Retention of Human iPSC-Derived or Primary Cells Following Xenotransplantation into Rat Immune-Privileged Sites
Abstract
:1. Introduction
2. Materials and Methods
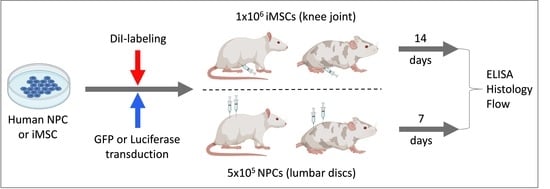
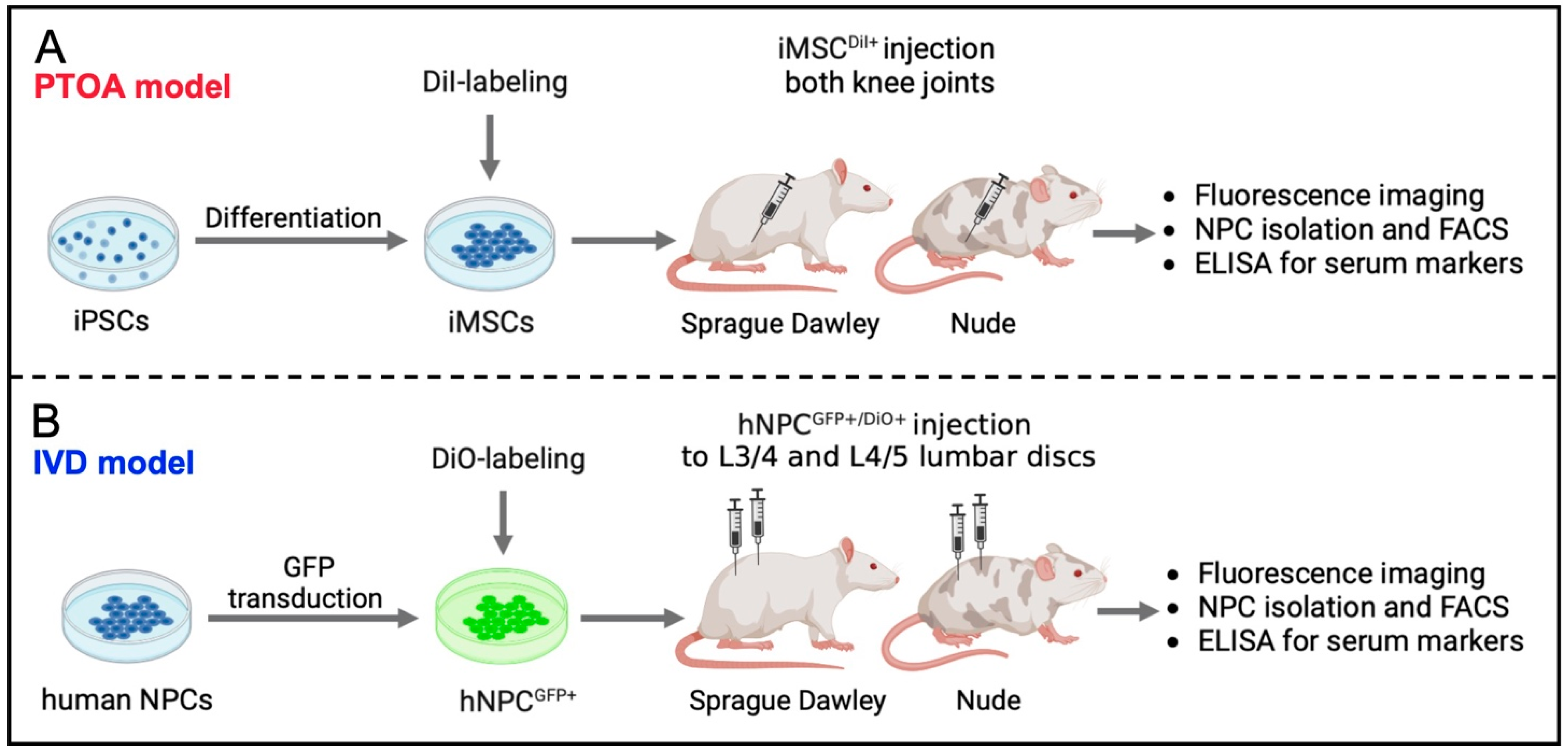
2.1. Study Design
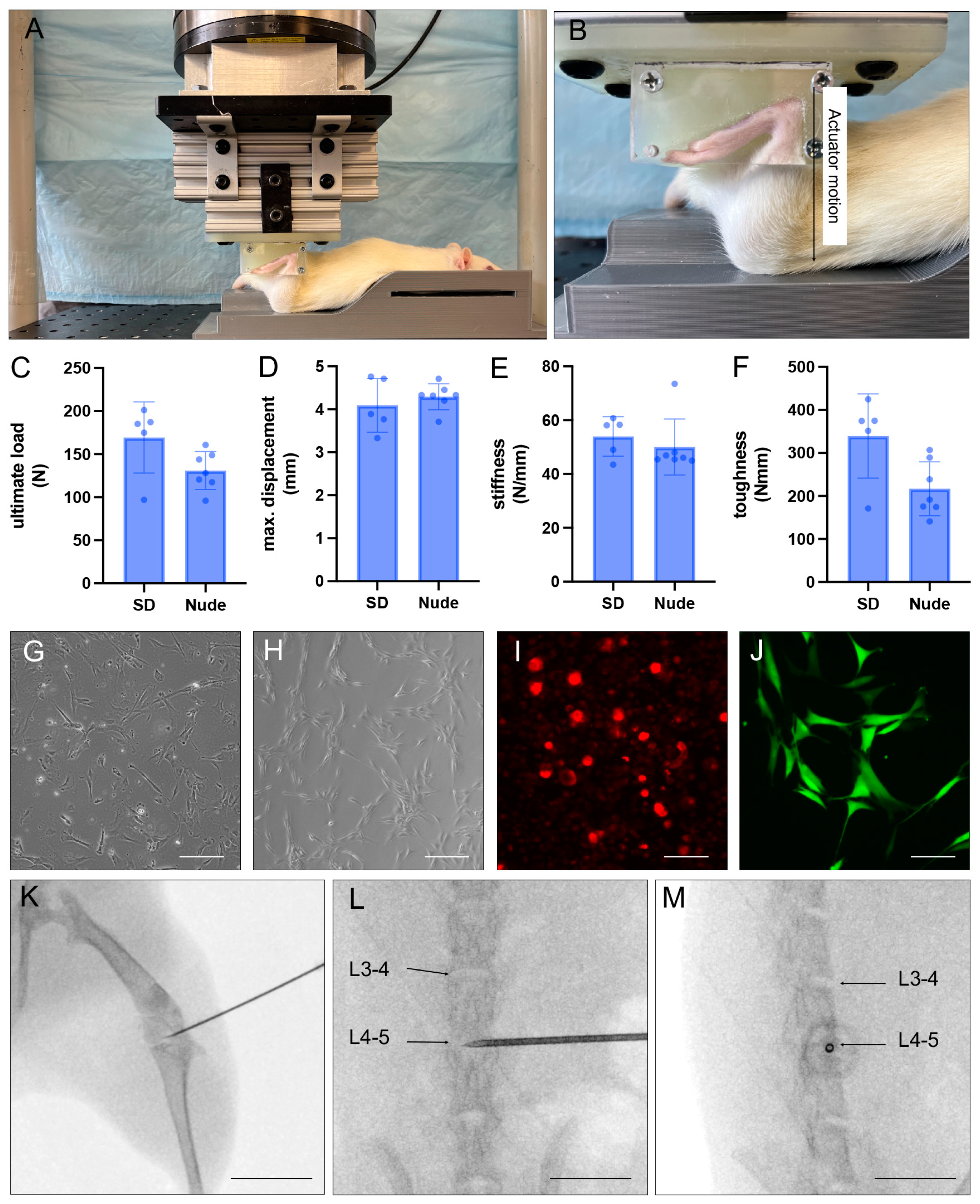
2.2. Non-Invasive Anterior Cruciate Ligament (ACL) Rupture
2.3. Generation of iMSCs from iPSC
2.4. Isolation of Human NPCs
2.5. Genetic Engineering of Human NPC to Overexpress GFP
2.6. Fluorescent Labeling of iMSC and NPCGFP+
2.7. X-ray-Guided Injection of iMSCDiI+ and NPCGFP+/DiO+
2.8. Blood Collection and Serum Preparation
2.9. Tissue Extraction and Digestion
2.10. Flow Cytometry
2.11. ELISA
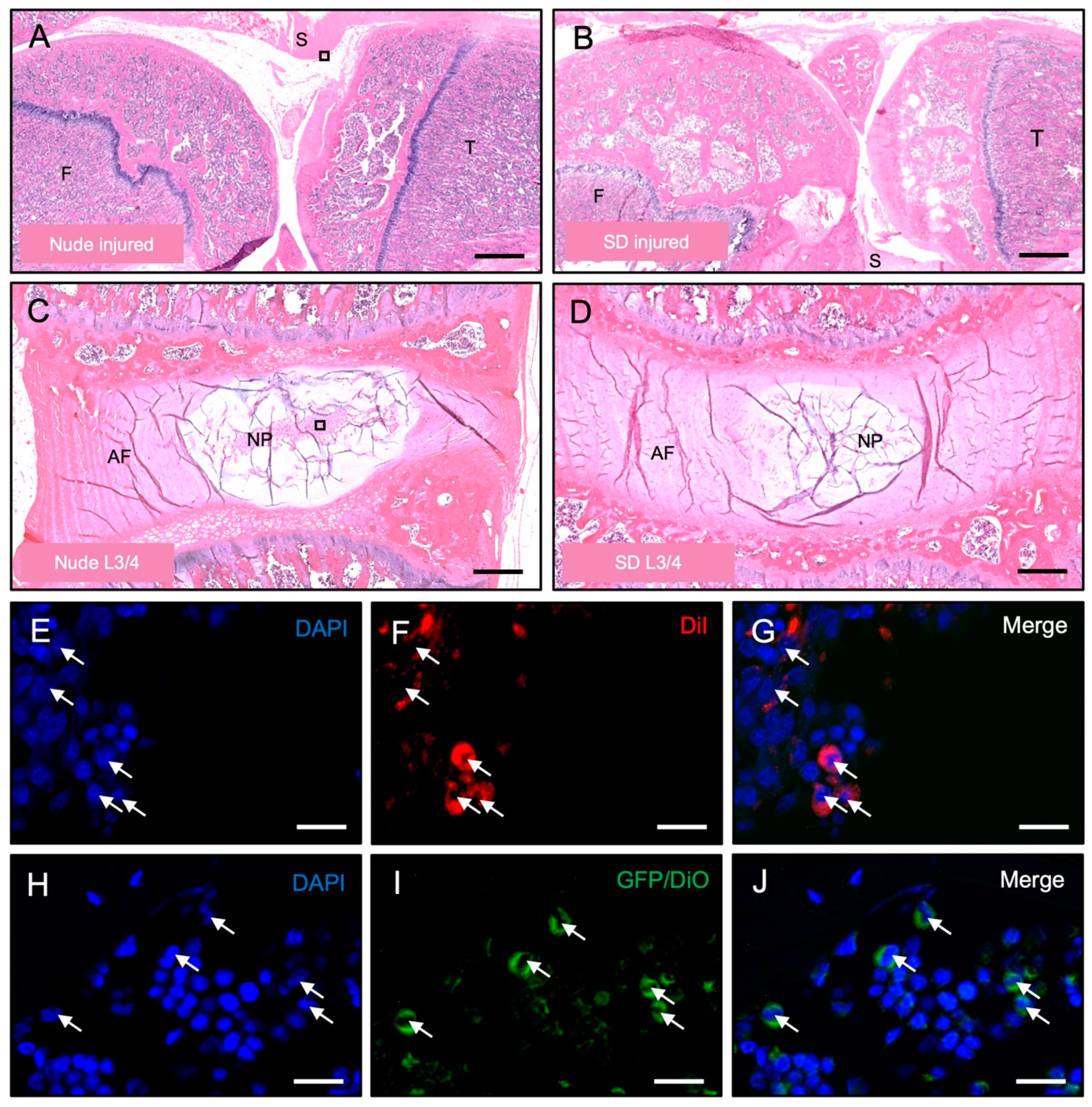
2.12. Histology and Immunofluorescence
2.13. Statistical Analysis
3. Results
3.1. Recovery of Injected Cells
3.2. Immune Response to Injected Cells
3.3. Histological Detection of Dye-Labeled Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Chen, J.; Chen, X.G.; Wang, L.; Gautam, S.C.; Xu, Y.X.; Katakowski, M.; Zhang, L.J.; Lu, M.; Janakiraman, N.; et al. Human marrow stromal cell therapy for stroke in rat: Neurotrophins and functional recovery. Neurology 2002, 59, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Bearzi, C.; Rota, M.; Hosoda, T.; Tillmanns, J.; Nascimbene, A.; De Angelis, A.; Yasuzawa-Amano, S.; Trofimova, I.; Siggins, R.W.; Lecapitaine, N.; et al. Human cardiac stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 14068–14073. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimmons, R.E.B.; Mazurek, M.S.; Soos, A.; Simmons, C.A. Mesenchymal Stromal/Stem Cells in Regenerative Medicine and Tissue Engineering. Stem Cells Int. 2018, 2018, 8031718. [Google Scholar] [CrossRef] [PubMed]
