The Effect of Transcranial Direct Current Stimulation on Lower-Limb Endurance Performance: A Systematic Review
Abstract
:1. Introduction
2. Methods
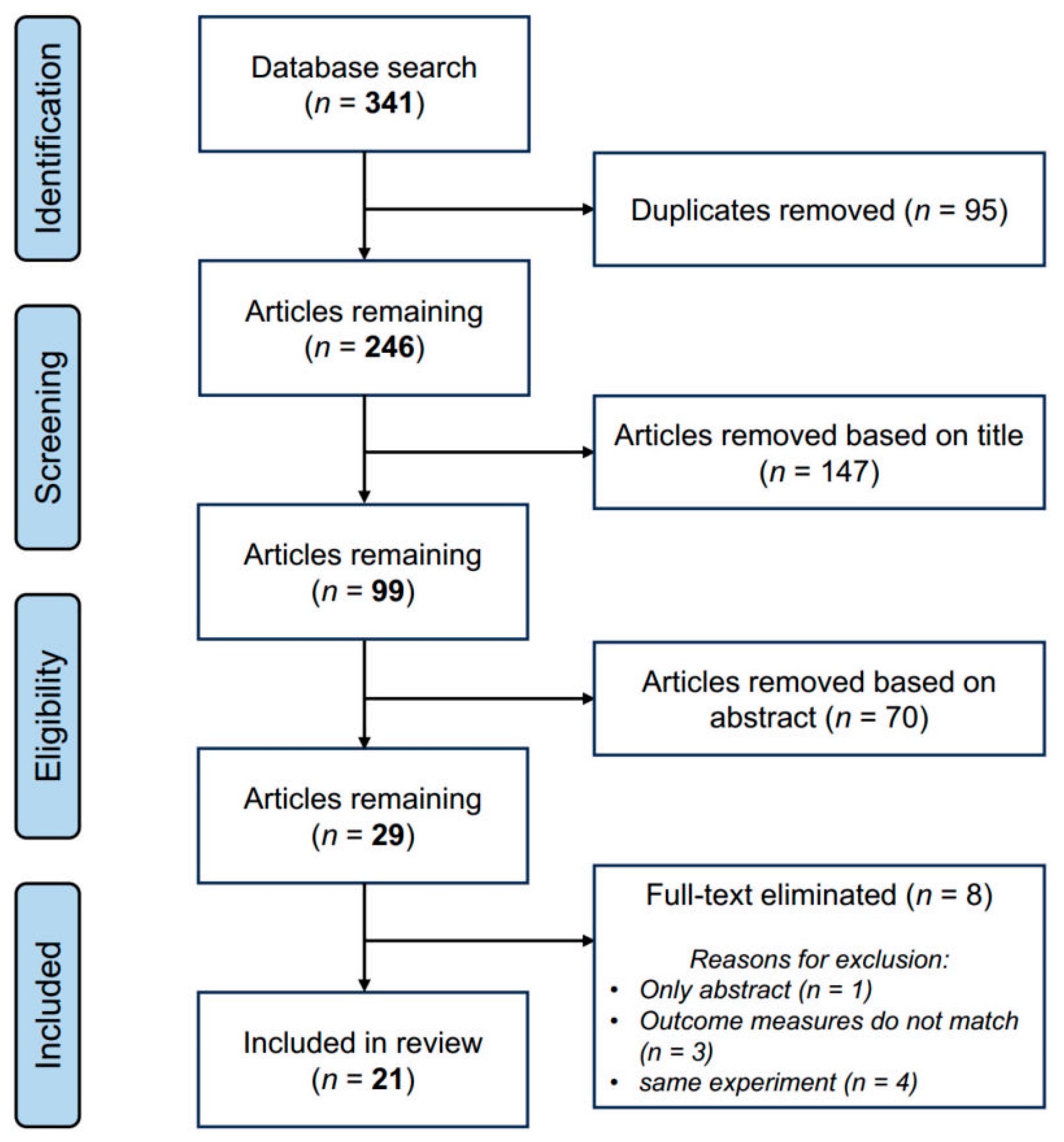
2.1. Search Strategy
2.2. Eligibility Criteria and Article Selection
2.3. Data Extraction
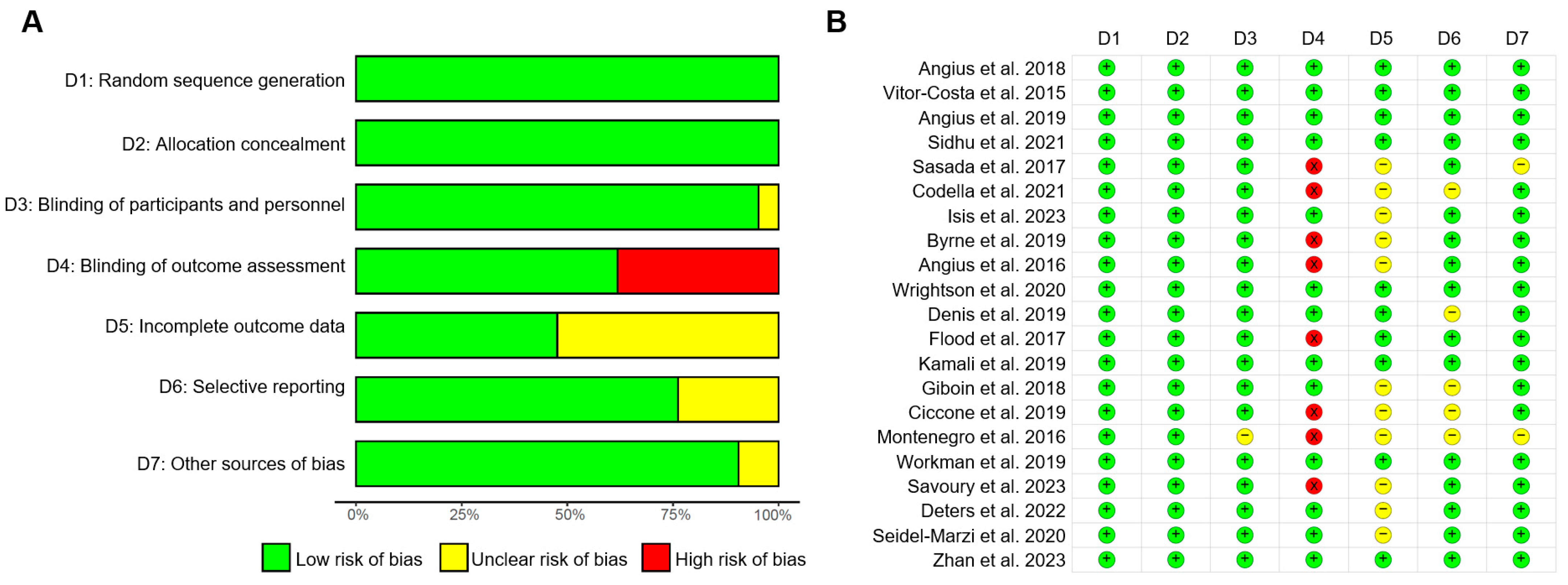
2.4. Risk of Bias Assessment
3. Results
3.1. Study Characteristics
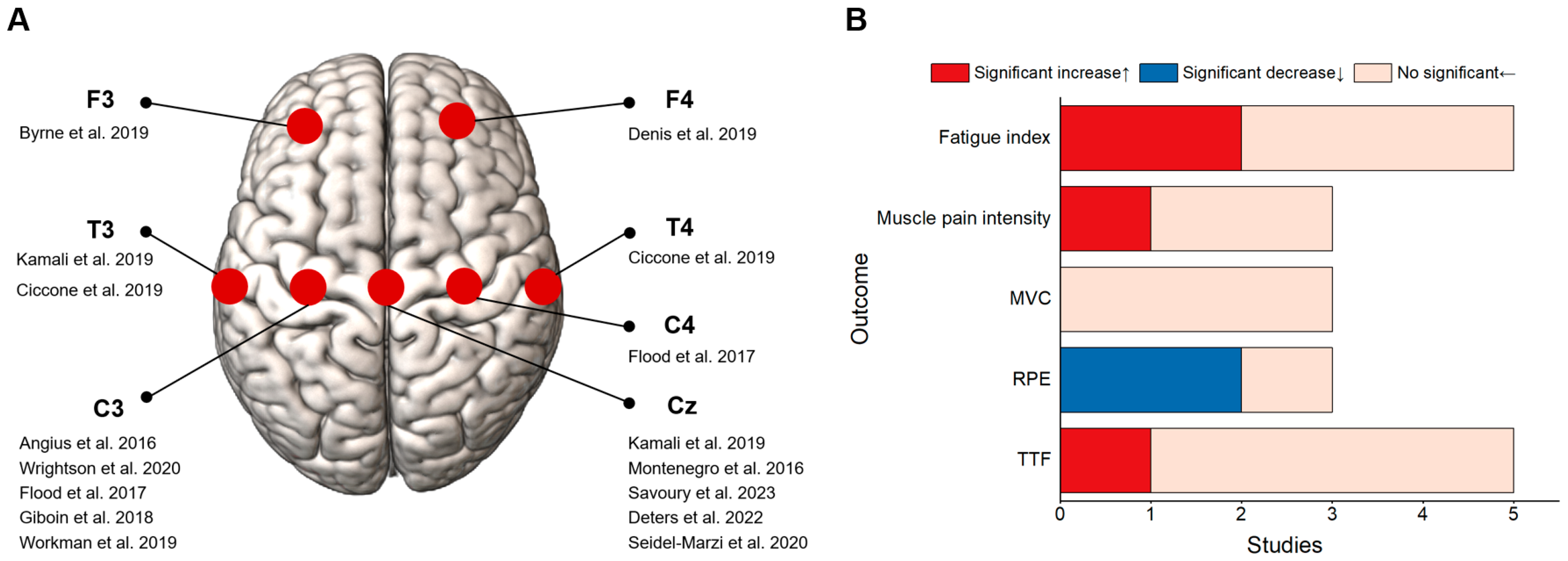
3.2. Effects of tDCS on Endurance Performance of Overall Lower Limbs
3.3. Effects of tDCS on Endurance Performance of a Single Joints/Segments of Lower Limbs
3.4. Risk of Bias Assessment
4. Discussion
4.1. Some Potential tDCS Mechanisms That Have Been Suggested to Improve Lower-Limb Endurance Performance
4.2. Possible Reasons for the Inconsistent Effects of tDCS Intervention
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Behrens, M.; Gube, M.; Chaabene, H.; Prieske, O.; Zenon, A.; Broscheid, K.C.; Schega, L.; Husmann, F.; Weippert, M. Fatigue and Human Performance: An Updated Framework. Sports Med. 2023, 53, 7–31. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Fan, Y. The Effect of Fatigue on Postural Control and Biomechanical Characteristic of Lunge in Badminton Players. Bioengineering 2023, 10, 301. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Li, L.; Zhang, S.; Xiao, S.; Sun, X.; Wang, S.; Fu, W. Acute effect of foot strike patterns on in vivo tibiotalar and subtalar joint kinematics during barefoot running. J. Sport Health Sci. 2024, 13, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Hornsby, W.G.; Sole, C.J.; Sato, K.; Stone, M.H. Effect of Neuromuscular Fatigue on the Countermovement Jump Characteristics: Basketball-Related High-Intensity Exercises. J. Strength Cond. Res. 2024, 38, 164–173. [Google Scholar] [CrossRef]
- Berger, N.J.A.; Best, R.; Best, A.W.; Lane, A.M.; Millet, G.Y.; Barwood, M.; Marcora, S.; Wilson, P.; Bearden, S. Limits of Ultra: Towards an Interdisciplinary Understanding of Ultra-Endurance Running Performance. Sports Med. 2024, 54, 73–93. [Google Scholar] [CrossRef]
- Xiao, S.; Shen, B.; Zhang, C.; Xu, Z.; Li, J.; Fu, W.; Jin, J. Effects of tDCS on Foot Biomechanics: A Narrative Review and Clinical Applications. Bioengineering 2023, 10, 1029. [Google Scholar] [CrossRef]
- Frohlich, F.; Riddle, J. Conducting double-blind placebo-controlled clinical trials of transcranial alternating current stimulation (tACS). Transl. Psychiatry 2021, 11, 284. [Google Scholar] [CrossRef]
- Sheffield, J.G.; Ramerpresad, S.; Brem, A.K.; Mansfield, K.; Orhan, U.; Dillard, M.; McKanna, J.; Plessow, F.; Thompson, T.; Santarnecchi, E.; et al. Blinding efficacy and adverse events following repeated transcranial alternating current, direct current, and random noise stimulation. Cortex 2022, 154, 77–88. [Google Scholar] [CrossRef]
