The 3D Printing of Nanocomposites for Wearable Biosensors: Recent Advances, Challenges, and Prospects
Abstract
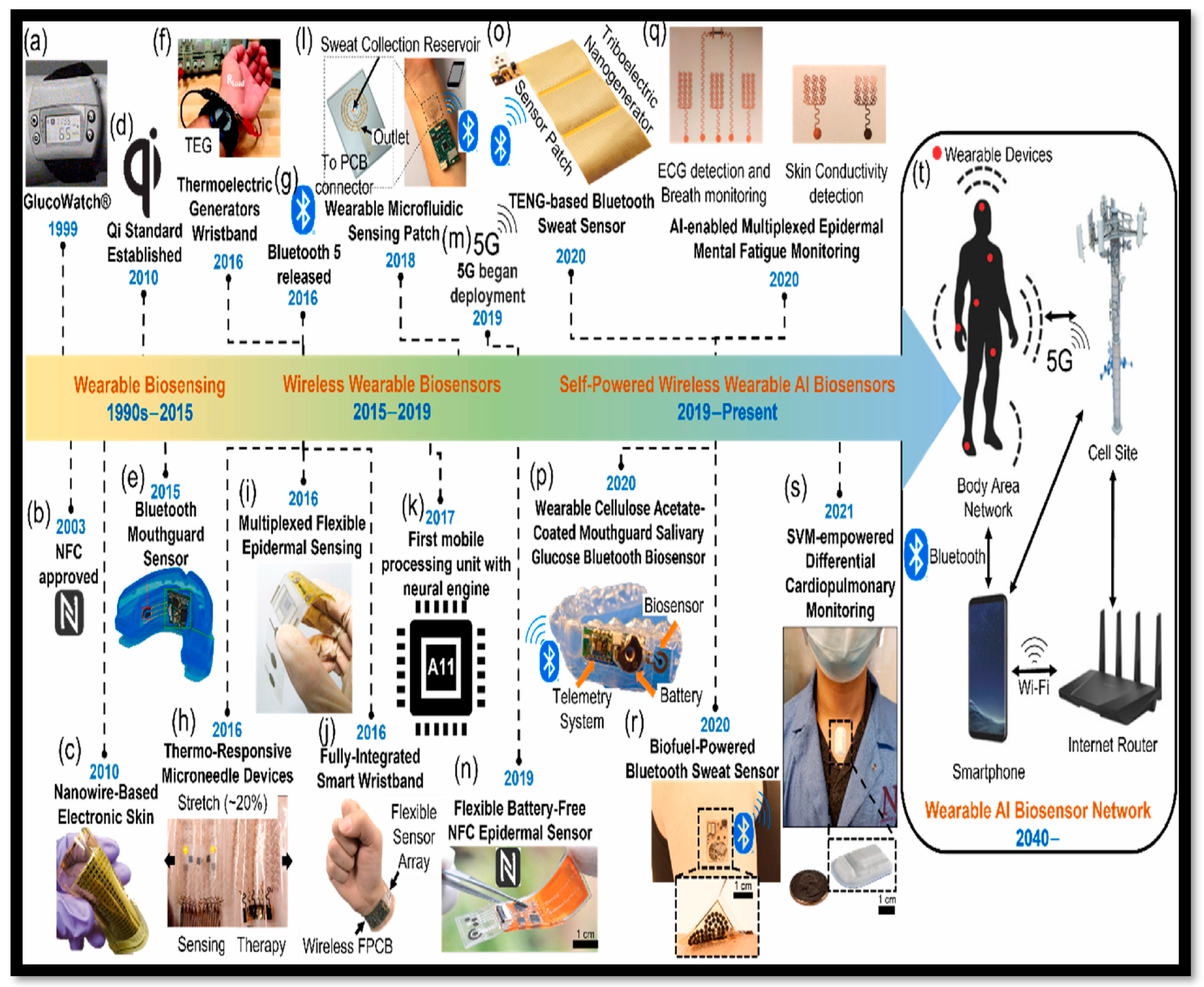
1. Introduction
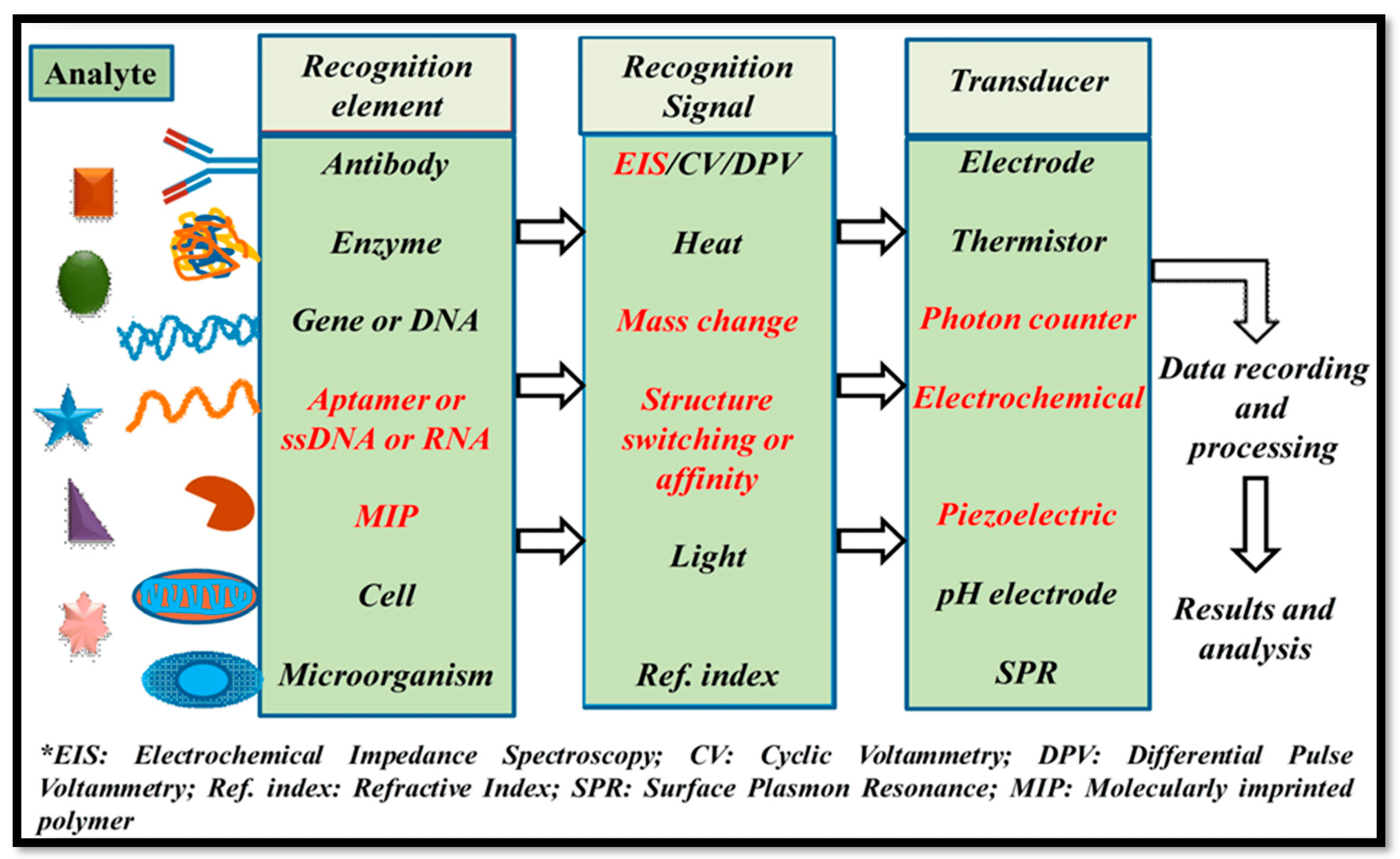
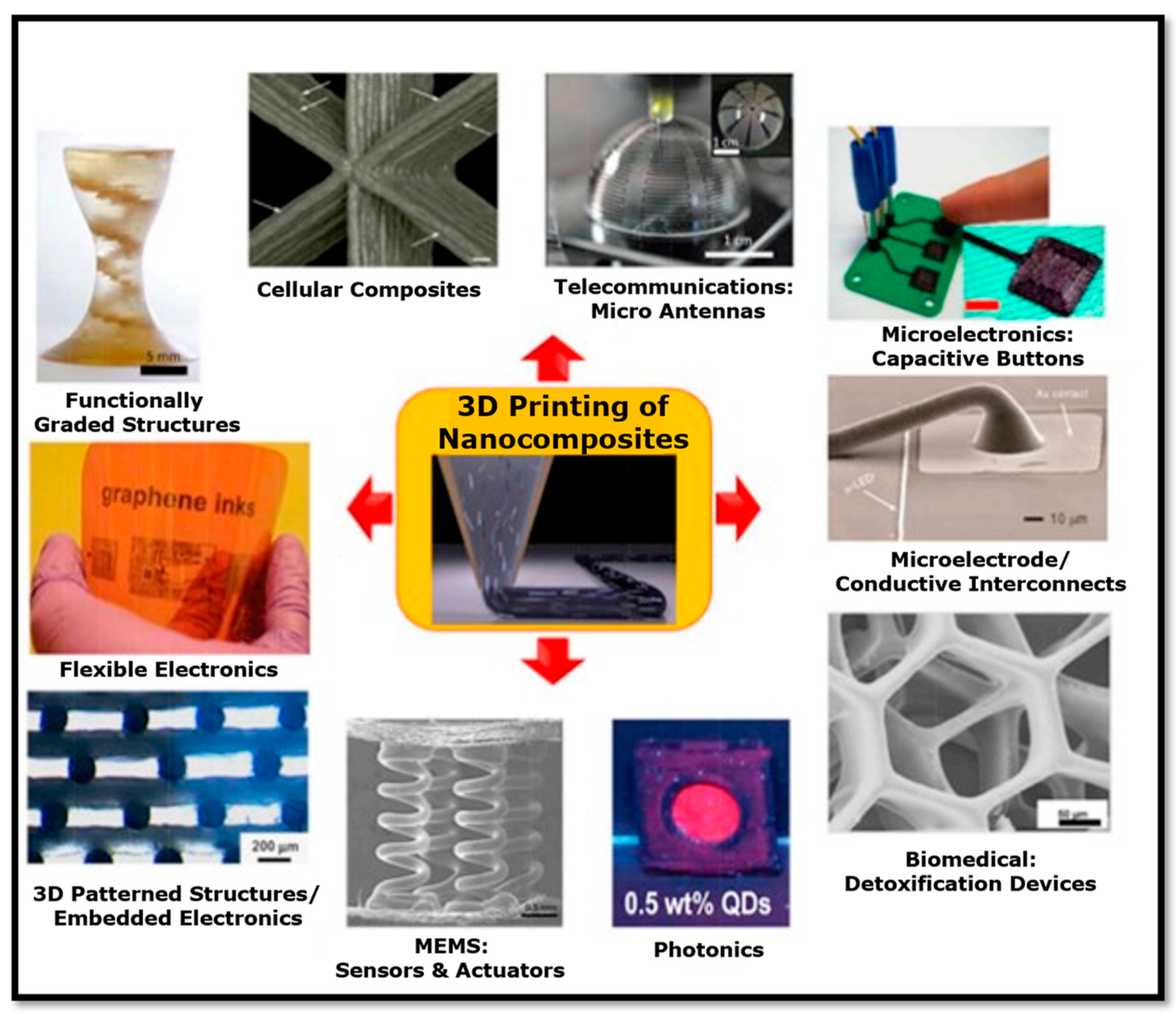
2. Nanocomposites
2.1. Polymer Matrix Nanocomposites (PMNCs)
2.2. Ceramic Matrix Nanocomposites (MMNCs)
2.3. Metal Matrix Nanocomposites (MMNCs)
3. Additive Manufacturing (3D Printing) Processes
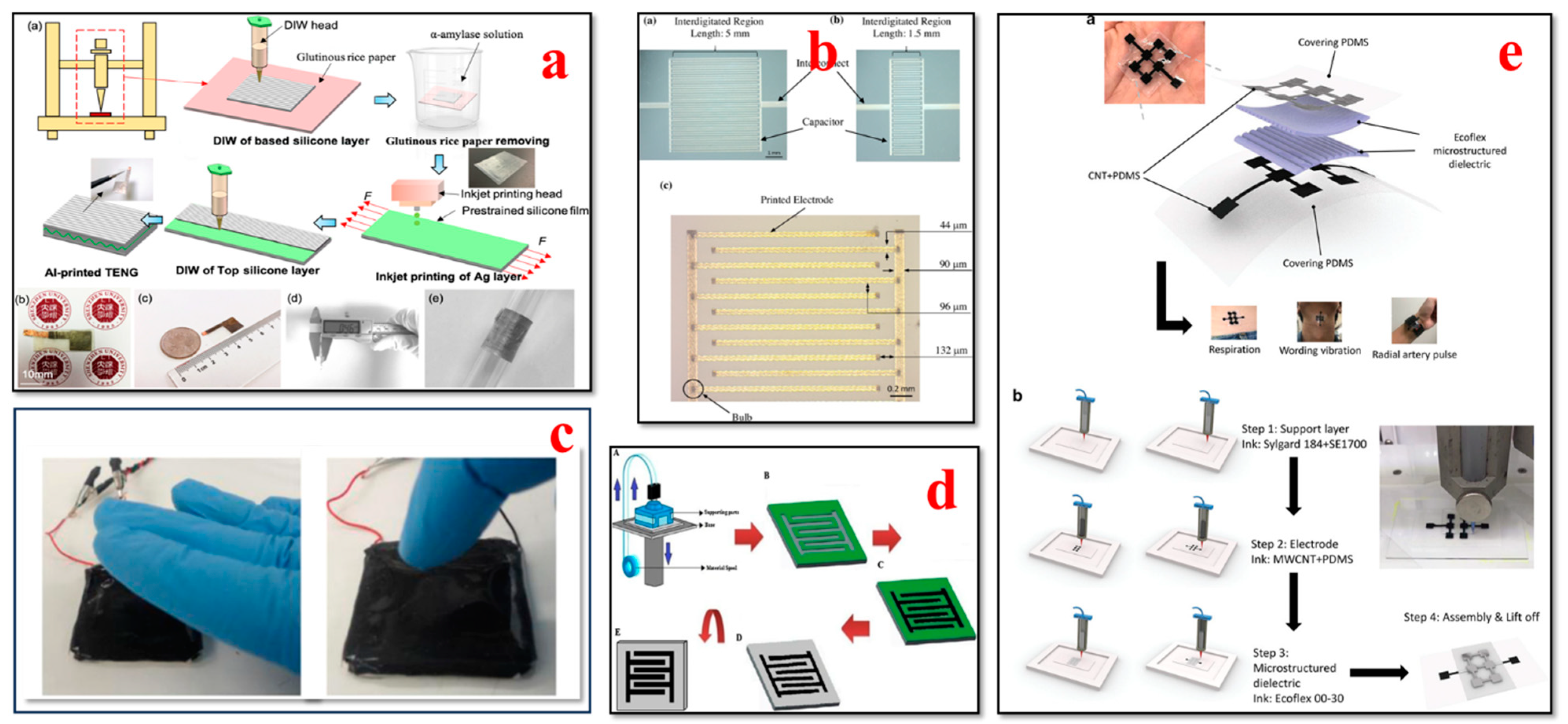
4. Recent Progress in Additive Manufacturing (3D Printing) of Wearable Biosensors
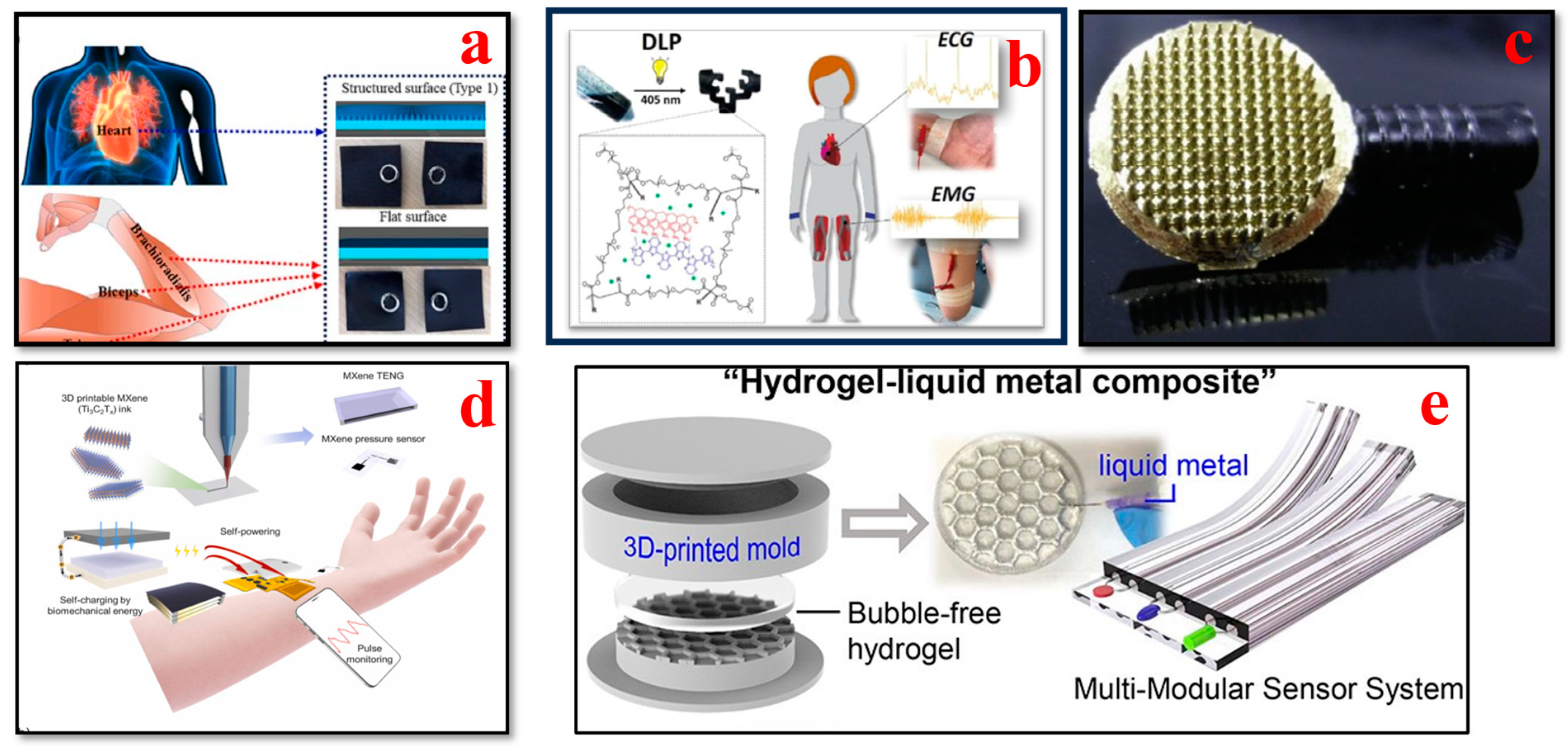
4.1. Electrocardiogram (ECG) Biosensors
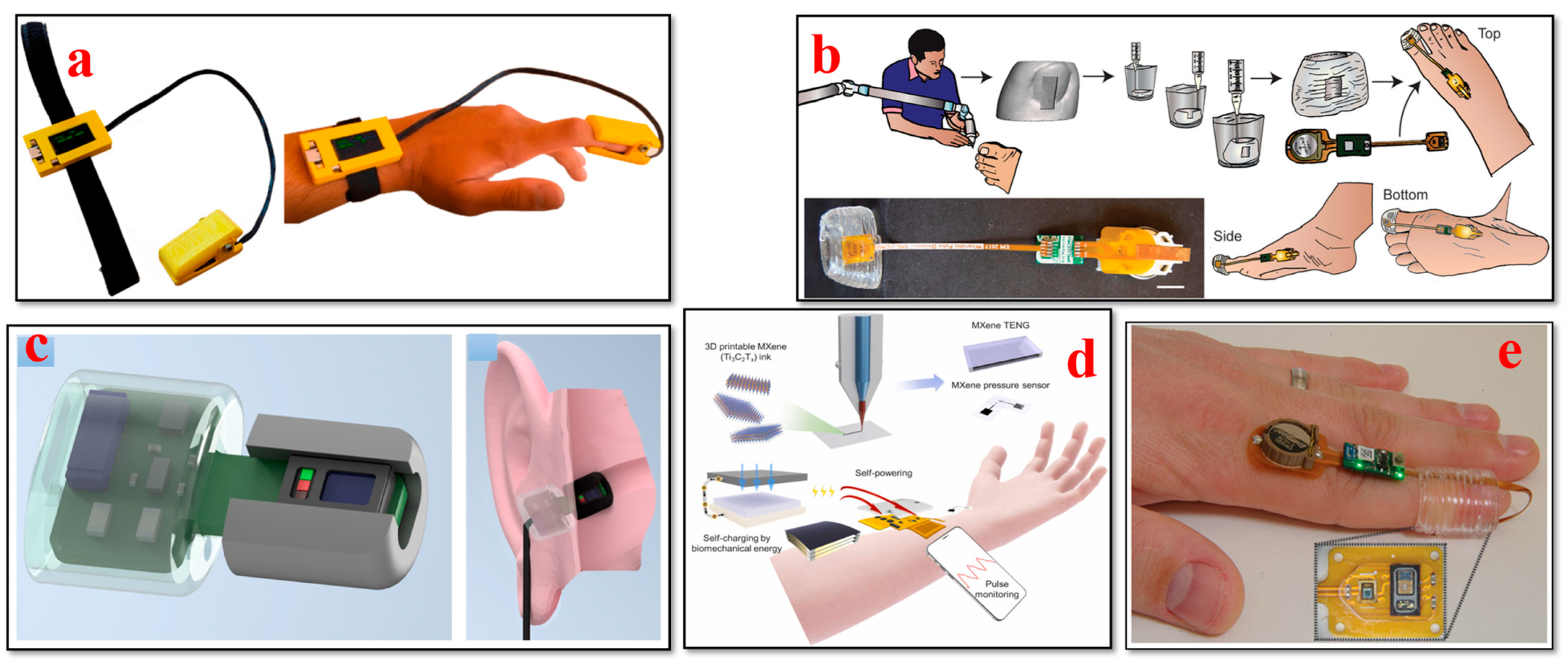
4.2. Electroencephalogram (EEG) Biosensors
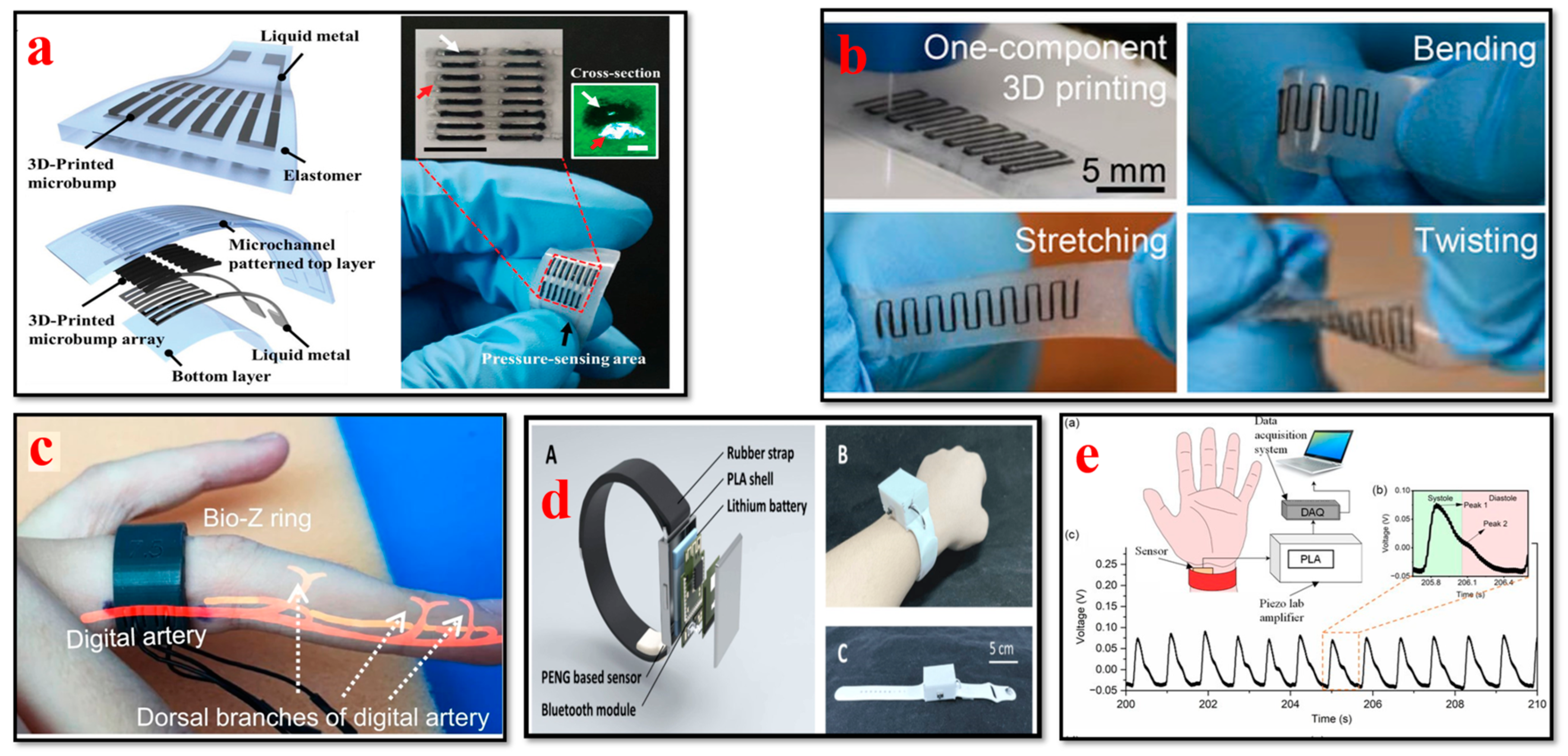
4.3. Blood Pressure Biosensors
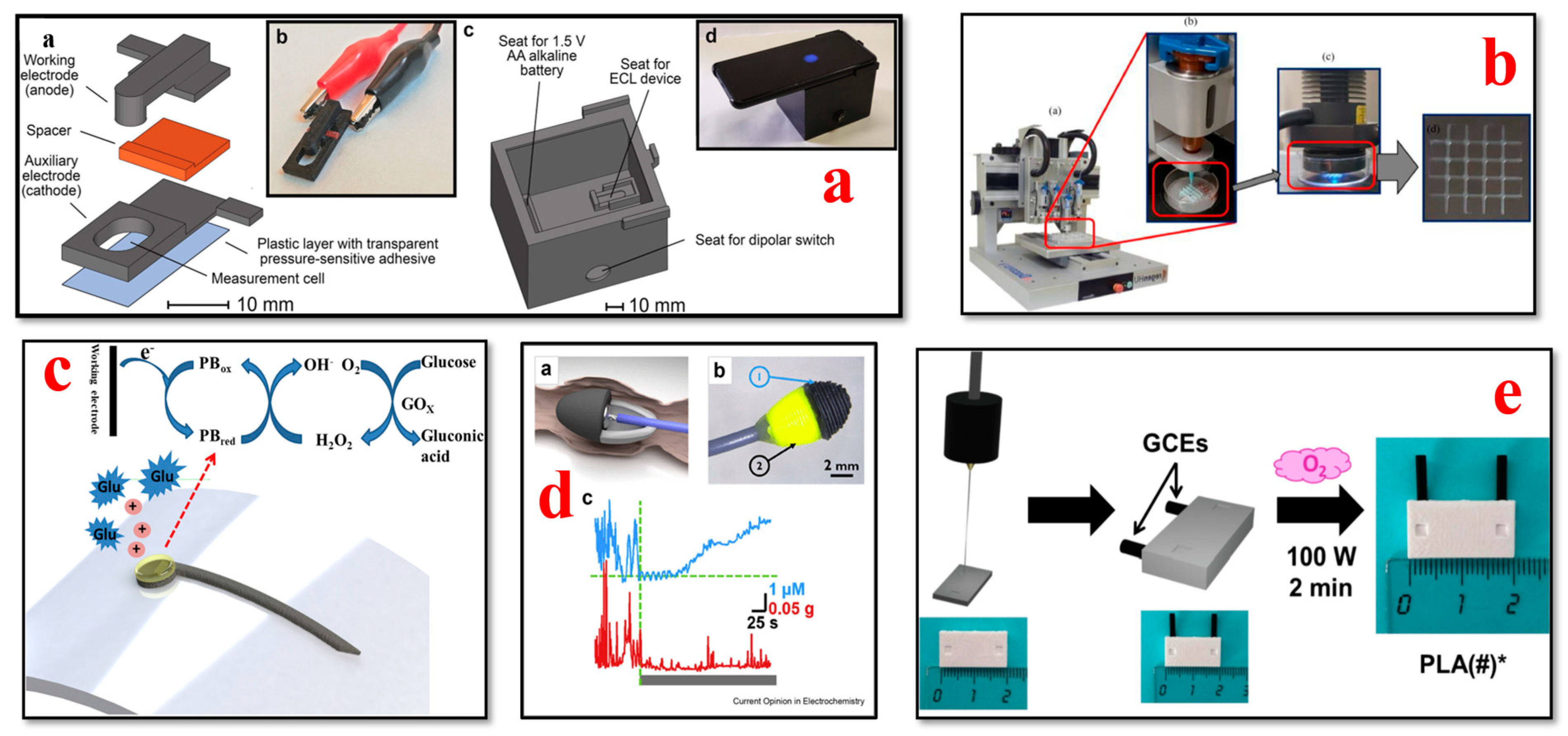
4.4. Glucose Biosensors
4.5. Oxygen Saturation (SpO2) Biosensors
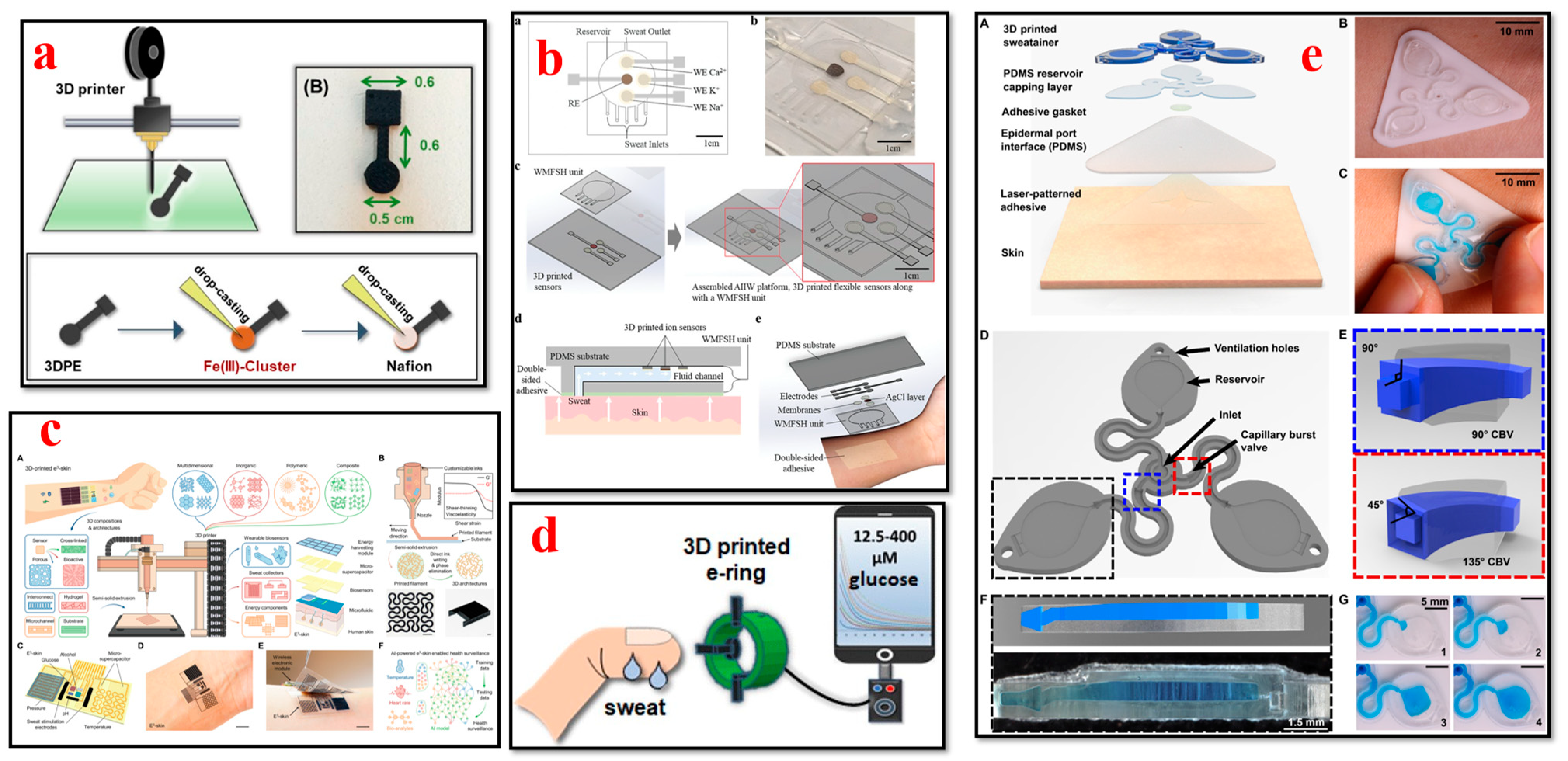
4.6. Sweat Biosensors
4.7. Tactile Biosensors
4.8. Respiratory Biosensors
4.9. A Comparison of 3D-Printed Wearable Biosensors with Traditional Wearable Biosensors
5. Challenges
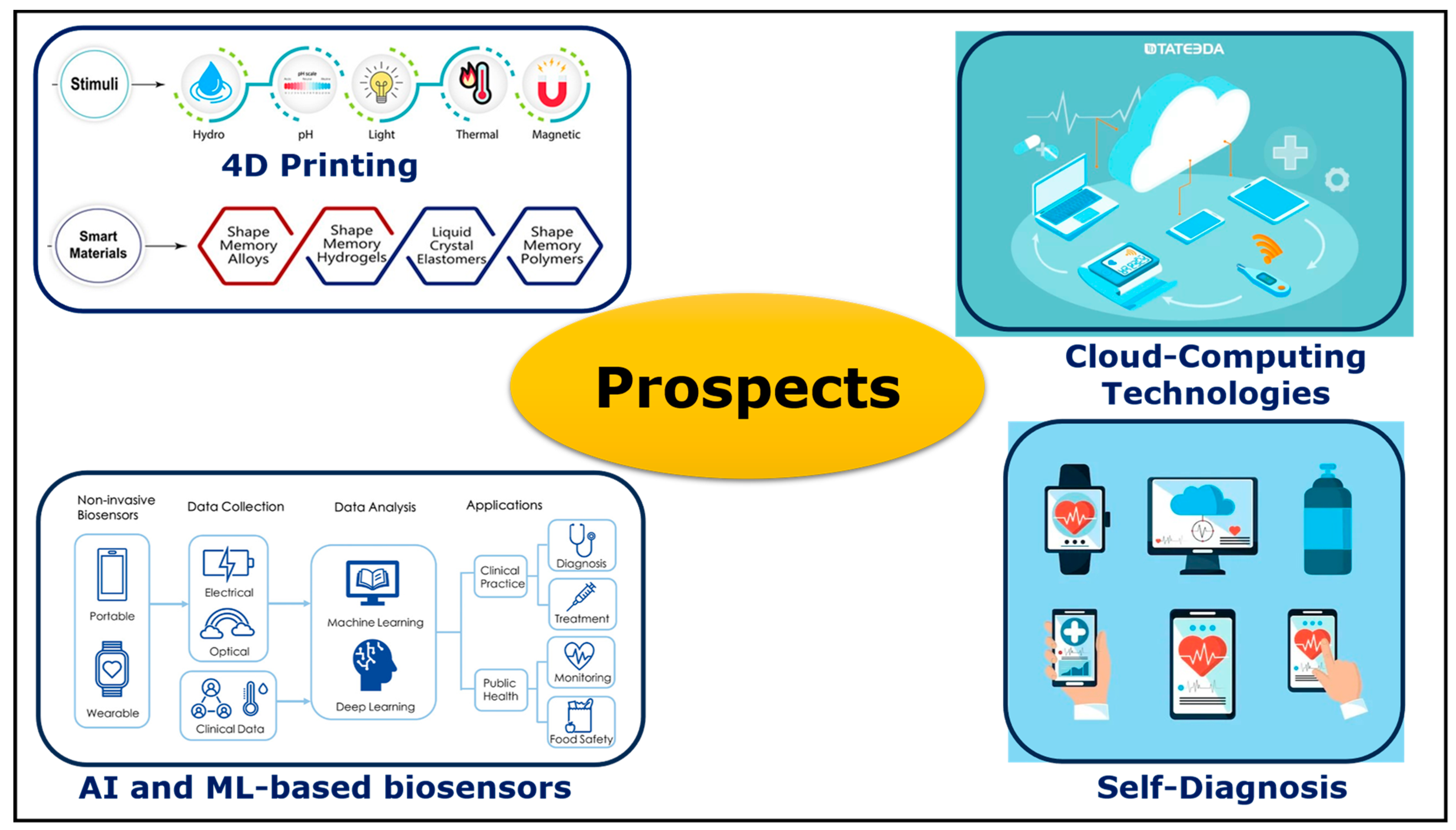
6. Prospects