- Marques, I.J.; Weiss, F.U.; Vlecken, D.H.; Nitsche, C.; Bakkers, J.; Lagendijk, A.K.; Partecke, L.I.; Heidecke, C.D.; Lerch, M.M.; Bagowski, C.P. Metastatic behaviour of primary human tumours in a zebrafish xenotransplantation model. BMC Cancer 2009, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef]
- Bekele, B.M.; Schowel-Wolf, V.; Kieshauer, J.; Marg, A.; Busjahn, A.; Davis, S.; Nugent, G.; Ebert, A.K.; Spuler, S. Human primary muscle stem cells regenerate injured urethral sphincter in athymic rats. Anim. Model. Exp. Med. 2022, 5, 453–460. [Google Scholar] [CrossRef]
- Rossignol, J.; Boyer, C.; Thinard, R.; Remy, S.; Dugast, A.S.; Dubayle, D.; Dey, N.D.; Boeffard, F.; Delecrin, J.; Heymann, D.; et al. Mesenchymal stem cells induce a weak immune response in the rat striatum after allo or xenotransplantation. J. Cell Mol. Med. 2009, 13, 2547–2558. [Google Scholar] [CrossRef]
- Chuang, C.K.; Lin, K.J.; Lin, C.Y.; Chang, Y.H.; Yen, T.C.; Hwang, S.M.; Sung, L.Y.; Chen, H.C.; Hu, Y.C. Xenotransplantation of human mesenchymal stem cells into immunocompetent rats for calvarial bone repair. Tissue Eng. Part. A 2010, 16, 479–488. [Google Scholar] [CrossRef]
- Zong, C.; Xue, D.; Yuan, W.; Wang, W.; Shen, D.; Tong, X.; Shi, D.; Liu, L.; Zheng, Q.; Gao, C.; et al. Reconstruction of rat calvarial defects with human mesenchymal stem cells and osteoblast-like cells in poly-lactic-co-glycolic acid scaffolds. Eur. Cell Mater. 