- Chase, H.W.; Boudewyn, M.A.; Carter, C.S.; Phillips, M.L. Transcranial direct current stimulation: A roadmap for research, from mechanism of action to clinical implementation. Mol. Psychiatry 2020, 25, 397–407. [Google Scholar] [CrossRef]
- Bourzac, K. Neurostimulation: Bright sparks. Nature 2016, 531, S6–S8. [Google Scholar] [CrossRef]
- Razza, L.B.; Luethi, M.S.; Zanão, T.; De Smet, S.; Buchpiguel, C.; Busatto, G.; Pereira, J.; Klein, I.; Kappen, M.; Moreno, M.; et al. Transcranial direct current stimulation versus intermittent theta-burst stimulation for the improvement of working memory performance. Int. J. Clin. Health Psychol. 2023, 23, 100334. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Shen, B.; Zhang, C.; Zhang, X.; Yang, S.; Zhou, J.; Fu, W. Anodal transcranial direct current stimulation enhances ankle force control and modulates the beta-band activity of the sensorimotor cortex. Cereb. Cortex 2023, 33, 7670–7677. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Wang, B.; Yu, C.; Shen, B.; Zhang, X.; Ye, D.; Deng, L.; Xu, Y.; Zhou, J.; Fu, W. Effects of intervention combining transcranial direct current stimulation and foot core exercise on sensorimotor function in foot and static balance. J. Neuroeng. Rehabil. 2022, 19, 98. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Zhang, Q.F.; Xiao, K.W.; Wang, L.; Yu, Q.P.; Xie, Q.; Poo, M.M.; Wen, Y. Neural Mechanism Underlying Task-Specific Enhancement of Motor Learning by Concurrent Transcranial Direct Current Stimulation. Neurosci. Bull. 2023, 39, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Zénon, A.; Sidibé, M.; Olivier, E. Disrupting the supplementary motor area makes physical effort appear less effortful. J. Neurosci. 2015, 35, 8737–8744. [Google Scholar] [CrossRef]
- Abdelmoula, A.; Baudry, S.; Duchateau, J. Anodal transcranial direct current stimulation enhances time to task failure of a submaximal contraction of elbow flexors without changing corticospinal excitability. Neuroscience 2016, 322, 94–103. [Google Scholar] [CrossRef]
- Oki, K.; Mahato, N.K.; Nakazawa, M.; Amano, S.; France, C.R.; Russ, D.W.; Clark, B.C. Preliminary Evidence That Excitatory Transcranial Direct Current Stimulation Extends Time to Task Failure of a Sustained, Submaximal Muscular Contraction in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1109–1112. [Google Scholar] [CrossRef]
- Vitor-Costa, M.; Okuno, N.M.; Bortolotti, H.; Bertollo, M.; Boggio, P.S.; Fregni, F.; Altimari, L.R. Improving Cycling Performance: Transcranial Direct Current Stimulation Increases Time to Exhaustion in Cycling. PLoS ONE 2015, 10, e0144916. [Google Scholar] [CrossRef]
- Angius, L.; Mauger, A.R.; Hopker, J.; Pascual-Leone, A.; Santarnecchi, E.; Marcora, S.M. Bilateral extracephalic transcranial direct current stimulation improves endurance performance in healthy individuals. Brain Stimul. 2018, 11, 108–117. [Google Scholar] [CrossRef]