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Straits Research. Wearable Sensors Market Size, Demand, Growth, Forecast Till 2030. August 2022. Available online: https://straitsresearch.com/report/wearable-sensors-market (accessed on 12 November 2023).
- Sharma, A.; Badea, M.; Tiwari, S.; Marty, J.L. Wearable biosensors: An alternative and practical approach in healthcare and disease monitoring. Molecules 2021, 26, 748. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Torrente-Rodríguez, R.M.; Wang, M.; Gao, W. The Era of Digital Health: A Review of Portable and Wearable Affinity Biosensors. Adv. Funct. Mater. 2020, 30, 1906713. [Google Scholar] [CrossRef]
- Lin, Y.; Bariya, M.; Javey, A. Wearable Biosensors for Body Computing. In Advanced Functional Materials; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2021; Volume 31. [Google Scholar] [CrossRef]
- Patel, S.; Park, H.; Bonato, P.; Chan, L.; Rodgers, M. A review of wearable sensors and systems with application in rehabilitation. J. Neuroeng. Rehabilitation 2012, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Chen, J.; He, B.-G.; Wang, W.; Zhu, Z.-L.; Lv, Z. Toward Wearable Sensors: Advances, Trends, and Challenges. ACM Comput. Surv. 2023, 55, 1–35. [Google Scholar] [CrossRef]