2010, 20, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Cizkova, D.; Rosocha, J.; Vanicky, I.; Jergova, S.; Cizek, M. Transplants of human mesenchymal stem cells improve functional recovery after spinal cord injury in the rat. Cell Mol. Neurobiol. 2006, 26, 1167–1180. [Google Scholar] [CrossRef]
- Min, J.Y.; Sullivan, M.F.; Yang, Y.; Zhang, J.P.; Converso, K.L.; Morgan, J.P.; Xiao, Y.F. Significant improvement of heart function by cotransplantation of human mesenchymal stem cells and fetal cardiomyocytes in postinfarcted pigs. Ann. Thorac. Surg. 2002, 74, 1568–1575. [Google Scholar] [CrossRef]
- Longoni, A.; Pennings, I.; Cuenca Lopera, M.; van Rijen, M.H.P.; Peperzak, V.; Rosenberg, A.; Levato, R.; Gawlitta, D. Endochondral Bone Regeneration by Non-autologous Mesenchymal Stem Cells. Front. Bioeng. Biotechnol. 2020, 8, 651. [Google Scholar] [CrossRef] [PubMed]
- Liston, A.; Carr, E.J.; Linterman, M.A. Shaping Variation in the Human Immune System. Trends Immunol. 2016, 37, 637–646. [Google Scholar] [CrossRef]
- Brodin, P.; Davis, M.M. Human immune system variation. Nat. Rev. Immunol. 2017, 17, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Streilein, J.W. Unraveling immune privilege. Science 1995, 270, 1158–1159. [Google Scholar] [CrossRef] [PubMed]
- Benhar, I.; London, A.; Schwartz, M. The privileged immunity of immune privileged organs: The case of the eye. Front. Immunol. 2012, 3, 296. [Google Scholar] [CrossRef]