- Isis, S.; Armele, D.; Paulo, G.L.; Raylene, A.; Luam, D.; Marina, B.R.; Adriana, B.; Katia, M.S. The effect of tDCS on improving physical performance and attenuating effort perception during maximal dynamic exercise in non-athletes. Neurosci. Lett. 2023, 794, 136991. [Google Scholar] [CrossRef]
- Muthalib, M.; Kan, B.; Nosaka, K.; Perrey, S. Effects of transcranial direct current stimulation of the motor cortex on prefrontal cortex activation during a neuromuscular fatigue task: An fNIRS study. Adv. Exp. Med. Biol. 2013, 789, 73–79. [Google Scholar] [CrossRef]
- Patel, R.; Dawidziuk, A.; Darzi, A.; Singh, H.; Leff, D.R. Systematic review of combined functional near-infrared spectroscopy and transcranial direct-current stimulation studies. Neurophotonics 2020, 7, 020901. [Google Scholar] [CrossRef] [PubMed]
- Cogiamanian, F.; Marceglia, S.; Ardolino, G.; Barbieri, S.; Priori, A. Improved isometric force endurance after transcranial direct current stimulation over the human motor cortical areas. Eur. J. Neurosci. 2007, 26, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.S.; Hoffman, R.L.; Clark, B.C. Preliminary evidence that anodal transcranial direct current stimulation enhances time to task failure of a sustained submaximal contraction. PLoS ONE 2013, 8, e81418. [Google Scholar] [CrossRef] [PubMed]
- Angius, L.; Hopker, J.; Mauger, A.R. The Ergogenic Effects of Transcranial Direct Current Stimulation on Exercise Performance. Front. Physiol. 2017, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.; Unal, G.; Andrade, S.M.; Moreira, A.; Altimari, L.R.; Brunoni, A.R.; Perrey, S.; Mauger, A.R.; Bikson, M.; Okano, A.H. Effect of transcranial direct current stimulation on exercise performance: A systematic review and meta-analysis. Brain Stimul. 2019, 12, 593–605. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, Ed000142. [Google Scholar] [CrossRef]
- Angius, L.; Santarnecchi, E.; Pascual-Leone, A.; Marcora, S.M. Transcranial Direct Current Stimulation over the Left Dorsolateral Prefrontal Cortex Improves Inhibitory Control and Endurance Performance in Healthy Individuals. Neuroscience 2019, 419, 34–45. [Google Scholar] [CrossRef]
- Sidhu, S.K. Remote muscle priming anodal transcranial direct current stimulation attenuates short interval intracortical inhibition and increases time to task failure of a constant workload cycling exercise. Exp. Brain Res. 2021, 239, 1975–1985. [Google Scholar] [CrossRef]
- Codella, R.; Alongi, R.; Filipas, L.; Luzi, L. Ergogenic Effects of Bihemispheric Transcranial Direct Current Stimulation on Fitness: A Randomized Cross-over Trial. Int. J. Sports Med. 2021, 42, 66–73. [Google Scholar] [CrossRef]