- Tan, M.; Xu, Y.; Gao, Z.; Yuan, T.; Liu, Q.; Yang, R.; Zhang, B.; Peng, L. Recent Advances in Intelligent Wearable Medical Devices Integrating Biosensing and Drug Delivery. Adv. Mater. 2022, 34, 2108491. [Google Scholar] [CrossRef] [PubMed]
- Vavrinsky, E.; Esfahani, N.E.; Hausner, M.; Kuzma, A.; Rezo, V.; Donoval, M.; Kosnacova, H. The Current State of Optical Sensors in Medical Wearables. Biosensors 2022, 12, 217. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.; Fortunato, G.; Radacsi, N. Wearable flexible sweat sensors for healthcare monitoring: A review. J. R. Soc. Interface 2019, 16, 20190217. [Google Scholar] [CrossRef]
- Dos Santos, C.C.; Lucena, G.N.; Pinto, G.C.; Júnior, M.J.; Marques, R.F. Advances and current challenges in non-invasive wearable sensors and wearable biosensors—A mini-review. Med. Devices Sens. 2021, 4, e10130. [Google Scholar] [CrossRef]
- Luo, H.; Gao, B. Development of smart wearable sensors for life healthcare. Eng. Regen. 2021, 2, 163–170. [Google Scholar] [CrossRef]
- Lee, E.K.; Yoo, H.; Lee, C.H. Advanced Materials and Assembly Strategies for Wearable Biosensors: A Review. Available online: www.intechopen.com (accessed on 24 October 2023).