- Garrity, C.; Arzi, B.; Haus, B.; Lee, C.A.; Vapniarsky, N. A Fresh Glimpse into Cartilage Immune Privilege. Cartilage 2022, 13, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Arzi, B.; DuRaine, G.D.; Lee, C.A.; Huey, D.J.; Borjesson, D.L.; Murphy, B.G.; Hu, J.C.Y.; Baumgarth, N.; Athanasiou, K.A. Cartilage immunoprivilege depends on donor source and lesion location. Acta Biomater. 2015, 23, 72–81. [Google Scholar] [CrossRef]
- Brown, S.B.; Hornyak, J.A.; Jungels, R.R.; Shah, Y.Y.; Yarmola, E.G.; Allen, K.D.; Sharma, B. Characterization of Post-Traumatic Osteoarthritis in Rats Following Anterior Cruciate Ligament Rupture by Non-Invasive Knee Injury (NIKI). J. Orthop. Res. 2020, 38, 356–367. [Google Scholar] [CrossRef]
- Khorasani, M.S.; Diko, S.; Hsia, A.W.; Anderson, M.J.; Genetos, D.C.; Haudenschild, D.R.; Christiansen, B.A. Effect of alendronate on post-traumatic osteoarthritis induced by anterior cruciate ligament rupture in mice. Arthritis Res. Ther. 2015, 17, 30. [Google Scholar] [CrossRef]
- Tao, Y.Q.; Liang, C.Z.; Li, H.; Zhang, Y.J.; Li, F.C.; Chen, G.; Chen, Q.X. Potential of co-culture of nucleus pulposus mesenchymal stem cells and nucleus pulposus cells in hyperosmotic microenvironment for intervertebral disc regeneration. Cell Biol. Int. 2013, 37, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Mochida, J.; Sakai, D.; Nakamura, Y.; Watanabe, T.; Yamamoto, Y.; Kato, S. Intervertebral disc repair with activated nucleus pulposus cell transplantation: A three-year, prospective clinical study of its safety. Eur. Cell Mater. 2015, 29, 202–212; Discussion 212. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.C.; Ducheyne, P.; Vresilovic, E.J.; Shapiro, I.M. Intervertebral disc tissue engineering II: Cultures of nucleus pulposus cells. Clin. Orthop. Relat. Res. 2003, 411, 315–324. [Google Scholar] [CrossRef]