- Sasada, S.; Endoh, T.; Ishii, T.; Komiyama, T. Polarity-dependent improvement of maximal-effort sprint cycling performance by direct current stimulation of the central nervous system. Neurosci. Lett. 2017, 657, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Wrightson, J.G.; Twomey, R.; Yeung, S.T.Y.; Millet, G.Y. No effect of tDCS of the primary motor cortex on isometric exercise performance or perceived fatigue. Eur. J. Neurosci. 2020, 52, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Denis, G.; Zory, R.; Radel, R. Testing the role of cognitive inhibition in physical endurance using high-definition transcranial direct current stimulation over the prefrontal cortex. Hum. Mov. Sci. 2019, 67, 102507. [Google Scholar] [CrossRef] [PubMed]
- Flood, A.; Waddington, G.; Keegan, R.J.; Thompson, K.G.; Cathcart, S. The effects of elevated pain inhibition on endurance exercise performance. PeerJ 2017, 5, e3028. [Google Scholar] [CrossRef]
- Byrne, R.; Flood, A. The influence of transcranial direct current stimulation on pain affect and endurance exercise. Psychol. Sport Exerc. 2019, 45, 101554. [Google Scholar] [CrossRef]
- Angius, L.; Pageaux, B.; Hopker, J.; Marcora, S.M.; Mauger, A.R. Transcranial direct current stimulation improves isometric time to exhaustion of the knee extensors. Neuroscience 2016, 339, 363–375. [Google Scholar] [CrossRef]
- Kamali, A.M.; Saadi, Z.K.; Yahyavi, S.S.; Zarifkar, A.; Aligholi, H.; Nami, M. Transcranial direct current stimulation to enhance athletic performance outcome in experienced bodybuilders. PLoS ONE 2019, 14, e0220363. [Google Scholar] [CrossRef]
- Giboin, L.S.; Gruber, M. Anodal and cathodal transcranial direct current stimulation can decrease force output of knee extensors during an intermittent MVC fatiguing task in young healthy male participants. J. Neurosci. Res. 2018, 96, 1600–1609. [Google Scholar] [CrossRef]
- Ciccone, A.B.; Deckert, J.A.; Schlabs, C.R.; Tilden, M.J.; Herda, T.J.; Gallagher, P.M.; Weir, J.P. Transcranial Direct Current Stimulation of the Temporal Lobe Does Not Affect High-Intensity Work Capacity. J. Strength Cond. Res. 2019, 33, 2074–2086. [Google Scholar] [CrossRef]
- Montenegro, R.A.; Farinatti, P.T.V.; de Lima, P.F.M.; Okano, A.; Meneses, A.; Oliveira-Neto, L.d.; Cavalcante, B.R.; Correia, M.d.A.; Fontes, E.B.; Ritti-Dias, R.M. Motor cortex tDCS does not modulate perceived exertion within multiple-sets of resistance exercises. Isokinet. Exerc. Sci. 2016, 24, 17–24. [Google Scholar] [CrossRef]
- Workman, C.D.; Kamholz, J.; Rudroff, T. The Tolerability and Efficacy of 4 mA Transcranial Direct Current Stimulation on Leg Muscle Fatigability. Brain Sci. 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Seidel-Marzi, O.; Ragert, P. Anodal transcranial direct current stimulation reduces motor slowing in athletes and non-athletes. BMC Neurosci. 2020, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Savoury, R.B.; Kibele, A.; Power, K.E.; Herat, N.; Alizadeh, S.; Behm, D.G. Reduced isometric knee extensor force following anodal transcranial direct current stimulation of the ipsilateral motor cortex. PLoS ONE 2023, 18, e0280129. [Google Scholar] [CrossRef] [PubMed]