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, M.; Peng, Y.; Li, S.; Han, D.; Ren, S.; Qin, K.; Li, S.; Han, T.; Wang, Y.; et al. Wearable biosensors for human fatigue diagnosis: A review. Bioeng. Transl. Med. 2023, 8, e10318. [Google Scholar] [CrossRef] [PubMed]
- Farooq, A.S.; Zhang, P. A comprehensive review on the prospects of next-generation wearable electronics for individualized health monitoring, assistive robotics, and communication. Sens. Actuators A Phys. 2022, 344, 113715. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Jeerapan, I.; Wang, J. Wearable Chemical Sensors: Present Challenges and Future Prospects. ACS Sens. 2016, 1, 464–482. [Google Scholar] [CrossRef]
- Janghorban, M.; Aradanas, I.; Kazemi, S.; Ngaju, P.; Pandey, R. Recent Advances, Opportunities, and Challenges in Developing Nucleic Acid Integrated Wearable Biosensors for Expanding the Capabilities of Wearable Technologies in Health Monitoring. Biosensors 2022, 12, 986. [Google Scholar] [CrossRef] [PubMed]
- Crean, C.; Mcgeough, C.; O’Kennedy, R. Wearable biosensors for medical applications. In Biosensors for Medical Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2012; pp. 301–330. [Google Scholar] [CrossRef]
- Khan, S.; Ali, S.; Bermak, A. Recent developments in printing flexible and wearable sensing electronics for healthcare applications. Sensors 2019, 19, 1230. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.D.; Turner, A.P.F. Historical perspective of biosensor and biochip development. In Handbook of Biosensors and Biochips; Wiley-Interscience: Hoboken, NJ, USA, 2007. [Google Scholar]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Palchetti, I.; Mascini, M. Biosensor technology: A brief history. In Sensors and Microsystems: AISEM 2009 Proceedings; Springer: Berlin/Heidelberg, Germany, 2009; pp. 15–23. [Google Scholar]
- Bahadır, E.B.; Sezgintürk, M.K. Applications of commercial biosensors in clinical, food, environmental, and biothreat/biowarfare analyses. Anal. Biochem. 2015, 478, 107–120. [Google Scholar] [CrossRef]
- Tierney, M.J.; Tamada, J.A.; Potts, R.O.; Jovanovic, L.; Garg, S.; Team, C.R. Clinical evaluation of the GlucoWatch® biographer: A continual, non-invasive glucose monitor for patients with diabetes. Biosens. Bioelectron. 2001, 16, 621–629. [Google Scholar] [CrossRef]
- Song, Y.; Mukasa, D.; Zhang, H.; Gao, W. Self-Powered Wearable Biosensors. Acc. Mater. Res. 2021, 2, 184–197. [Google Scholar] [CrossRef]
- Gualandi, I.; Marzocchi, M.; Achilli, A.; Cavedale, D.; Bonfiglio, A.; Fraboni, B. Textile Organic Electrochemical Transistors as a Platform for Wearable Biosensors. Sci. Rep. 2016, 6, 33637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, Y.; Jiang, N.; Yetisen, A.K. Wearable artificial intelligence biosensor networks. Biosens. Bioelectron. 2023, 219, 114825. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Piwek, L.; Ellis, D.A.; Andrews, S.; Joinson, A. The Rise of Consumer Health Wearables: Promises and Barriers. PLoS Med. 2016, 13, e1001953. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Y.; Ching, C.T.-S.; Wang, H.-M.D.; Liao, L.-D. Emerging Wearable Biosensor Technologies for Stress Monitoring and Their Real-World Applications. Biosensors 2022, 12, 1097. [Google Scholar] [CrossRef] [PubMed]
- Salim, A.; Lim, S. Recent advances in noninvasive flexible and wearable wireless biosensors. Biosens. Bioelectron. 2019, 141, 111422. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Ong, J.J.; Goyanes, A.; Orlu, M.; Gaisford, S.; Elbadawi, M.; Basit, A.W. Electrochemical biosensors: A nexus for precision medicine. Drug Discov. Today 2021, 26, 69–79. [Google Scholar] [CrossRef]
- Nam, D.; Cha, J.M.; Park, K. Next-generation wearable biosensors developed with flexible bio-chips. Micromachines 2021, 12, 64. [Google Scholar] [CrossRef]
- Yao, K.; Yang, Y.; Wu, P.; Zhao, G.; Wang, L.; Yu, X. Recent Advances in Materials, Designs and Applications of Skin Electronics. IEEE Open J. Nanotechnol. 2023, 4, 55–70. [Google Scholar] [CrossRef]
- Singh, S.U.; Chatterjee, S.; Lone, S.A.; Ho, H.-H.; Kaswan, K.; Peringeth, K.; Khan, A.; Chiang, Y.-W.; Lee, S.; Lin, Z.-H. Advanced wearable biosensors for the detection of body fluids and exhaled breath by graphene. Microchim. Acta 2022, 189, 236. [Google Scholar] [CrossRef]
- Shetti, N.P.; Mishra, A.; Basu, S.; Mascarenhas, R.J.; Kakarla, R.R.; Aminabhavi, T.M. Skin-Patchable Electrodes for Biosensor Applications: A Review. ACS Biomater. Sci. Eng. 2020, 6, 1823–1835. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.A.; Li, R.; Tse, Z.T.H. Reshaping healthcare with wearable biosensors. Sci. Rep. 2023, 13, 4998. [Google Scholar] [CrossRef] [PubMed]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J.; et al. Wearable sensors: Modalities, challenges, and prospects. Lab Chip 2018, 18, 217–248. [Google Scholar] [CrossRef] [PubMed]
- De Tommasi, F.; Massaroni, C.; Caponero, M.A.; Schena, E.; Presti, D.L.; Carassiti, M. Wearable 3D-Printed Thumb-Shaped Device Based on Fiber Bragg Grating Sensor for Epidural Space Detection. IEEE Sens. J. 2023, 23, 16907–16914. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, Q.; Zhan, R.; Xu, K.; Wang, Y.; Zhang, X.; Li, B.; Luo, G.; Xing, M.; Zhong, W. Tough but self-healing and 3D printable hydrogels for E-skin, E-noses and laser controlled actuators. J. Mater. Chem. A 2019, 7, 24814–24829. [Google Scholar] [CrossRef]
- Ramasamy, M.; Varadan, V.K. 3D printing of wearable fractal-based sensor systems for neurocardiology and healthcare. In Proceedings of the Nanosensors, Biosensors, Info-Tech Sensors and 3D Systems 2017, SPIE, Portland, OR, USA, 24 April 2017; p. 1016709. [Google Scholar]
- Ali, A.; Hu, C.; Yttri, E.A.; Panat, R. Recent Advances in 3D Printing of Biomedical Sensing Devices. Adv. Funct. Mater. 2022, 32, 2107671. [Google Scholar] [CrossRef]
- Parupelli, S.K.; Desai, S. Hybrid additive manufacturing (3D printing) and characterization of functionally gradient materials via in situ laser curing. Int. J. Adv. Manuf. Technol. 2020, 110, 543–556. [Google Scholar] [CrossRef]
- Agarwal, S.; Saha, S.; Balla, V.K.; Pal, A.; Barui, A.; Bodhak, S. Current Developments in 3D Bioprinting for Tissue and Organ Regeneration—A Review. Front. Mech. Eng. 2020, 6, 589171. [Google Scholar] [CrossRef]
- Muldoon, K.; Song, Y.; Ahmad, Z.; Chen, X.; Chang, M.-W. High Precision 3D Printing for Micro to Nano Scale Biomedical and Electronic Devices. Micromachines 2022, 13, 642. [Google Scholar] [CrossRef]
- Han, T.; Kundu, S.; Nag, A.; Xu, Y. 3D Printed sensors for biomedical applications: A review. Sensors 2019, 19, 1706. [Google Scholar] [CrossRef]
- Parupelli, S.K.; Aljohani, A.; Desai, S.; Khanal, S.; Bhattarai, N. Direct jet printing and characterization of calcium alginate microcapsules for biomedical applications. In Proceedings of the IIE Annual Conference. Proceedings, Institute of Industrial and Systems Engineers (IISE), Orlando, FL, USA, 18–21 May 2019; pp. 300–305. [Google Scholar]
- Ogunsanyaa, M.; Desai, S. Predictive modeling of additive manufacturing process using deep learning algorithm. In Proceedings of the IISE Annual Conference & Expo 2022, Seattle, WA, USA, 21–24 May 2022. [Google Scholar]
- Aldawood, F.; Desai, S. Additive Manufacturing of Compensator Devices for Radiation Therapy. In Proceedings of the 2020 IISE Annual Conference, New Orleans, LA, USA, 30 May–2 June 2020. [Google Scholar]
- Parupelli, S.K.; Desai, S. Understanding Hybrid Additive Manufacturing of Functional Devices. Am. J. Eng. Appl. Sci. 2017, 10, 264–271. [Google Scholar] [CrossRef][Green Version]
- Elhoone, H.; Zhang, T.; Anwar, M.; Desai, S. Cyber-based design for additive manufacturing using artificial neural networks for Industry 4.0. Int. J. Prod. Res. 2020, 58, 2841–2861. [Google Scholar] [CrossRef]
- McKenzie, J.; Parupelli, S.; Martin, D.; Desai, S. Additive manufacturing of multiphase materials for electronics. In Proceedings of the IIE Annual Conference. Proceedings, Institute of Industrial and Systems Engineers (IISE), Pittsburgh, PA, USA, 20–23 May 2017; pp. 1133–1138. [Google Scholar]
- Aldawood, F.K.; Chang, S.X.; Desai, S. Design and Manufacture of a High Precision Personalized Electron Bolus Device for Radiation Therapy. Med. Devices Sens. 2020, 3, e10077. [Google Scholar] [CrossRef]
- Tan, H.W.; Choong, Y.Y.C.; Kuo, C.N.; Low, H.Y.; Chua, C.K. 3D printed electronics: Processes, materials and future trends. Prog. Mater. Sci. 2022, 127, 100945. [Google Scholar] [CrossRef]
- Khosravani, M.R.; Reinicke, T. 3D-printed sensors: Current progress and future challenges. Sens. Actuators A Phys. 2020, 305, 111916. [Google Scholar] [CrossRef]
- Marzo, A.M.L.; Mayorga-Martinez, C.C.; Pumera, M. 3D-printed graphene direct electron transfer enzyme biosensors. Biosens. Bioelectron. 2020, 151, 111980. [Google Scholar] [CrossRef]
- Cheng, M.; Zhu, G.; Zhang, F.; Tang, W.-L.; Jianping, S.; Yang, J.-Q.; Zhu, L.-Y. A review of flexible force sensors for human health monitoring. J. Adv. Res. 2020, 26, 53–68. [Google Scholar] [CrossRef]
- Huang, Z.; Shao, G.; Li, L. Micro/nano functional devices fabricated by additive manufacturing. Prog. Mater. Sci. 2023, 131, 101020. [Google Scholar] [CrossRef]
- Wu, Y.; Kakaraparthi, V.; Li, Z.; Pham, T.; Liu, J.; Nguyen, P. BioFace-3D: Continuous 3d facial reconstruction through lightweight single-ear biosensors. In Proceedings of the MobiCom ‘21: Proceedings of the 27th Annual International Conference on Mobile Computing and Networking, New Orleans, LA, USA, 28 March–1 April 2022; Association for Computing Machinery (ACM): New York, NY, USA, 2021; pp. 350–363. [Google Scholar] [CrossRef]
- Zou, Z.; Chen, Y.; Yuan, S.; Luo, N.; Li, J.; He, Y. 3D Printing of Liquid Metals: Recent Advancements and Challenges. Adv. Funct. Mater. 2023, 33, 2213312. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, R.; Wu, X. Recent progress in the development of conductive hydrogels and the application in 3D printed wearable sensors. RSC Appl. Polym. 2023, 1, 132–157. [Google Scholar] [CrossRef]
- Padash, M.; Enz, C.; Carrara, S. Microfluidics by additive manufacturing for wearable biosensors: A review. Sensors 2020, 20, 4236. [Google Scholar] [CrossRef]
- Ogunsanya, M.; Isichei, J.; Parupelli, S.K.; Desai, S.; Cai, Y. In-situ droplet monitoring of inkjet 3D printing process using image analysis and machine learning models. Procedia Manuf. 2021, 53, 427–434. [Google Scholar] [CrossRef]
- Aruna Kumari, M.L.; Lokanatha Reddy, P.; Theerthagiri, J.; Lee, S.J.; Murthy, A.P.; Yu, Y.; Choi, M.Y. Additively Manufactured Electrochemical Sensors and Biosensors. Nanotechnol. -Based Addit. Manuf. Prod. Des. Prop. Appl. 2023, 2, 399–434. [Google Scholar] [CrossRef]
- McKenzie, J.; Desai, S. Investigating sintering mechanisms for additive manufacturing of conductive traces. Am. J. Eng. Appl. Sci. 2018, 11, 652–662. [Google Scholar] [CrossRef]
- Aljohani, A.; Desai, S. 3D Printing of porous scaffolds for medical applications. Am. J. Eng. Appl. Sci. 2018, 11, 1076–1085. [Google Scholar] [CrossRef]
- Zhang, L.; Forgham, H.; Shen, A.; Wang, J.; Zhu, J.; Huang, X.; Tang, S.-Y.; Xu, C.; Davis, T.P.; Qiao, R. Nanomaterial integrated 3D printing for biomedical applications. J. Mater. Chem. B 2022, 10, 7473–7490. [Google Scholar] [CrossRef] [PubMed]
- Sharafeldin, M.; Jones, A.; Rusling, J.F. 3D-printed biosensor arrays for medical diagnostics. Micromachines 2018, 9, 394. [Google Scholar] [CrossRef] [PubMed]
- Ogunsanya, M.; Isichei, J.; Desai, S. Grid search hyperparameter tuning in additive manufacturing processes. Manuf. Lett. 2023, 35, 1031–1042. [Google Scholar] [CrossRef]
