Lipid Alterations in Chronic Nonspecific Low Back Pain in the Chinese Population: A Metabolomic and Lipidomic Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Metabolomic Measurement
2.3. Lipidomic Analysis
2.4. Statistical Analysis
3. Results
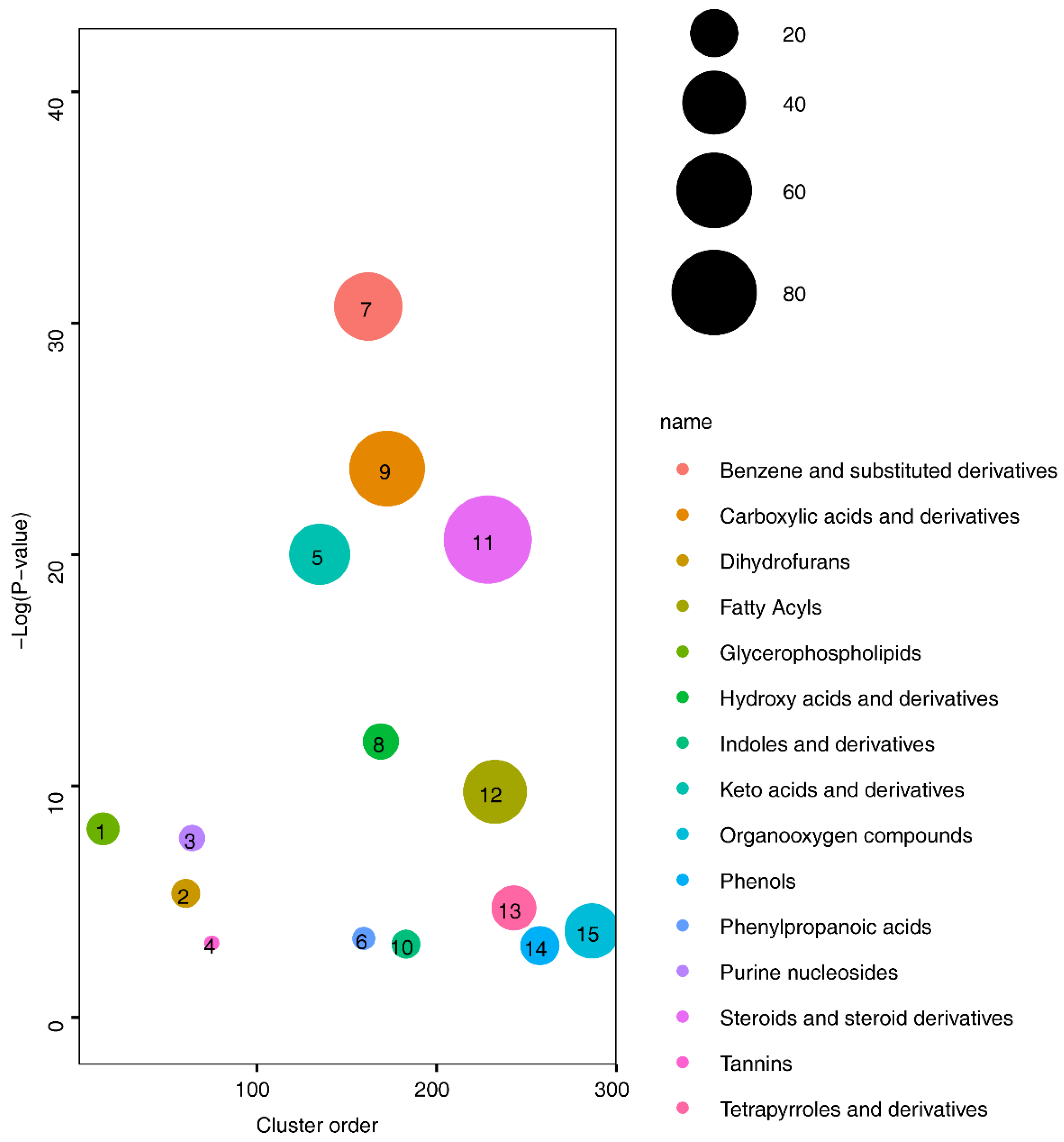
3.1. Metabolite Alterations Between Healthy Volunteers and cNLBP Patients
3.2. Lipid Composition Analysis in Healthy Volunteers and cNLBP Patients
3.3. Altered Glycerolipid Levels in cNLBP Patients
3.4. Increased Levels of PS in Patients with cNLBP
4. Discussion
5. Conclusions and Clinical Perspective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knezevic, N.N.; Candido, K.D.; Vlaeyen, J.W.S.; Van Zundert, J.; Cohen, S.P. Low back pain. Lancet 2021, 398, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Hartvigsen, J.; Hancock, M.J.; Kongsted, A.; Louw, Q.; Ferreira, M.L.; Genevay, S.; Hoy, D.; Karppinen, J.; Pransky, G.; Sieper, J.; et al. What low back pain is and why we need to pay attention. Lancet 2018, 391, 2356–2367. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Song, K.S.; Cho, J.H.; Lee, J.H.; Kim, N.H. An Updated Overview of Low Back Pain Management. Asian Spine J. 2022, 16, 968–982. [Google Scholar] [CrossRef] [PubMed]
- Herman, P.M.; Coulter, I.D.; Hays, R.D.; Rodriguez, A.; Edelen, M.O. A Scoping Review of Chronic Low Back Pain Classification Schemes Based on Patient-Reported Outcomes. Pain Physician 2022, 25, 471–482. [Google Scholar]
- Dagenais, S.; Caro, J.; Haldeman, S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008, 8, 8–20. [Google Scholar] [CrossRef]
- Patrick, N.; Emanski, E.; Knaub, M.A. Acute and chronic low back pain. Med. Clin. N. Am. 2014, 98, 777–789. [Google Scholar] [CrossRef]
- Hoy, D.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Bain, C.; Williams, G.; Smith, E.; Vos, T.; Barendregt, J.; et al. The global burden of low back pain: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 968–974. [Google Scholar] [CrossRef]
- Xu, S.; Qi, J.; Liu, C.; Xia, W.; Wang, Z.; Li, K.; Zhou, M.; Liu, H. Evaluation of three decades of the burden of low back pain in China before COVID-19: Estimates from the Global Burden of Disease Database 2019. J. Glob. Health 2024, 14, 04006. [Google Scholar] [CrossRef]
- Gomez-Varela, D.; Barry, A.M.; Schmidt, M. Proteome-based systems biology in chronic pain. J. Proteom. 2019, 190, 1–11. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Snyder, M.P. Integrative omics for health and disease. Nat. Rev. Genet. 2018, 19, 299–310. [Google Scholar] [CrossRef]
- Babu, M.; Snyder, M. Multi-Omics Profiling for Health. Mol. Cell. Proteom. MCP 2023, 22, 100561. [Google Scholar] [CrossRef] [PubMed]