- Deters, J.R.; Fietsam, A.C.; Workman, C.D.; Rudroff, T. High Estrogen Levels Cause Greater Leg Muscle Fatigability in Eumenorrheic Young Women after 4 mA Transcranial Direct Current Stimulation. Brain Sci. 2022, 12, 506. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Yu, C.; Xiao, S.; Shen, B.; Zhang, C.; Zhou, J.; Fu, W. Effects of high-definition transcranial direct current stimulation on the cortical-muscular functional coupling and muscular activities of ankle dorsi-plantarflexion under running-induced fatigue. Front. Physiol. 2023, 14, 1263309. [Google Scholar] [CrossRef]
- van Duinen, H.; Renken, R.; Maurits, N.; Zijdewind, I. Effects of motor fatigue on human brain activity, an fMRI study. Neuroimage 2007, 35, 1438–1449. [Google Scholar] [CrossRef]
- de Morree, H.M.; Klein, C.; Marcora, S.M. Perception of effort reflects central motor command during movement execution. Psychophysiology 2012, 49, 1242–1253. [Google Scholar] [CrossRef]
- Liu, N.; Yang, C.; Song, Q.; Yang, F.; Chen, Y. Patients with chronic ankle instability exhibit increased sensorimotor cortex activation and correlation with poorer lateral balance control ability during single-leg stance: A FNIRS study. Front. Hum. Neurosci. 2024, 18, 1366443. [Google Scholar] [CrossRef]
- Haggard, P.; Whitford, B. Supplementary motor area provides an efferent signal for sensory suppression. Brain Res. Cogn. Brain Res. 2004, 19, 52–58. [Google Scholar] [CrossRef]
- Liu, J.Z.; Dai, T.H.; Sahgal, V.; Brown, R.W.; Yue, G.H. Nonlinear cortical modulation of muscle fatigue: A functional MRI study. Brain Res. 2002, 957, 320–329. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 2001, 57, 1899–1901. [Google Scholar] [CrossRef] [PubMed]
- Devanathan, D.; Madhavan, S. Effects of anodal tDCS of the lower limb M1 on ankle reaction time in young adults. Exp. Brain Res. 2016, 234, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, C.; Yang, H.; Shao, Q.; Niu, W.; Yang, Y.; Zheng, F. Halo Sport Transcranial Direct Current Stimulation Improved Muscular Endurance Performance and Neuromuscular Efficiency During an Isometric Submaximal Fatiguing Elbow Flexion Task. Front. Hum. Neurosci. 2022, 16, 758891. [Google Scholar] [CrossRef] [PubMed]
- Teymoori, H.; Amiri, E.; Tahmasebi, W.; Hoseini, R.; Grospretre, S.; Machado, D. Effect of tDCS targeting the M1 or left DLPFC on physical performance, psychophysiological responses, and cognitive function in repeated all-out cycling: A randomized controlled trial. J. Neuroeng. Rehabil. 2023, 20, 97. [Google Scholar] [CrossRef]
- Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef]