- Clarissa, W.H.-Y.; Chia, C.H.; Zakaria, S.; Evyan, Y.C.-Y. Recent advancement in 3-D printing: Nanocomposites with added functionality. Prog. Addit. Manuf. 2022, 7, 325–350. [Google Scholar] [CrossRef]
- Zhu, W.; Webster, T.J.; Zhang, L.G.; Costa, J.B.; Silva-Correia, J.; Reis, R.L.; Oliveira, J.M.; Mazur, J.; Roy, K.; Kanwar, J.R.; et al. How can 3D printing be a powerful tool in nanomedicine? Nanomedicine 2018, 13, 251–253. [Google Scholar] [CrossRef]
- Desai, S.; Bidanda, B.; Bártolo, P.J. Emerging trends in the applications of metallic and ceramic biomaterials. In Bio-Materials and Prototyping Applications in Medicine; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2021; pp. 1–17. [Google Scholar]
- Desai, S.; Shankar, M.R. Emerging trends in polymers, composites, and nano biomaterial applications. In Bio-Materials and Prototyping Applications in Medicine; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2021; pp. 19–34. [Google Scholar]
- Kalkal, A.; Kumar, S.; Kumar, P.; Pradhan, R.; Willander, M.; Packirisamy, G.; Kumar, S.; Malhotra, B.D. Recent advances in 3D printing technologies for wearable (bio)sensors. Addit. Manuf. 2021, 46, 102088. [Google Scholar] [CrossRef]
- Yi, Q.; Najafikhoshnoo, S.; Das, P.; Noh, S.; Hoang, E.; Kim, T.; Esfandyarpour, R. All-3D-Printed, Flexible, and Hybrid Wearable Bioelectronic Tactile Sensors Using Biocompatible Nanocomposites for Health Monitoring. Adv. Mater. Technol. 2021, 7, 2101034. [Google Scholar] [CrossRef]
- Banigo, A.; Azeez, T.; Ejeta, K.; Lateef, A.; Ajuogu, E. Nanobiosensors: Applications in biomedical technology. IOP Conf.Ser. Mater. Sci. Eng. 2020, 805, 012028. [Google Scholar] [CrossRef]
- Remaggi, G.; Zaccarelli, A.; Elviri, L. 3D Printing Technologies in Biosensors Production: Recent Developments. Chemosensors 2022, 10, 65. [Google Scholar] [CrossRef]
- Ni, Y.; Ji, R.; Long, K.; Bu, T.; Chen, K.; Zhuang, S. A review of 3D-printed sensors. Appl. Spectrosc. Rev. 2017, 52, 623–652. [Google Scholar] [CrossRef]
- Khan, A.; Haque, N.; Kabiraz, D.C.; Yeasin, A.; Al Rashid, H.; Sarker, A.C.; Hossain, G. A review on advanced nanocomposites materials based smart textile biosensor for healthcare monitoring from human sweat. Sens. Actuators A Phys. 2023, 350, 114093. [Google Scholar] [CrossRef]
- Huynh, N.; Ho, B. 3D Printing Technology in Low-Cost Diagnostic Sensors for Neurological Disorders. Doctoral Dissertation, Washington State University, Pullman, WA, USA, 2023. [Google Scholar]
- Xu, Y.; Wu, X.; Guo, X.; Kong, B.; Zhang, M.; Qian, X.; Mi, S.; Sun, W. The Boom in 3D-Printed Sensor Technology. Sensors 2017, 17, 1166. [Google Scholar] [CrossRef] [PubMed]
- Aldawood, F.K.; Andar, A.; Desai, S. A comprehensive review of microneedles: Types, materials, processes, characterizations and applications. Polymers 2021, 13, 2815. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Li, C.; Chou, T.-W. Nanocomposites in context. Compos. Sci. Technol. 2005, 65, 491–516. [Google Scholar] [CrossRef]
- Vaia, R.A.; Wagner, H.D. Framework for nanocomposites. Mater. Today 2004, 7, 32–37. [Google Scholar] [CrossRef]
- Omanović-Mikličanin, E.; Badnjević, A.; Kazlagić, A.; Hajlovac, M. Nanocomposites: A brief review. Health Technol. 2019, 10, 51–59. [Google Scholar] [CrossRef]
- Okpala, C.C. Nanocomposites-An Overview. 2013. Available online: www.ijerd.com (accessed on 27 October 2023).
- Zare, Y.; Shabani, I. Polymer/metal nanocomposites for biomedical applications. Mater. Sci. Eng. C 2016, 60, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-M.; Awaji, H. Nanocomposites—A new material design concept. Sci. Technol. Adv. Mater. 2005, 6, 2–10. [Google Scholar] [CrossRef]
- Camargo, P.H.C.; Satyanarayana, K.G.; Wypych, F. Nanocomposites: Synthesis, Structure, Properties and New Application Opportunities. Mater. Res. 2009, 12, 1–39. [Google Scholar] [CrossRef]
- Ogasawara, T.; Ishida, Y.; Ishikawa, T.; Yokota, R. Characterization of multi-walled carbon nanotube/phenylethynyl terminated polyimide composites. Compos. Part A Appl. Sci. Manuf. 2004, 35, 67–74. [Google Scholar] [CrossRef]
- Bhushan, I.; Kumar, V.; Durgesh, S.; Tripathi, K. Nanotechnology in the Life Sciences Nanomaterials and Environmental Biotechnology. Available online: http://www.springer.com/series/15921 (accessed on 20 October 2023).
- Komarneni, S. Feature article. Nanocomposites. J. Mater. Chem. 1992, 2, 1219–1230. [Google Scholar] [CrossRef]
- Shanmugam, V.; Velu, R. 3D Printing of Nanocomposites. In Smart 3D Nanoprinting; CRC Press: Boca Raton, FL, USA, 2022; pp. 149–164. [Google Scholar] [CrossRef]
- Malik, P.; Gupta, R.; Malik, V.; Ameta, R.K. Emerging nanomaterials for improved biosensing. Meas. Sensors 2021, 16, 100050. [Google Scholar] [CrossRef]
- Praveena, B.A.; Lokesh, N.; Buradi, A.; Santhosh, N.; Praveena, B.L.; Vignesh, R. A comprehensive review of emerging additive manufacturing (3D printing technology): Methods, materials, applications, challenges, trends and future potential. Mater. Today: Proc. 2022, 52, 1309–1313. [Google Scholar] [CrossRef]
- Desai, S.; Parupelli, S. Maynard’s Industrial and Systems Engineering Handbook; Mcgraw Hill LLC: New York, NY, USA, 2022. [Google Scholar]
- Ghazy, M.M. Development of an Additive Manufacturing Decision Support System (AMDSS). Ph.D. Thesis, Faculty of Science, Agriculture and Engineering, School of Mechanical and Systems Engineering, Newcastle University, Newcastle, UK, 2012; pp. 1–17. Available online: https://theses.ncl.ac.uk/dspace/handle/10443/1692 (accessed on 27 September 2023).
- ISO/ASTM 52900:2021(en); Additive manufacturing—General principles—Fundamentals and vocabulary, International Organization for Standardization, Geneva, Switzerland. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso-astm:52900:ed-2:v1:en (accessed on 10 October 2023).
- ASTM F2792-12a; Standard Terminology for Additive Manufacturing Technologies. Rapid Manufacturing Association; ASTM International: West Conshohocken, PA, USA, 2013; pp. 10–12.
- Mamo, H.B.; Adamiak, M.; Kunwar, A. 3D printed biomedical devices and their applications: A review on state-of-the-art technologies, existing challenges, and future perspectives. J. Mech. Behav. Biomed. Mater. 2023, 143, 105930. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B. Additive Manufacturing Technologies 3D Printing, Rapid Prototyping, and Direct Digital Manufacturing; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Parupelli, S.K.; Desai, S. A Comprehensive Review of Additive Manufacturing (3D Printing): Processes, Applications and Future Potential. Am. J. Appl. Sci. 2019, 16, 244–272. [Google Scholar] [CrossRef]
- Aldawood, F.K.; Desai, S.; Parupelli, S.K.; Andar, A. Polymeric Microneedles Using Additive Manufacturing for Therapeutic Applications. In Proceedings of the IIE Annual Conference. Proceedings, Institute of Industrial and Systems Engineers (IISE), New Orleans, LA, USA, 21–23 May 2023; pp. 1–6. [Google Scholar]
- Adarkwa, E.; Roy, A.; Ohodnicki, J.; Lee, B.; Kumta, P.N.; Desai, S. 3D printing of drug-eluting bioactive multifunctional coatings for orthopedic applications. Int. J. Bioprinting 2023, 9, 158–175. [Google Scholar] [CrossRef]
- Olowe, M.; Parupelli, S.K.; Desai, S. A Review of 3D-Printing of Microneedles. Pharmaceutics 2022, 14, 2693. [Google Scholar] [CrossRef]
- Hassan, M.; Dave, K.; Chandrawati, R.; Dehghani, F.; Gomes, V.G. 3D printing of biopolymer nanocomposites for tissue engineering: Nanomaterials, processing and structure-function relation. Eur. Polym. J. 2019, 121, 109340. [Google Scholar] [CrossRef]
- Tettey, F.; Saudi, S.; Davies, D.; Shrestha, S.; Johnson, K.; Fialkova, S.; Subedi, K.; Bastakoti, B.P.; Sankar, J.; Desai, S.; et al. Fabrication and Characterization of Zn Particle Incorporated Fibrous Scaffolds for Potential Application in Tissue Healing and Regeneration. ACS Appl. Mater. Interfaces 2023, 15, 48913–48929. [Google Scholar] [CrossRef]
- Parupelli, S.K.; Saudi, S.; Bhattarai, N.; Desai, S. 3D printing of PCL-ceramic composite scaffolds for bone tissue engineering applications. Int. J. Bioprinting 2023, 9, 0196. [Google Scholar] [CrossRef]
- Ogunsanya, M.; Desai, S. Physics-based and data-driven modeling for biomanufacturing 4.0. Manuf. Lett. 2023, 36, 91–95. [Google Scholar] [CrossRef]
- Bártolo, P.; Bidanda, B. Bio-Materials and Prototyping Applications in Medicine; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Desai, S.; Shankar, M.R. Polymers, composites and nano biomaterials: Current and future developments. In Bio-Materials and Prototyping Applications in Medicine; Springer: Berlin/Heidelberg, Germany, 2008; pp. 15–26. [Google Scholar]
- Desai, S.; Bidanda, B.; Bartolo, P. Metallic and Ceramics Biomaterials: Current and Future Developments; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Perkins, J.; Desai, S.; Wagner, W.; Hong, Y. Biomanufacturing: Direct-writing of controlled release coatings for cardiovascular (Stents) applications. In Proceedings of the IIE Annual Conference. Proceedings, Institute of Industrial and Systems Engineers (IISE), Reno, NV, USA, 21–25 May 2011; p. 1. [Google Scholar]
- Wang, C.; Gong, D.; Feng, P.; Cheng, Y.; Cheng, X.; Jiang, Y.; Zhang, D.; Cai, J. Ultra-sensitive and wide sensing-range flexible pressure sensors based on the carbon nanotube film/stress-induced square frustum structure. ACS Appl. Mater. Interfaces 2023, 15, 8546–8554. [Google Scholar] [CrossRef]
- Cheng, X.; Cai, J.; Xu, J.; Gong, D. High-performance strain sensors based on au/graphene composite films with hierarchical cracks for wide linear-range motion monitoring. ACS Appl. Mater. Interfaces 2022, 14, 39230–39239. [Google Scholar] [CrossRef]
- Perkins, J.; Hong, Y.; Ye, S.-H.; Wagner, W.R.; Desai, S. Direct writing of bio-functional coatings for cardiovascular applications. J. Biomed. Mater. Res. Part A 2014, 102, 4290–4300. [Google Scholar] [CrossRef]
- Li, W.; Ruff, B.; Yin, J.; Venkatasubramanian, R.; Mast, D.; Sowani, A.; Krishnaswamy, A.; Shanov, V.; Alvarez, N.; Malik, R.; et al. Tiny Medicine. In Nanotube Superfiber Materials; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Chapter 25. [Google Scholar]
- Desai, S.; Craps, M.; Esho, T. Direct writing of nanomaterials for flexible thin-film transistors (fTFTs). Int. J. Adv. Manuf. Technol. 2013, 64, 537–543. [Google Scholar] [CrossRef]
- Bayoumy, K.; Gaber, M.; Elshafeey, A.; Mhaimeed, O.; Dineen, E.H.; Marvel, F.A.; Martin, S.S.; Muse, E.D.; Turakhia, M.P.; Tarakji, K.G.; et al. Smart wearable devices in cardiovascular care: Where we are and how to move forward. Nat. Rev. Cardiol. 2021, 18, 581–599. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-W.; Lee, J.C.-M.; Chuang, K.-C.; Chiu, C.-W. Photocured, highly flexible, and stretchable 3D-printed graphene/polymer nanocomposites for electrocardiography and electromyography smart clothing. Prog. Org. Coatings 2023, 176, 107378. [Google Scholar] [CrossRef]
- Salvo, P.; Raedt, R.; Carrette, E.; Schaubroeck, D.; Vanfleteren, J.; Cardon, L. A 3D printed dry electrode for ECG/EEG recording. Sens. Actuators A Phys. 2012, 174, 96–102. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Y.; Lu, J.; Cai, R.; Zhao, T.; Chen, Y.; Zhang, M.; Lu, X.; Chen, Y. Injectable, tough and adhesive zwitterionic hydrogels for 3D-printed wearable strain sensors. Chem. Eng. J. 2023, 475, 146340. [Google Scholar] [CrossRef]
- Demircioğlu, P.; Böğrekci, İ.; Uymaz, Ş.C. 3D printed holter electrocardiogram (ECG). Int. J. 3D Print. Technol. Digit. Ind. 2022, 6, 429–437. [Google Scholar] [CrossRef]
- Abdou, A.; Mistry, N.; Krishnan, S. 3D Printed Dry Electrodes for Single-Lead Newborn ECG Monitoring, Computing in Cardiology. Available online: https://cinc.org/2023/Program/accepted/30_CinCFinalPDF.pdf (accessed on 12 October 2023).
