influence of Bone Conditions on the Accuracy of Implant Placement. Bioengineering, 11(11), 1161. https://doi.org/10.3390/bioengineering11111161