- Tornero-Aguilera, J.F.; Jimenez-Morcillo, J.; Rubio-Zarapuz, A.; Clemente-Suárez, V.J. Central and Peripheral Fatigue in Physical Exercise Explained: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 3909. [Google Scholar] [CrossRef]
- Sun, J.; Liu, G.; Sun, Y.; Lin, K.; Zhou, Z.; Cai, J. Application of Surface Electromyography in Exercise Fatigue: A Review. Front. Syst. Neurosci. 2022, 16, 893275. [Google Scholar] [CrossRef]
- Salehinejad, M.A.; Vosough, Y.; Nejati, V. The Impact of Bilateral Anodal tDCS over Left and Right DLPFC on Executive Functions in Children with ADHD. Brain Sci. 2022, 12, 1098. [Google Scholar] [CrossRef]
- Ma, Y.; Yin, K.; Zhuang, W.; Zhang, C.; Jiang, Y.; Huang, J.; Manor, B.; Zhou, J.; Liu, Y. Effects of Combining High-Definition Transcranial Direct Current Stimulation with Short-Foot Exercise on Chronic Ankle Instability: A Pilot Randomized and Double-Blinded Study. Brain Sci. 2020, 10, 749. [Google Scholar] [CrossRef]
- Santos Ferreira, I.; Teixeira Costa, B.; Lima Ramos, C.; Lucena, P.; Thibaut, A.; Fregni, F. Searching for the optimal tDCS target for motor rehabilitation. J. Neuroeng. Rehabil. 2019, 16, 90. [Google Scholar] [CrossRef]
- Labree, B.; Hoare, D.J.; Gascoyne, L.E.; Scutt, P.; Del Giovane, C.; Sereda, M. Determining the Effects of Transcranial Direct Current Stimulation on Tinnitus, Depression, and Anxiety: A Systematic Review. Brain Sci. 2022, 12, 484. [Google Scholar] [CrossRef] [PubMed]
- Dmochowski, J.P.; Datta, A.; Bikson, M.; Su, Y.; Parra, L.C. Optimized multi-electrode stimulation increases focality and intensity at target. J. Neural. Eng. 2011, 8, 046011. [Google Scholar] [CrossRef] [PubMed]
- Jaberzadeh, S.; Zoghi, M. Transcranial Direct Current Stimulation Enhances Exercise Performance: A Mini Review of the Underlying Mechanisms. Front. Neuroergon. 2022, 3, 841911. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, S.; Larochelle-Brunet, F.; Lafleur, L.P.; El Mouderrib, S.; Lepage, J.F.; Théoret, H. Systematic assessment of duration and intensity of anodal transcranial direct current stimulation on primary motor cortex excitability. Eur. J. Neurosci. 2016, 44, 2184–2190. [Google Scholar] [CrossRef] [PubMed]
- Batsikadze, G.; Moliadze, V.; Paulus, W.; Kuo, M.F.; Nitsche, M.A. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol 2013, 591, 1987–2000. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xiao, S.; Shen, B.; Zhang, C.; Zhan, J.; Li, J.; Li, J.; Zhou, J.; Fu, W. Gray Matter Volumes Mediate the Relationship Between Disease Duration and Balance Control Performance in Chronic Ankle Instability. Scand. J. Med. Sci. Sports 2024, 34, e14725. [Google Scholar] [CrossRef]
- Shen, B.; Xiao, S.; Yu, C.; Zhang, C.; Zhan, J.; Liu, Y.; Fu, W. Cerebral hemodynamics underlying ankle force sense modulated by high-definition transcranial direct current stimulation. Cereb. Cortex 2024, 34, bhae226. [Google Scholar] [CrossRef]