- Lopez-Larrea, N.; Criado-Gonzalez, M.; Dominguez-Alfaro, A.; Alegret, N.; del Agua, I.; Marchiori, B.; Mecerreyes, D. Digital Light 3D Printing of PEDOT-Based Photopolymerizable Inks for Biosensing. ACS Appl. Polym. Mater. 2022, 4, 6749–6759. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Ho, D.H.; Cho, J.H. Self-healable hydrogel–liquid metal composite platform enabled by a 3d printed stamp for a multimodular sensor system. ACS Appl. Mater. Interfaces 2020, 12, 9824–9832. [Google Scholar] [CrossRef]
- Alsharif, A.; Cucuri, N.; Dakhaikh, L.; Al-Modaf, F.; El-Atab, N. Structured 3D Printed Dry ECG Electrodes Using Copper Based Filament. ECS Trans. 2022, 109, 3–8. [Google Scholar] [CrossRef]
- Foster, M.; Erb, P.; Plank, B.; West, H.; Russenberger, J.; Gruen, M.; Daniele, M.; Roberts, D.L.; Bozkurt, A. 3D-Printed Electrocardiogram Electrodes for Heart Rate Detection in Canines. In Proceedings of the 2018 IEEE Biomedical Circuits and Systems Conference, BioCAS, Cleveland, OH, USA, 17–19 October 2018. [Google Scholar]
- Ahmmed, P.; Reynolds, J.; Hamada, S.; Regmi, P.; Bozkurt, A. Novel 3D-printed Electrodes for Implantable Biopotential Monitoring. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Mexico, Mexico, 1–5 November 2021; pp. 7120–7123. [Google Scholar]
- Casson, A.J.; Abdulaal, M.; Dulabh, M.; Kohli, S.; Krachunov, S.; Trimble, E. Electroencephalogram. In Seamless Healthcare Monitoring: Advancements in Wearable, Attachable, and Invisible Devices; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 45–81. [Google Scholar] [CrossRef]
- Alsharif, A.A.; Cucuri, N.S.M.; Mishra, R.B.; El-Atab, N. 3D Printed Dry Electrodes for Electrophysiological Signal Monitoring: A Review. Adv. Mater. Technol. 2023, 8, 2201677. [Google Scholar] [CrossRef]
- Lopez-Gordo, M.A.; Sanchez-Morillo, D.; Valle, F.P. Dry EEG Electrodes. Sensors 2014, 14, 12847–12870. [Google Scholar] [CrossRef]
- Casson, A.J. Wearable EEG and beyond. Biomed. Eng. Lett. 2019, 9, 53–71. [Google Scholar] [CrossRef]
- Xing, L.; Batchelor, J.C.; Casson, A.J. Opportunities and challenges for flexible and printable electrodes in electroencephalography. In Proceedings of the 2021 IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), Manchester, UK, 20–23 June 2021. [Google Scholar]
- Xing, L.; Casson, A.J. 3D-printed, directly conductive and flexible electrodes for personalized electroencephalography. Sens. Actuators A Phys. 2023, 349, 114062. [Google Scholar] [CrossRef]
- Krachunov, S.; Casson, A.J. 3D Printed Dry EEG Electrodes. Sensors 2016, 16, 1635. [Google Scholar] [CrossRef] [PubMed]
- Velcescu, A.; Lindley, A.; Cursio, C.; Krachunov, S.; Beach, C.; Brown, C.A.; Jones, A.K.P.; Casson, A.J. Flexible 3D-printed EEG electrodes. Sensors 2019, 19, 1650. [Google Scholar] [CrossRef] [PubMed]
- Krieger, K.J.; Liegey, J.; Cahill, E.M.; Bertollo, N.; Lowery, M.M.; O’Cearbhaill, E.D. Development and evaluation of 3D-printed dry microneedle electrodes for surface electromyography. Adv. Mater. Technol. 2020, 5, 2000518. [Google Scholar] [CrossRef]
- Ho, D.H.; Hong, P.; Han, J.T.; Kim, S.; Kwon, S.J.; Cho, J.H. 3D-Printed Sugar Scaffold for High-Precision and Highly Sensitive Active and Passive Wearable Sensors. Adv. Sci. 2020, 7, 1902521. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-J.; Byun, D.; Nam, T.-S.; Choi, S.-Y.; Lee, B.-G.; Kim, M.-K.; Kim, S. A 3D-Printed Sensor for Monitoring Biosignals in Small Animals. J. Health Eng. 2017, 2017, 9053764. [Google Scholar] [CrossRef] [PubMed]
- Schuhknecht, A.; Fadanelli, E.; Patel, M.; Hanson, A.J.; Maddipatla, D.; Atashbar, M.Z. Development of a Flexible and Conformable EEG Sensors Using 3D Printing Process. In Proceedings of the 2021 IEEE Sensors, Sydney, Australia, 31 October–3 November 2021. [Google Scholar] [CrossRef]
- Niiranen, T.J.; Hänninen, M.-R.; Johansson, J.; Reunanen, A.; Jula, A.M. Home-measured blood pressure is a stronger predictor of cardiovascular risk than office blood pressure. Hypertension 2010, 55, 1346–1351. [Google Scholar] [CrossRef] [PubMed]
- Arnold, R.; Karrett, T.; Melville, C. Biosensor for Ischemic Stroke Detection Major Qualifying Project. 2021. Available online: https://digital.wpi.edu/downloads/kk91fp28w (accessed on 29 October 2023).
- Panula, T.; Koivisto, T.; Pänkäälä, M.; Niiranen, T.; Kantola, I.; Kaisti, M. An instrument for measuring blood pressure and assessing cardiovascular health from the fingertip. Biosens. Bioelectron. 2020, 167, 112483. [Google Scholar] [CrossRef]
- Li, J.; Jia, H.; Zhou, J.; Huang, X.; Xu, L.; Jia, S.; Gao, Z.; Yao, K.; Li, D.; Zhang, B.; et al. Thin, soft, wearable system for continuous wireless monitoring of artery blood pressure. Nat. Commun. 2023, 14, 5009. [Google Scholar] [CrossRef]
- Tay, R.Y.; Song, Y.; Yao, D.R.; Gao, W. Direct-ink-writing 3D-printed bioelectronics. Mater. Today 2023, V71, 135–151. [Google Scholar] [CrossRef]
- Sel, K.; Osman, D.; Huerta, N.; Edgar, A.; Pettigrew, R.I.; Jafari, R. Continuous cuffless blood pressure monitoring with a wearable ring bioimpedance device. NPJ Digit. Med. 2023, 6, 59. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Long, Y.; Yang, F.; Wei, H.; Zhang, Z.; Wang, Y.; Wang, J.; Li, C.; Carlos, C.; Dong, Y.; et al. Multifunctional Artificial Artery from Direct 3D Printing with Built-In Ferroelectricity and Tissue-Matching Modulus for Real-Time Sensing and Occlusion Monitoring. Adv. Funct. Mater. 2020, 30, 2002868. [Google Scholar] [CrossRef] [PubMed]
- Ganti, V.G.; Carek, A.M.; Nevius, B.N.; Heller, J.A.; Etemadi, M.; Inan, O.T. Wearable Cuff-Less Blood Pressure Estimation at Home via Pulse Transit Time. IEEE J. Biomed. Health Inform. 2021, 25, 1926–1937. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Choi, J.; Jeong, Y.; Cho, I.; Kim, M.; Kim, S.; Oh, Y.; Park, I. Highly sensitive and wearable liquid metal-based pressure sensor for health monitoring applications: Integration of a 3D-printed microbump array with the microchannel. Adv. Health Mater. 2019, 8, e1900978. [Google Scholar] [CrossRef] [PubMed]
- Shar, A.; Glass, P.; Park, S.H.; Joung, D. 3D Printable One-Part Carbon Nanotube-Elastomer Ink for Health Monitoring Applications. Adv. Funct. Mater. 2023, 33, 2211079. [Google Scholar] [CrossRef]
- Tan, P.; Xi, Y.; Chao, S.; Jiang, D.; Liu, Z.; Fan, Y.; Li, Z. An artificial intelligence-enhanced blood pressure monitor wristband based on piezoelectric nanogenerator. Biosensors 2022, 12, 234. [Google Scholar] [CrossRef]
- Mandal, A.; Morali, A.; Skorobogatiy, M.; Bodkhe, S. 3D printing of Polyvinylidene Fluoride Based Piezoelectric sensors for Non-Invasive Continuous Blood Pressure Monitoring. Adv. Eng. Mater. 2023, 2301292. [Google Scholar] [CrossRef]
- Xuan, Y.; Fascetti, A.J.; Barry, C.; Wang, E.J. Development of a One Dollar Blood Pressure Monitor. arXiv 2023, arXiv:2308.05897. [Google Scholar]
- Park, J.; Kim, J.-K.; Kim, D.-S.; Shanmugasundaram, A.; Park, S.A.; Kang, S.; Kim, S.-H.; Jeong, M.H.; Lee, D.-W. Wireless pressure sensor integrated with a 3D printed polymer stent for smart health monitoring. Sens. Actuators B Chem. 2019, 280, 201–209. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Lin, C.-C.; Huang, G.-W.; Wu, J.-P.; Kuo, L.-C.; Su, F.-C.; Yang, Y.-C. BP-ExerGuide: Smart Blood Pressure Monitor System for Personalized Exercise Safety Guidelines in Senior Communities. In Proceedings of the 2023 IEEE 5th Eurasia Conference on Biomedical Engineering, Healthcare and Sustainability (ECBIOS), Tainan, Taiwan, 2–4 June 2023; pp. 140–143. [Google Scholar]
- Young, B.; Luo, W.; Young, D.J. A 3D-Printed Wearable Ring Sensor For Long-Term Accurate Monitoring of Human Cardiovascular Condition. In Proceedings of the 2022 IEEE Sensors, Dallas, TX, USA, 30 October–2 November 2022. [Google Scholar]
- Scognamiglio, V.; Arduini, F. The technology tree in the design of glucose biosensors. TrAC Trends Anal. Chem. 2019, 120, 115642. [Google Scholar] [CrossRef]
- Cardoso, R.M.; Silva, P.R.; Lima, A.P.; Rocha, D.P.; Oliveira, T.C.; do Prado, T.M.; Fava, E.L.; Fatibello-Filho, O.; Richter, E.M.; Muñoz, R.A. 3D-Printed graphene/polylactic acid electrode for bioanalysis: Biosensing of glucose and simultaneous determination of uric acid and nitrite in biological fluids. Sens. Actuators B Chem. 2020, 307, 127621. [Google Scholar] [CrossRef]
- Podunavac, I.; Djocos, M.; Vejin, M.; Birgermajer, S.; Pavlovic, Z.; Kojic, S.; Petrovic, B.; Radonic, V. 3D-Printed Microfluidic Chip for Real-Time Glucose Monitoring in Liquid Analytes. Micromachines 2023, 14, 503. [Google Scholar] [CrossRef] [PubMed]
- Nesaei, S.; Song, Y.; Wang, Y.; Ruan, X.; Du, D.; Gozen, A.; Lin, Y. Micro additive manufacturing of glucose biosensors: A feasibility study. Anal. Chim. Acta 2018, 1043, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, Q.; Luo, X.; Yang, L.; Cui, Y. Continuous monitoring of diabetes with an integrated microneedle biosensing device through 3D printing. Microsyst. Nanoeng. 2021, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Rahman, T.; Du, D.; Panat, R.; Lin, Y. 3-D printed adjustable microelectrode arrays for electrochemical sensing and biosensing. Sens. Actuators B Chem. 2016, 230, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Kurochkin, A.Y.; Ripka, D.S. System for Continuous Monitoring of Oxygen Saturation Level. In Proceedings of the 2022 Conference of Russian Young Researchers in Electrical and Electronic Engineering (ElConRus), Saint Petersburg, Russia, 25–28 January 2022; pp. 1542–1544. [Google Scholar]
- Ma, M.; Zhe, T.; Ma, Y.; Wang, Z.; Chen, Q.; Wang, J. Highly sensitive and reproducible non-enzymatic glucose sensor fabricated by drop-casting novel nanocomposite with 3D architecture and tailorable properties prepared in controllable way. Talanta 2018, 180, 133–143. [Google Scholar] [CrossRef]
- Calabria, D.; Lazzarini, E.; Pace, A.; Trozzi, I.; Zangheri, M.; Cinti, S.; Difonzo, M.; Valenti, G.; Guardigli, M.; Paolucci, F.; et al. Smartphone-based 3D-printed electrochemiluminescence enzyme biosensor for reagentless glucose quantification in real matrices. Biosens. Bioelectron. 2023, 227, 115146. [Google Scholar] [CrossRef]
- Krstić, N.; Jüttner, J.; Giegerich, L.; Mayer, M.; Knuth, M.; Müller, A.; Thielemann, C. 3D printed biosensor for continuous glucose measurement in cell cultures. Ann. 3D Print. Med. 2023, 10, 100111. [Google Scholar] [CrossRef]