- Sadee, W.; Wang, D.; Hartmann, K.; Toland, A.E. Pharmacogenomics: Driving Personalized Medicine. Pharmacol. Rev. 2023, 75, 789–814. [Google Scholar] [CrossRef] [PubMed]
- Teckchandani, S.; Nagana Gowda, G.A.; Raftery, D.; Curatolo, M. Metabolomics in chronic pain research. Eur. J. Pain 2021, 25, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.; Wilson, I.D.; Nicholson, J.K. Metabolic phenotyping in health and disease. Cell 2008, 134, 714–717. [Google Scholar] [CrossRef]
- Zetterman, T.; Nieminen, A.I.; Markkula, R.; Kalso, E.; Lötsch, J. Machine learning identifies fatigue as a key symptom of fibromyalgia reflected in tyrosine, purine, pyrimidine, and glutaminergic metabolism. Clin. Transl. Sci. 2024, 17, e13740. [Google Scholar] [CrossRef]
- Finco, G.; Locci, E.; Mura, P.; Massa, R.; Noto, A.; Musu, M.; Landoni, G.; d’Aloja, E.; De-Giorgio, F.; Scano, P.; et al. Can Urine Metabolomics Be Helpful in Differentiating Neuropathic and Nociceptive Pain? A Proof-of-Concept Study. PLoS ONE 2016, 11, e0150476. [Google Scholar] [CrossRef]
- Livshits, G.; Macgregor, A.J.; Gieger, C.; Malkin, I.; Moayyeri, A.; Grallert, H.; Emeny, R.T.; Spector, T.; Kastenmüller, G.; Williams, F.M.K. An omics investigation into chronic widespread musculoskeletal pain reveals epiandrosterone sulfate as a potential biomarker. Pain 2015, 156, 1845–1851. [Google Scholar] [CrossRef]
- Parker, K.S.; Crowley, J.R.; Stephens-Shields, A.J.; van Bokhoven, A.; Lucia, M.S.; Lai, H.H.; Andriole, G.L.; Hooton, T.M.; Mullins, C.; Henderson, J.P. Urinary Metabolomics Identifies a Molecular Correlate of Interstitial Cystitis/Bladder Pain Syndrome in a Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network Cohort. EBioMedicine 2016, 7, 167–174. [Google Scholar] [CrossRef]
- Jensen, J.R.; Pitcher, M.H.; Yuan, Z.X.; Ramsden, C.E.; Domenichiello, A.F. Concentrations of oxidized linoleic acid derived lipid mediators in the amygdala and periaqueductal grey are reduced in a mouse model of chronic inflammatory pain. Prostaglandins Leukot. Essent. Fat. Acids 2018, 135, 128–136. [Google Scholar] [CrossRef]
- Ren, J.; Lin, J.; Yu, L.; Yan, M. Lysophosphatidylcholine: Potential Target for the Treatment of Chronic Pain. Int. J. Mol. Sci. 2022, 23, 8274. [Google Scholar] [CrossRef]
- Khoury, S.; Colas, J.; Breuil, V.; Kosek, E.; Ahmed, A.S.; Svensson, C.I.; Marchand, F.; Deval, E.; Ferreira, T. Identification of Lipid Biomarkers for Chronic Joint Pain Associated with Different Joint Diseases. Biomolecules 2023, 13, 342. [Google Scholar] [CrossRef] [PubMed]