| Study | Sample (Male/Female) | Age (Years) | tDCS Protocol | Fatigue Protocol | Main Outcomes | |||
|---|---|---|---|---|---|---|---|---|
| Anodal/Cathodal Location | Electrode Size (cm2) | Current (mA) | Duration (min) | |||||
| Angius et al, 2018 [19] | 8/4 | 24 ± 5 | A: bilateral M1, C: ipsilateral shoulders/A: ipsilateral shoulders, C: bilateral M1 | A: 35, C: 25 A: 25, C: 35 | 2 | 10 | 70% Wpeak Cycling | TTF↑ corticospinal excitability (VL)↑ ∆B[La−]↑ RPE↓ |
| Vitor-Costa et al, 2015 [18] | 11/0 | 26 ± 4 | A: Cz, R: occipital protuberance/C: Cz, R: occipital protuberance | A: 36, C: 35 | 2 | 13 | 80% Wpeak Cycling | TTF↑ peak power← RPE← HR← sEMG activity (VL)← |
| Angius et al, 2019 [28] | 9/3 | 23 ± 3 | A: F3, C: Fp2 | A: 35, C: 25 | 2 | 30 | 70% Wpeak Cycling | TTF↑ RPE↓ ∆B[La−]↑ HR↓ |
| Sidhu et al, 2021 [29] | 12/0 | 20.8 ± 0.4 | A: left M1, C: right supraorbital | 25 | 2 | 10 | 80% Wpeak Cycling | TTF↑ RPE← HR← |
| Sasada et al, 2017 [31] | 17/6 | 21~30 | A: vertex, C: right forehead/A: right forehead, C: vertex | 35 | 2 | 15 | 30s maximum effort sprint cycling | pooled mean power← peak power← |
| Codella et al, 2021 [30] | 17/0 | 30.9 ± 6.5 | portable tDCS headset: Cz, C1–C6 | 3 × 28 | 2 | 20 | modified Bruce ramp protocol | VO2peak↑ RPE↓ |
| Zhan et al, 2023 [45] | 24/0 | A: 21.5 ± 2.2 C: 21.7 ± 2.3 | A: Cz, C:C3, C4, Fz, Pz | 4 × 1 HD-tDCS | 2 | 20 | running-induced fatigue | sEMG activity (TA)↑ CMC (beta: C1-TA)↑ CMC (gamma: C1-TA, Cz-TA)↑ |
| Isis et al, 2023 [20] | 6/9 | 25.8 ± 5 | A: MI, C: T3/A: T3, C: M1 | 35 | 2 | 20 | maximal incremental exercise test (cycling) | TTF← sEMG activity (VL, RF, VM)← cortical excitability (VL)↑ |
| Study | Sample (Male/Female) | Age (Years) | tDCS Protocol | Fatigue Protocol | Main Outcomes | |||
|---|---|---|---|---|---|---|---|---|
| Anodal/Cathodal Location | Electrode Size (cm2) | Current (mA) | Duration (min) | |||||
| Byrne et al, 2019 [35] | 11/12 | 26 ± 5 | A: F3, C: Fp2 | 35 | 2 | 20 | 25% MVC isometric KE | TTF← MVC← muscle pain intensity← |
| Angius et al, 2016 [36] | 9/0 | 23 ± 2 | A: left M1, C: right prefrontal cortex/A: left M1, C: left shoulder | 12 | 2 | 10 | 20% MVC isometric KE | TTF↑ RPE↓ muscle pain intensity← |
| Wrightson et al, 2020 [32] | 11/9 | 23.8 ± 4.7 | A: hotspot for the right VL, C: left deltoid | 35 | 1, 2 | 10 | 20% MVC isometric KE | TTF← sEMG activity (VL)← cortical excitability (VL)← perceived fatigue← MVC← |
| Denis et al, 2019 [33] | 7/13 | 20.6 ± 1.7 | A: right dorsolateral prefrontal cortex, C: distance of 3.5 cm around the anode electrode | 4 × 1 HD-tDCS | 2 | 10+ (online) | 30% MVC isometric KE | TTF← RPE← |
| Flood et al, 2017 [34] | 12/0 | 24.4 ± 3.9 | A: C3, C: Cz, F3, T7, P3/A: C4, C: Cz, F4, T8, P4 | 4 × 1 HD-tDCS | 2 | 20 | 30% MVC isometric KE | TTF← endogenous pain inhibition↑ MVC← |