- Tang, X.; Jing, L.; Richardson, W.J.; Isaacs, R.E.; Fitch, R.D.; Brown, C.R.; Erickson, M.M.; Setton, L.A.; Chen, J. Identifying molecular phenotype of nucleus pulposus cells in human intervertebral disc with aging and degeneration. J. Orthop. Res. 2016, 34, 1316–1326. [Google Scholar] [CrossRef]
- Sheyn, D.; Ben-David, S.; Shapiro, G.; De Mel, S.; Bez, M.; Ornelas, L.; Sahabian, A.; Sareen, D.; Da, X.; Pelled, G.; et al. Human Induced Pluripotent Stem Cells Differentiate Into Functional Mesenchymal Stem Cells and Repair Bone Defects. Stem Cells Transl. Med. 2016, 5, 1447–1460. [Google Scholar] [CrossRef]
- Basatvat, S.; Bach, F.C.; Barcellona, M.N.; Binch, A.L.; Buckley, C.T.; Bueno, B.; Chahine, N.O.; Chee, A.; Creemers, L.B.; Dudli, S.; et al. Harmonization and standardization of nucleus pulposus cell extraction and culture methods. JOR SPINE 2023, 6, e1238. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Glaeser, J.D.; Salehi, K.; Kaneda, G.; Mathkar, P.; Wagner, A.; Ho, R.; Sheyn, D. Single-cell Atlas Unveils Cellular Heterogeneity and Novel Markers in Human Neonatal and Adult Intervertebral Discs. iScience 2022, 25, 104504. [Google Scholar] [CrossRef]
- Glaeser, J.D.; Salehi, K.; Kanim, L.E.A.; NaPier, Z.; Kropf, M.A.; Cuellar, J.M.; Perry, T.G.; Bae, H.W.; Sheyn, D. NF-kappaB inhibitor, NEMO-binding domain peptide attenuates intervertebral disc degeneration. Spine J. 2020, 20, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- White, M.S.; Brancati, R.J.; Lepley, L.K. Relationship between altered knee kinematics and subchondral bone remodeling in a clinically translational model of ACL injury. J. Orthop. Res. 2022, 40, 74–86. [Google Scholar] [CrossRef]