- Meints, S.M.; Mawla, I.; Napadow, V.; Kong, J.; Gerber, J.; Chan, S.T.; Wasan, A.D.; Kaptchuk, T.J.; McDonnell, C.; Carriere, J.; et al. The relationship between catastrophizing and altered pain sensitivity in patients with chronic low-back pain. Pain 2019, 160, 833–843. [Google Scholar] [CrossRef] [PubMed]
- van Dieën, J.H.; Reeves, N.P.; Kawchuk, G.; van Dillen, L.R.; Hodges, P.W. Motor Control Changes in Low Back Pain: Divergence in Presentations and Mechanisms. J. Orthop. Sports Phys. Ther. 2019, 49, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Price, D.D. Psychological and neural mechanisms of the affective dimension of pain. Science 2000, 288, 1769–1772. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, T.; Shen, X.; Liu, J.; Zhao, D.; Sun, Y.; Wang, L.; Liu, Y.; Gong, X.; Liu, Y.; et al. Serum metabolomics for early diagnosis of esophageal squamous cell carcinoma by UHPLC-QTOF/MS. Metabolomics 2016, 12, 116. [Google Scholar] [CrossRef]
- Xuan, Q.; Zheng, F.; Yu, D.; Ouyang, Y.; Zhao, X.; Hu, C.; Xu, G. Rapid lipidomic profiling based on ultra-high performance liquid chromatography-mass spectrometry and its application in diabetic retinopathy. Anal. Bioanal. Chem. 2020, 412, 3585–3594. [Google Scholar] [CrossRef]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar] [CrossRef]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC-HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef]
- Nicol, V.; Verdaguer, C.; Daste, C.; Bisseriex, H.; Lapeyre, É.; Lefèvre-Colau, M.M.; Rannou, F.; Rören, A.; Facione, J.; Nguyen, C. Chronic Low Back Pain: A Narrative Review of Recent International Guidelines for Diagnosis and Conservative Treatment. J. Clin. Med. 2023, 12, 1685. [Google Scholar] [CrossRef]
- Pousinis, P.; Gowler, P.R.W.; Burston, J.J.; Ortori, C.A.; Chapman, V.; Barrett, D.A. Lipidomic identification of plasma lipids associated with pain behaviour and pathology in a mouse model of osteoarthritis. Metabolomics 2020, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Liu, M.; Tian, J.; Zhai, G.; Cicuttini, F.; Schooneveldt, Y.L.; Meikle, P.J.; Jones, G.; Pan, F. Lipidomic Profiling Identifies Serum Lipids Associated with Persistent Multisite Musculoskeletal Pain. Metabolites 2022, 12, 206. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, P.A.; Simcox, J.; Raff, H.; Wade, G.; Von Bank, H.; Weisman, S.; Hainsworth, K. Lipid signatures of chronic pain in female adolescents with and without obesity. Lipids Health Dis. 2022, 21, 80. [Google Scholar] [CrossRef] [PubMed]
- Farese, R.V., Jr.; Walther, T.C. Glycerolipid Synthesis and Lipid Droplet Formation in the Endoplasmic Reticulum. Cold Spring Harb. Perspect. Biol. 2023, 15, a041246. [Google Scholar] [CrossRef] [PubMed]