| Kamali et al, 2019 [37] | 12/0 | 18~40 | A: M1 + T3, C: bilateral shoulder | A: 35, C:16/A:16, C: 16 | 2 | 13 | 30% 1 RM isotonic KE | SEI↑ RPE↓ HR↓ 1 RM↑ sEMG activity (RF)↑ |
| Giboin et al, 2018 [38] | 14/0 | 26 ± 3 | A: hotspot for the right VL, C: contra lateral orbit | 35 | 2 | 10 (online) | 35 × 5 s MVC isometric KE | amplitude of MVC↓ sEMG activity (VL)↓ |
| Ciccone et al, 2019 [39] | 10/10 | 21.0 ± 1.5 | A: T3, C: Fp2 /A: T4, C: Fp1 | 25 | 2 | 30 | 50 maximal effort isokinetic KE | fatigue index← mean torque integral← HR variability← |
| Montenegro et al, 2016 [40] | 13/0 | 26 ± 4 | A: M1, C: Fp2 | 35 | 2 | 20 | 3 × 10 maximal effort isokinetic KE | total work← work fatigue percentage← peak torque← sEMG activity (VM, RF, BF, ST)← |
| Workman et al, 2019 [41] | 12/22 | 24 ±3.6 | A: C3, C: contralateral supraorbital area | 35 | 4 | 20 (online) | 40 maximal effort isokinetic KE and KF | fatigue index (KF muscle group)↑ fatigue index (KE muscle group)← |
| Savoury et al, 2023 [43] | 8/8 | males 24.1 ± 2.8 females 21.9 ± 1.6 | A: M1, C: ipsilateral shoulder area | A: 25; C: 35 | 2 | 10 | 12 × 5 s maximal effort isokinetic KE | MVC (KE)↓ normalized MVC (KE)↓ fatigue index← |
| Deters et al, 2022 [44] | 0/10 | 24.3 ± 5.5 | A: M1, C: Fp2 | A: 25; C: 35 | 4 | 20 | 40 120°/s maximal effort isokinetic KE and KF | fatigue index (during high-estrogen level)↑ sEMG activity (KE and KF)↑ |
| Seidel-Marzi et al, 2020 [42] | FB: 10/3 HB: 7/5 NA: 10/11 | FB: 24.0 ± 3.9 HB: 22.5 ± 4.3 NA: 27.0 ± 3.4 | A: Cz, C: Fz | A: 35; C: 100 | 2 | 20 (online) | 20 s foot-tapping tasks | maintenance of tapping frequency↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Shen, B.; Xiao, S.; Zhang, C.; Zhan, J.; Li, J.; Fu, W.; Jin, J. The Effect of Transcranial Direct Current Stimulation on Lower-Limb Endurance Performance: A Systematic Review. Bioengineering 2024, 11, 1088. https://doi.org/10.3390/bioengineering11111088
Xu Z, Shen B, Xiao S, Zhang C, Zhan J, Li J, Fu W, Jin J. The Effect of Transcranial Direct Current Stimulation on Lower-Limb Endurance Performance: A Systematic Review. Bioengineering. 2024; 11(11):1088. https://doi.org/10.3390/bioengineering11111088
Chicago/Turabian StyleXu, Zhen, Bin Shen, Songlin Xiao, Chuyi Zhang, Jianglong Zhan, Jingjing Li, Weijie Fu, and Jing Jin. 2024. "The Effect of Transcranial Direct Current Stimulation on Lower-Limb Endurance Performance: A Systematic Review" Bioengineering 11, no. 11: 1088. https://doi.org/10.3390/bioengineering11111088
APA StyleXu, Z., Shen, B., Xiao, S., Zhang, C., Zhan, J., Li, J., Fu, W., & Jin, J. (2024). The Effect of Transcranial Direct Current Stimulation on Lower-Limb Endurance Performance: A Systematic Review. Bioengineering, 11(11), 1088. https://doi.org/10.3390/bioengineering11111088