- Abdalla, A.; Patel, B.A. 3D-printed electrochemical sensors: A new horizon for measurement of biomolecules. Curr. Opin. Electrochem. 2020, 20, 78–81. [Google Scholar] [CrossRef]
- Fontana-Escartín, A.; Lanzalaco, S.; Bertran, O.; Alemán, C. Electrochemical multi-sensors obtained by applying an electric discharge treatment to 3D-printed poly(lactic acid). Appl. Surf. Sci. 2022, 597, 153623. [Google Scholar] [CrossRef]
- Wei, H.; Han, L.; Yin, R.; Yang, T.; Liu, Y.; Mou, C.; Pang, F.; Wang, T. Micro-3D printed Concanavalin A hydrogel based photonic devices for high-sensitivity glucose sensing. Sens. Actuators B: Chem. 2023, 386, 133707. [Google Scholar] [CrossRef]
- Lee, J.; Maji, S.; Lee, H. Fabrication and Integration of a Low-cost 3D Printing-based Glucose Biosensor for Bioprinted Liver-on-a-chip. Biotechnol. J. 2023, V1, e2300154. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.; Malkoc, A.; La Belle, J.T. The Development of a Glucose Dehydrogenase 3D-Printed Glucose Sensor: A Proof-of-Concept Study. J. Diabetes Sci. Technol. 2018, 12, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.N.T.; Rocha, R.G.; Richter, E.M.; Munoz, R.A.A.; Nossol, E. Nickel Oxy-Hydroxy/Multi-Wall Carbon Nanotubes Film Coupled with a 3D-Printed Device as a Nonenzymatic Glucose Sensor. Biosensors 2023, 13, 646. [Google Scholar] [CrossRef] [PubMed]
- Youssef, K.; Ullah, A.; Rezai, P.; Hasan, A.; Amirfazli, A. Recent advances in biosensors for real time monitoring of pH, temperature, and oxygen in chronic wounds. Mater. Today Bio 2023, 22, 100764. [Google Scholar] [CrossRef] [PubMed]
- Capobussi, M.; Moja, L. 3D printing technology and internet of things prototyping in family practice: Building pulse oximeters during COVID-19 pandemic. 3D Print. Med. 2020, 6, 32. [Google Scholar] [CrossRef]
- Osten, W.; Faulhaber, M.A.; Herkommer, A.; Reichert, C. Design and Implementation of a Low-Cost Pulse Oximeter Kit for Developing and Educational Purposes. Master’s Thesis, University of Stuttgart, Stuttgart, Germany, 2016. [Google Scholar]
- Otero, J.; Ulldemolins, A.; Farré, R.; Almendros, I. Oxygen biosensors and control in 3d physiomimetic experimental models. Antioxidants 2021, 10, 1165. [Google Scholar] [CrossRef]
- Davies, H.J.; Williams, I.; Peters, N.S.; Mandic, D.P. In-ear SpO2: A tool for wearable, unobtrusive monitoring of core blood oxygen saturation. Sensors 2020, 20, 4879. [Google Scholar] [CrossRef]
- Rivera, K.R.; Pozdin, V.A.; Young, A.T.; Erb, P.D.; Wisniewski, N.A.; Magness, S.T.; Daniele, M. Integrated phosphorescence-based photonic biosensor (iPOB) for monitoring oxygen levels in 3D cell culture systems. Biosens. Bioelectron. 2019, 123, 131–140. [Google Scholar] [CrossRef]
- Contardi, U.A.; Morikawa, M.; Brunelli, B.; Thomaz, D.V. MAX30102 Photometric Biosensor Coupled to ESP32-Webserver Capabilities for Continuous Point of Care Oxygen Saturation and Heartrate Monitoring. Eng. Proc. 2022, 16, 9. [Google Scholar]
- Abdollahi, S.; Markvicka, E.J.; Majidi, C.; Feinberg, A.W. 3D Printing Silicone Elastomer for Patient-Specific Wearable Pulse Oximeter. Adv. Health Mater. 2020, 9, e1901735. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.; Pei, X.; Das, P.; Qin, H.; Lee, S.W.; Esfandyarpour, R. A self-powered triboelectric MXene-based 3D-printed wearable physiological biosignal sensing system for on-demand, wireless, and real-time health monitoring. Nano Energy 2022, 101, 107511. [Google Scholar] [CrossRef]
- Zirath, H.; Rothbauer, M.; Spitz, S.; Bachmann, B.; Jordan, C.; Müller, B.; Ehgartner, J.; Priglinger, E.; Mühleder, S.; Redl, H.; et al. Every breath you take: Non-invasive real-time oxygen biosensing in two- and three-dimensional microfluidic cell models. Front. Physiol. 2018, 9, 815. [Google Scholar] [CrossRef] [PubMed]
- Kostecki, R.; Arman, A.; Zhang, B.; Yang, K.; Narayan, R.J.; Hutchinson, M.R.; Ebendorff-Heidepriem, H. Dynamic in vivo protein carbonyl biosensor for measuring oxidative stress. Med. Devices Sens. 2020, 3, e10135. [Google Scholar] [CrossRef]
- Dcosta, J.V.; Ochoa, D.; Sanaur, S. Recent Progress in Flexible and Wearable All Organic Photoplethysmography Sensors for SpO2 Monitoring. Adv. Sci. 2023, 10, e2302752. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liu, C.; Zhang, L.; Liu, T.; Wang, Z.; Song, Z.; Cai, H.; Fang, Z.; Chen, J.; Wang, J.; et al. Wearable and flexible electrochemical sensors for sweat analysis: A review. Microsystems Nanoeng. 2023, 9, 1. [Google Scholar] [CrossRef]
- Ghaffari, R.; Rogers, J.A.; Ray, T.R. Recent progress, challenges, and opportunities for wearable biochemical sensors for sweat analysis. Sens. Actuators B Chem. 2021, 332, 129447. [Google Scholar] [CrossRef]
- Ji, W.; Zhu, J.; Wu, W.; Wang, N.; Wang, J.; Wu, J.; Wu, Q.; Wang, X.; Yu, C.; Wei, G.; et al. Wearable Sweat Biosensors Refresh Personalized Health/Medical Diagnostics. Research 2021, 2021, 9757126. [Google Scholar] [CrossRef]
- Jo, S.; Sung, D.; Kim, S.; Koo, J. A review of wearable biosensors for sweat analysis. Biomed. Eng. Lett. 2021, 11, 117–129. [Google Scholar] [CrossRef]
- Koukouviti, E.; Plessas, A.K.; Economou, A.; Thomaidis, N.; Papaefstathiou, G.S.; Kokkinos, C. 3D Printed Voltammetric Sensor Modified with an Fe(III)-Cluster for the Enzyme-Free Determination of Glucose in Sweat. Biosensors 2022, 12, 1156. [Google Scholar] [CrossRef]
- Kim, T.; Yi, Q.; Hoang, E.; Esfandyarpour, R. A 3D Printed Wearable Bioelectronic Patch for Multi-Sensing and In Situ Sweat Electrolyte Monitoring. Adv. Mater. Technol. 2021, 6, 2001021. [Google Scholar] [CrossRef]
- Padash, M.; Carrara, S. A 3D printed wearable device for sweat analysis. In Proceedings of the 2020 IEEE international symposium on medical measurements and applications (MeMeA), IEEE, Bari, Italy, 1 June–1 July 2020; pp. 1–5. [Google Scholar]
- Song, Y.; Tay, R.Y.; Li, J.; Xu, C.; Min, J.; Sani, E.S.; Kim, G.; Heng, W.; Kim, I.; Gao, W. 3D-printed epifluidic electronic skin for machine learning–powered multimodal health surveillance. Sci. Adv. 2023, 9, eadi6492. [Google Scholar] [CrossRef] [PubMed]
- Katseli, V.; Economou, A.; Kokkinos, C. Smartphone-addressable 3d-printed electrochemical ring for nonenzymatic self-monitoring of glucose in human sweat. Anal. Chem. 2021, 93, 3331–3336. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Ma, H.J.H.; Baessler, P.; Balanay, R.K.; Ray, T.R. Skin-interfaced microfluidic systems with spatially engineered 3D fluidics for sweat capture and analysis. Sci. Adv. 2023, 9, eadg4272. Available online: https://www.science.org (accessed on 27 October 2023). [CrossRef]
- Weng, X.; Fu, Z.; Zhang, C.; Jiang, W.; Jiang, H. A Portable 3D Microfluidic Origami Biosensor for Cortisol Detection in Human Sweat. Anal. Chem. 2022, 94, 3526–3534. [Google Scholar] [CrossRef] [PubMed]
- Xuan, X.; Pérez-Ràfols, C.; Chen, C.; Cuartero, M.; Crespo, G.A. Lactate Biosensing for Reliable On-Body Sweat Analysis. ACS Sens. 2021, 6, 2763–2771. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, C.; Shang, F.; Niu, S.; Ke, L.; Zhang, N.; Ma, B.; Li, R.; Sun, X.; Zhang, S. Tactile sensing technology in bionic skin: A review. Biosens. Bioelectron. 2023, 220, 114882. [Google Scholar] [CrossRef]
- Gardner, S.D.; Alexander, J.I.D.; Massoud, Y.; Haider, M.R. Minimally produced inkjet-printed tactile sensor model for improved data reliability. In Proceedings of the 2020 11th International Conference on Electrical and Computer Engineering (ICECE), Dhaka, Bangladesh, 17–19 December 2020; pp. 49–52. [Google Scholar]
- Haque, R.I.; Chandran, O.; Lani, S.; Briand, D. Self-powered triboelectric touch sensor made of 3D printed materials. Nano Energy 2018, 52, 54–62. [Google Scholar] [CrossRef]
- Bao, C. 3D Printed Neuromorphic Sensing Systems. Ph.D. Thesis, Simon Fraser University, Burnaby, BC, Canada, 2021. [Google Scholar]
- Li, H.; Fang, X.; Li, R.; Liu, B.; Tang, H.; Ding, X.; Xie, Y.; Zhou, R.; Zhou, G.; Tang, Y. All-printed soft triboelectric nanogenerator for energy harvesting and tactile sensing. Nano Energy 2020, 78, 105288. [Google Scholar] [CrossRef]
- Nag, A.; Feng, S.; Mukhopadhyay, S.; Kosel, J.; Inglis, D. 3D printed mould-based graphite/PDMS sensor for low-force applications. Sens. Actuators A Phys. 2018, 280, 525–534. [Google Scholar] [CrossRef]
- Qu, J.; Wu, Q.; Clancy, T.; Fan, Q.; Wang, X.; Liu, X. 3D-Printed Strain-Gauge Micro Force Sensors. IEEE Sens. J. 2020, 20, 6971–6978. [Google Scholar] [CrossRef]
- Rahman, T.; Rahimi, A.; Gupta, S.; Panat, R. Microscale additive manufacturing and modeling of interdigitated capacitive touch sensors. Sens. Actuators A Phys. 2016, 248, 94–103. [Google Scholar] [CrossRef]
- Presti, D.L.; Dimo, A.; Zoboli, L.; Bianchi, D.; Massaroni, C.; Altomare, V.; Grasso, A.; Oddo, C.M.; Gizzi, A.; Schena, E. A 3D-printed tactile probe based on fiber Bragg grating sensors for non-invasive breast cancer identification. IEEE Sens. J. 2023, 23, 24489–24499. [Google Scholar] [CrossRef]
- Simić, M.; Stavrakis, A.K.; Sinha, A.; Premčevski, V.; Markoski, B.; Stojanović, G.M. Portable Respiration Monitoring System with an Embroidered Capacitive Facemask Sensor. Biosensors 2022, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Annabestani, M.; Esmaeili-Dokht, P.; Nejad, S.K.; Fardmanesh, M. NAFAS: Non-Rigid Air Flow Active Sensor, a Cost-Effective, Wearable, and Ubiquitous Respiratory Bio-Sensor. IEEE Sens. J. 2021, 21, 9530–9537. [Google Scholar] [CrossRef]
- Zhang, Q.; Rawal, G.; Qian, J.; Ibrahim, H.; Zhang, J.; Dong, L.; Lu, M. An integrated magneto-opto-fluidic biosensor for rapid on-chip assay of respiratory viruses of livestock. Lab Chip 2022, 22, 3236–3244. [Google Scholar] [CrossRef] [PubMed]
- Tavares, C.; Leitão, C.; Presti, D.L.; Domingues, M.d.F.F.; Alberto, N.; Silva, H.; Antunes, P. Respiratory and heart rate monitoring using an FBG 3D-printed wearable system. Biomed. Opt. Express 2022, 13, 2299–2311. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zhang, G.F.; Hu, C.; Yuan, B.; Jahan, S.; Kitsios, G.D.; Morris, A.; Gao, S.; Panat, R. Ultrarapid and ultrasensitive detection of SARS-CoV-2 antibodies in COVID-19 patients via a 3D-printed nanomaterial-based biosensing platform. J. Med. Virol. 2022, 94, 5808–5826. [Google Scholar] [CrossRef]