- Renbäck, K.; Inoue, M.; Yoshida, A.; Nyberg, F.; Ueda, H. Vzg-1/lysophosphatidic acid-receptor involved in peripheral pain transmission. Brain Res. Mol. Brain Res. 2000, 75, 350–354. [Google Scholar] [CrossRef]
- Inoue, M.; Xie, W.; Matsushita, Y.; Chun, J.; Aoki, J.; Ueda, H. Lysophosphatidylcholine induces neuropathic pain through an action of autotaxin to generate lysophosphatidic acid. Neuroscience 2008, 152, 296–298. [Google Scholar] [CrossRef]
- Camprubí-Robles, M.; Mair, N.; Andratsch, M.; Benetti, C.; Beroukas, D.; Rukwied, R.; Langeslag, M.; Proia, R.L.; Schmelz, M.; Ferrer Montiel, A.V.; et al. Sphingosine-1-phosphate-induced nociceptor excitation and ongoing pain behavior in mice and humans is largely mediated by S1P3 receptor. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 2582–2592. [Google Scholar] [CrossRef]
- Poursharifi, P.; Madiraju, S.R.M.; Prentki, M. Monoacylglycerol signalling and ABHD6 in health and disease. Diabetes Obes. Metab. 2017, 19 (Suppl. S1), 76–89. [Google Scholar] [CrossRef]
- Calignano, A.; La Rana, G.; Giuffrida, A.; Piomelli, D. Control of pain initiation by endogenous cannabinoids. Nature 1998, 394, 277–281. [Google Scholar] [CrossRef]
- Deng, H.; Li, W. Monoacylglycerol lipase inhibitors: Modulators for lipid metabolism in cancer malignancy, neurological and metabolic disorders. Acta Pharm. Sin. B 2020, 10, 582–602. [Google Scholar] [CrossRef]
- Beaulieu, P.; Bisogno, T.; Punwar, S.; Farquhar-Smith, W.P.; Ambrosino, G.; Di Marzo, V.; Rice, A.S. Role of the endogenous cannabinoid system in the formalin test of persistent pain in the rat. Eur. J. Pharmacol. 2000, 396, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Guindon, J.; Desroches, J.; Beaulieu, P. The antinociceptive effects of intraplantar injections of 2-arachidonoyl glycerol are mediated by cannabinoid CB2 receptors. Br. J. Pharmacol. 2007, 150, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Khasabova, I.A.; Chandiramani, A.; Harding-Rose, C.; Simone, D.A.; Seybold, V.S. Increasing 2-arachidonoyl glycerol signaling in the periphery attenuates mechanical hyperalgesia in a model of bone cancer pain. Pharmacol. Res. 2011, 64, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Guindon, J.; Guijarro, A.; Piomelli, D.; Hohmann, A.G. Peripheral antinociceptive effects of inhibitors of monoacylglycerol lipase in a rat model of inflammatory pain. Br. J. Pharmacol. 2011, 163, 1464–1478. [Google Scholar] [CrossRef]
- Hohmann, A.G.; Suplita, R.L.; Bolton, N.M.; Neely, M.H.; Fegley, D.; Mangieri, R.; Krey, J.F.; Walker, J.M.; Holmes, P.V.; Crystal, J.D.; et al. An endocannabinoid mechanism for stress-induced analgesia. Nature 2005, 435, 1108–1112. [Google Scholar] [CrossRef]
- Guindon, J.; Lai, Y.; Takacs, S.M.; Bradshaw, H.B.; Hohmann, A.G. Alterations in endocannabinoid tone following chemotherapy-induced peripheral neuropathy: Effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following cisplatin treatment. Pharmacol. Res. 2013, 67, 94–109. [Google Scholar]
- Ghosh, S.; Wise, L.E.; Chen, Y.; Gujjar, R.; Mahadevan, A.; Cravatt, B.F.; Lichtman, A.H. The monoacylglycerol lipase inhibitor JZL184 suppresses inflammatory pain in the mouse carrageenan model. Life Sci. 2013, 92, 498–505. [Google Scholar] [CrossRef]
- Kinsey, S.G.; Nomura, D.K.; O’Neal, S.T.; Long, J.Z.; Mahadevan, A.; Cravatt, B.F.; Grider, J.R.; Lichtman, A.H. Inhibition of monoacylglycerol lipase attenuates nonsteroidal anti-inflammatory drug-induced gastric hemorrhages in mice. J. Pharmacol. Exp. Ther. 2011, 338, 795–802. [Google Scholar] [CrossRef]
- Kinsey, S.G.; Long, J.Z.; Cravatt, B.F.; Lichtman, A.H. Fatty acid amide hydrolase and monoacylglycerol lipase inhibitors produce anti-allodynic effects in mice through distinct cannabinoid receptor mechanisms. J. Pain 2010, 11, 1420–1428. [Google Scholar] [CrossRef]
- Kinsey, S.G.; Long, J.Z.; O’Neal, S.T.; Abdullah, R.A.; Poklis, J.L.; Boger, D.L.; Cravatt, B.F.; Lichtman, A.H. Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain. J. Pharmacol. Exp. Ther. 2009, 330, 902–910. [Google Scholar] [CrossRef]
- Sugiura, T.; Kondo, S.; Kishimoto, S.; Miyashita, T.; Nakane, S.; Kodaka, T.; Suhara, Y.; Takayama, H.; Waku, K. Evidence that 2-arachidonoylglycerol but not N-palmitoylethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor. Comparison of the agonistic activities of various cannabinoid receptor ligands in HL-60 cells. J. Biol. Chem. 2000, 275, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elsayed, A.; Gyorfi, M. Chronic low back pain and cognitive function. Pain Pract. Off. J. World Inst. Pain 2023, 23, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Loo, L.; Wright, B.D.; Zylka, M.J. Lipid kinases as therapeutic targets for chronic pain. Pain 2015, 156 (Suppl. S1), S2–S10. [Google Scholar] [CrossRef] [PubMed]
- Mifflin, K.A.; Kerr, B.J. The transition from acute to chronic pain: Understanding how different biological systems interact. Can. J. Anaesth. 2014, 61, 112–122. [Google Scholar] [CrossRef]
- Glade, M.J.; Smith, K. Phosphatidylserine and the human brain. Nutrition 2015, 31, 781–786. [Google Scholar] [CrossRef]
- Zeng, X.; Tang, W.; Gao, F.; Tang, Z.; Zhang, Z.; Zhang, J.; Du, M.; Chen, Z.; Chen, X.; Yuan, Z. Behavioral modeling and neuroimaging of impaired risky decision making in patients with chronic musculoskeletal pain. Neurophotonics 2023, 10, 020901. [Google Scholar] [CrossRef]