- Martins, G.; Gogola, J.L.; Budni, L.H.; Janegitz, B.C.; Marcolino-Junior, L.H.; Bergamini, M.F. 3D-printed electrode as a new platform for electrochemical immunosensors for virus detection. Anal. Chim. Acta 2020, 1147, 30–37. [Google Scholar] [CrossRef]
- Muñoz, J.; Pumera, M. 3D-Printed COVID-19 immunosensors with electronic readout. Chem. Eng. J. 2021, 425, 131433. [Google Scholar] [CrossRef]
- Chen, X.; Ma, K.; Ou, J.; Mo, D.; Lian, H.; Li, X.; Cui, Z.; Luo, Y. Fast-Response Non-Contact Flexible Humidity Sensor Based on Direct-Writing Printing for Respiration Monitoring. Biosensors 2023, 13, 792. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, F.; Carhart, M.; Andreescu, S. A 3D-Printed Breath Analyzer Incorporating CeO2 Nanoparticles for Colorimetric Enzyme-Based Ethanol Sensing. ACS Appl. Nano Mater. 2021, 4, 9361–9369. [Google Scholar] [CrossRef]
- Jodat, Y.A.; Kiaee, K.; Jarquin, D.V.; Hernández, R.L.D.l.G.; Wang, T.; Joshi, S.; Rezaei, Z.; de Melo, B.A.G.; Ge, D.; Mannoor, M.S.; et al. A 3D-Printed Hybrid Nasal Cartilage with Functional Electronic Olfaction. Adv. Sci. 2020, 7, 1901878. [Google Scholar] [CrossRef] [PubMed]
- Panahi, M.; Masihi, S.; Hanson, A.J.; Maddipatla, D.; Zhang, X.; Palaniappan, V.; Narakathu, B.B.; Bazuin, B.J.; Atashbar, M.Z. Highly Sensitive Cone-Structured Porous Pressure Sensors for Respiration Monitoring Applications. In Proceedings of the 2021 IEEE Sensors, Sydney, Australia, 31 October 2021–3 November 2021. [Google Scholar] [CrossRef]
- Tettey, F.; Parupelli, S.K.; Desai, S. A Review of Biomedical Devices: Classification, Regulatory Guidelines, Human Factors, Software as a Medical Device, and Cybersecurity. In Biomedical Materials & Devices; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–26. [Google Scholar]
- Shuvo, M.H.; Titirsha, T.; Amin, N.; Islam, S.K. Energy harvesting in implantable and wearable medical devices for enduring precision healthcare. Energies 2022, 15, 7495. [Google Scholar] [CrossRef]
- Merrett, G.V.; Huang, H.; White, N.M. Modeling the effect of orientation on human-powered inertial energy harvesters. IEEE Sens. J. 2014, 15, 434–441. [Google Scholar] [CrossRef]
- Wu, T.; Redoute, J.-M.; Yuce, M.R. A Wireless implantable sensor design with subcutaneous energy harvesting for long-term IoT healthcare applications. IEEE Access 2018, 6, 35801–35808. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Y.; Avila, R.; Liu, C.; Xie, Z.; Wang, L.; Yu, X. 3D printed microstructures for flexible electronic devices. Nanotechnology 2019, 30, 414001. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, S.; Liu, H.; Chen, X.; Chen, X.; Li, B.; Tang, W.; Meng, Q.; Ding, P.; Tian, H.; et al. 3D printed piezoelectric BNNTs nanocomposites with tunable interface and microarchitectures for self-powered conformal sensors. Nano Energy 2020, 77, 105300. [Google Scholar] [CrossRef]
- Isichei, J.C.; Khorsandroo, S.; Desai, S. Cybersecurity and privacy in smart bioprinting. Bioprinting 2023, 36, e00321. [Google Scholar] [CrossRef]
- Almakayeel, N.; Desai, S.; Alghamdi, S.; Qureshi, M.R.N.M. Smart Agent System for Cyber Nano-Manufacturing in Industry 4.0. Appl. Sci. 2022, 12, 6143. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, J.; Liu, T.; Luo, Y.; Loh, X.J.; Chen, X. Machine learning-reinforced noninvasive biosensors for healthcare. Adv. Health Mater. 2021, 10, 2100734. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, R.; Chauhdary, S.T.; Anwar, M.T.; Zahid, A.; Khosa, A.A.; Imran, M.; Sajjad, M.H. A Review of 4D Printing-Technologies, Shape Shifting, Smart Materials, and Biomedical Applications. Adv. Ind. Eng. Polym.Res. 2023, in press. [CrossRef]




| Company | Model | Analyte | Measuring Range (mM) |
|---|---|---|---|
| Yellow Springs Instruments, Yellow Springs, OH, USA | 23 A 13 L | Glucose Lactate | 1–45 0–15 |
| Zentrum für Wissenschaftlichen Geratebau, Berlin, Germany | Gluco- meter | Glucose Uric acid | 0.5–50 0.1–1.2 |
| Abbott, Abbott Park, Illinois, USA | FreeStyle InsuLinx | Glucose, insulin | 20–500 mg/dL |
| Lifestream cholesterol monitor Alcosan saliva alcohol dipstick | i-STAT PCA | Glucose Urea nitrogen, Cl, K, Na+, hematocrit blood gases | 20–700 mg/dL - |
| DEX blood glucose meter | Bio-scanner 2000 | Glucose, cholesterol, HDL, blood ketone, triglyceride | 55–230 mg/dL - |
| Germaine Laboratories, Inc., San Antonio, TX, USA | AimStrip hemoglobin meter | Hemoglobin | 5.6 to 23.5 g/dL |
| Class | Examples | Properties |
|---|---|---|
| Polymer | Thermoplastics/layered silicates/thermoset polymers, polymer/CNT, polyester/TiO2, polymer/layered double hydroxides. | Enhanced electrical conductivity and colloidal stability, biodegradability |
| Ceramic | Al2O3/CNT Polymer, Al2O3/TiO2, SiO2/Ni, Al2O3/SiC, Al2O3/SiO2 | High toughness and superior failure properties |
| Metal | Fe-Cr/Al2O3, Al/CNT, Mg/CNT, Co/Cr, Fe/MgO, Ni/Al2O3 | Strong ductility and excellent shear/compression practices |
| Process | Technology | Materials | Minimum Layer Resolution | Max Build Volume (LxWxH-mm3) and Applications |
|---|---|---|---|---|
| Vat photopolymerization | Stereolithography (SLA) Digital light processing (DLP) Continuous liquid interface production (CLIP) Scan, spin, and selectively photocure (3SP) | Photopolymers | 50–100 µm 25–150 µm 50–100 µm 25–100 µm | 1500 × 750 × 550 192 × 120 × 230 190 × 112 × 325 266 × 175 × 193 Rapid prototypes, tooling, end-user parts, and mold patterns. |
| Extrusion-based systems | Fused deposition modeling (FDM) | Thermoplastics (PLA, ABS, HIPS, Nylon, PC) | 10–100 µm | 1500 × 1100 × 1500 Spare parts, automotive, testing tool designs, and jigs |
| Powder bed fusion | Selective laser sintering (SLS) Electron beam melting (EBM) Selective laser melting (SLM) Selective heat sintering (SHS) Direct metal laser sintering (DMLS) | Polymers, metals and ceramic powder | 80 µm 70 µm 20–50 µm 100 µm 20–40 µm | 381 × 330 × 460 6096 × 1194 × 1524 300 × 300 × 300 160 × 140 × 150 250 × 250 × 325 Aerospace, automotive, dental, rapid prototyping, and jewelry |
| Material jetting | Multi-jet modeling, drop-on-demand, thermo-jet printing, and inkjet printing | Polymers, plastics, and waxes | 13 µm | 300 × 185 × 200 Casting patterns, prototypes, and electronics |
| Binder jetting | 3D printing | Polymers, waxes, metals, and foundry sand | 90 µm | 2200 × 1200 × 600 Prototypes, casting patterns, and molds |
| Directed energy deposition | Laser engineering net shape (LENS) | Metals | 50–100 µm | 1500 × 1500 × 2100 Aerospace, military, repair metal objects and satellites |
| Sheet lamination processes | Laminated Object manufacturing (LOM) | Metals, paper, plastic film | 100 µm | 256 × 169 × 150 Prototypes, plastic parts, and end-user parts |
| Methods | Biomarker | Sensor Structures (3D and Non-3D) | Detection Range | LoD | Sensing Capabilities and Remarks |
|---|---|---|---|---|---|
| 3D printing (SLA) | Prostate-specific antigen | 3D-printed channels; immunoarray | 0.5 pg mL−1 to 5 ng mL−1 | 0.5 pg mL−1 | Customizability and rapid prototyping capability. Automated detection system and assay time ≈ 30 min. Accuracy comparable with ELISA and commercial devices such as Abbott Diagnostics (0.008 ng mL−1), Roche (0.002 ng mL−1), Beckman Coulter (0.008 ng mL−1), and Diagnostic Products Corporation (0.04 ng mL−1) |
| Commercial SPR biochip (TRD) | Self-assembled monolayered Au | 1–1000 ng mL−1 | 18.1 ng mL−1 | Assay time ≈ 14 min. Sensing with buffer solution and human serum | |
| Microfabrication (TRD) | Self-assembled monolayered Au | 0–4 µg mL−1 | 0.2 µg mL−1 | Single-use biosensor, sensing with serum samples and good sensitivity | |
| 3D printing (AJP) | Dopamine | Micropillar array electrode | 100 am–1 mm | am | Low LoD ≈ 500 attomoles, breaking the barrier described in the literature [47] through multi-length-scale electrode structure. Rapid prototyping capability and waste minimization due to the small microfluidic volume required for testing. |
| 3D printing (2PP) | 3D carbon electrode | 0.5–100 µm | nm | High sensitivity to multiple neurochemicals, high reproducibility, and capability for both in vitro and in vivo. | |
| Lithography (TRD) | Graphene | 0.5–120 µm | nm | Good sensitivity in urine samples | |
| Screen-printed electrode (TRD) | Conducting polymer–Pd composite | 0.1 to 200 μm | nm | In vitro sensing capabilities | |
| 3D printing (AJP) | Glucose | Polymer nanocomposite | 0–10 mm | 6.9 μm | Multimaterial printing, customizability, and rapid prototyping. High sensitivity. |
| 3D printing (Inkjet printing) | PEDOT.PSS | 0.25–0.9 mm | μm | Rapid, fully printed, and customizable biosensor. Noninvasive, good sensitivity in saliva, stability ≈ 1 month, and response time ≈ 1 min | |
| Electrodeposition (TRD) | MnO2/MWCNTs | 10μm–28 mm | μm | Low-potential, stable, and fast detection time | |
| 3D printing (SLM) | Ascorbic acid | Au electrode | 0.1–1 mm | 2.1 μm | Multimaterial printing and rapid prototyping. |
| Glassy carbon electrode (TRD) | Carbon nanoplatelets | 0.1 µm–1.8 mm | 1.09 μm | Sensing ability with soft drinks, orange juice, and urine. | |
| Lithography (TRD) | Indium tin oxide (ITO) electrode | 0.058 to 0.71 mm | 8.4 μm | Response time ≈ 40 s, shelf life ≈ 1.5 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parupelli, S.K.; Desai, S. The 3D Printing of Nanocomposites for Wearable Biosensors: Recent Advances, Challenges, and Prospects. Bioengineering 2024, 11, 32. https://doi.org/10.3390/bioengineering11010032
Parupelli SK, Desai S. The 3D Printing of Nanocomposites for Wearable Biosensors: Recent Advances, Challenges, and Prospects. Bioengineering. 2024; 11(1):32. https://doi.org/10.3390/bioengineering11010032
Chicago/Turabian StyleParupelli, Santosh Kumar, and Salil Desai. 2024. "The 3D Printing of Nanocomposites for Wearable Biosensors: Recent Advances, Challenges, and Prospects" Bioengineering 11, no. 1: 32. https://doi.org/10.3390/bioengineering11010032
APA StyleParupelli, S. K., & Desai, S. (2024). The 3D Printing of Nanocomposites for Wearable Biosensors: Recent Advances, Challenges, and Prospects. Bioengineering, 11(1), 32. https://doi.org/10.3390/bioengineering11010032