- Kroeze, W.K.; Sheffler, D.J.; Roth, B.L. G-protein-coupled receptors at a glance. J. Cell Sci. 2003, 116 Pt 24, 4867–4869. [Google Scholar] [CrossRef]
- Mobasheri, A.; Henrotin, Y. Biomarkers of (osteo)arthritis. Biomarkers 2015, 20, 513–518. [Google Scholar] [CrossRef]
- Baggelaar, M.P.; Chameau, P.J.; Kantae, V.; Hummel, J.; Hsu, K.L.; Janssen, F.; van der Wel, T.; Soethoudt, M.; Deng, H.; den Dulk, H.; et al. Highly Selective, Reversible Inhibitor Identified by Comparative Chemoproteomics Modulates Diacylglycerol Lipase Activity in Neurons. J. Am. Chem. Soc. 2015, 137, 8851–8857. [Google Scholar] [CrossRef]
- Ogasawara, D.; Deng, H.; Viader, A.; Baggelaar, M.P.; Breman, A.; den Dulk, H.; van den Nieuwendijk, A.M.; Soethoudt, M.; van der Wel, T.; Zhou, J.; et al. Rapid and profound rewiring of brain lipid signaling networks by acute diacylglycerol lipase inhibition. Proc. Natl. Acad. Sci. USA 2016, 113, 26–33. [Google Scholar] [CrossRef]








| Healthy Volunteers | cNLBP Patients | p | |
|---|---|---|---|
| N (male/total count) | 11/26 | 9/30 | |
| Age (years) | 24.65 ± 4.49 | 28.50 ± 9.19 | 0.2806 |
| Height (m) | 1.66 ± 0.08 | 1.67 ± 0.11 | 0.8037 |
| Weight (kg) | 59.42 ± 13.52 | 57.00 ± 9.90 | 0.8074 |
| BMI (kg/m2) | 21.38 ± 3.50 | 21.87 ± 2.52 | 0.5523 |
| VAS | 0 | 4.50 ± 0.71 | 4.91044 × 10−25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, W.; Wang, H.-J.; Luo, S.-Y.; Zhang, S.-Y.; Xie, H.; Chen, H.-Q.; Wang, C.-H.; Zhang, Z. Lipid Alterations in Chronic Nonspecific Low Back Pain in the Chinese Population: A Metabolomic and Lipidomic Study. Bioengineering 2024, 11, 1114. https://doi.org/10.3390/bioengineering11111114
Tang W, Wang H-J, Luo S-Y, Zhang S-Y, Xie H, Chen H-Q, Wang C-H, Zhang Z. Lipid Alterations in Chronic Nonspecific Low Back Pain in the Chinese Population: A Metabolomic and Lipidomic Study. Bioengineering. 2024; 11(11):1114. https://doi.org/10.3390/bioengineering11111114
Chicago/Turabian StyleTang, Wen, Hong-Jiang Wang, Su-Ying Luo, Si-Yun Zhang, Hao Xie, Hua-Qing Chen, Chu-Huai Wang, and Zhou Zhang. 2024. "Lipid Alterations in Chronic Nonspecific Low Back Pain in the Chinese Population: A Metabolomic and Lipidomic Study" Bioengineering 11, no. 11: 1114. https://doi.org/10.3390/bioengineering11111114
APA StyleTang, W., Wang, H.-J., Luo, S.-Y., Zhang, S.-Y., Xie, H., Chen, H.-Q., Wang, C.-H., & Zhang, Z. (2024). Lipid Alterations in Chronic Nonspecific Low Back Pain in the Chinese Population: A Metabolomic and Lipidomic Study. Bioengineering, 11(11), 1114. https://doi.org/10.3390/bioengineering11111114