- Antebi, B.; Zhang, L.; Sheyn, D.; Pelled, G.; Zhang, X.; Gazit, Z.; Schwarz, E.M.; Gazit, D. Controlling Arteriogenesis and Mast Cells Are Central to Bioengineering Solutions for Critical Bone Defect Repair Using Allografts. Bioengineering 2016, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yu, F.; Yan, G.; Hu, Y.; Sun, H.; Ding, L. Human endometrial perivascular stem cells exhibit a limited potential to regenerate endometrium after xenotransplantation. Hum. Reprod. 2021, 36, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cooper, D.K.C.; Wang, H.; Chen, P.; He, C.; Cai, Z.; Mou, L.; Luan, S.; Gao, H. Potential pathological role of pro-inflammatory cytokines (IL-6, TNF-alpha, and IL-17) in xenotransplantation. Xenotransplantation 2019, 26, e12502. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, P.; Forsman, A.; Andersson, G.; Blomberg, J.; Korsgren, O. Pig islet xenotransplantation: Activation of porcine endogenous retrovirus in the immediate post-transplantation period. Xenotransplantation 2005, 12, 450–456. [Google Scholar] [CrossRef]
- Yin, D.; Ma, L.L.; Blinder, L.; Shen, J.; Sankary, H.; Williams, J.W.; Chong, A.S. Induction of species-specific host accommodation in the hamster-to-rat xenotransplantation model. J. Immunol. 1998, 161, 2044–2051. [Google Scholar] [CrossRef] [PubMed]
- Saenz, N.C.; Hendren, R.B.; Schoof, D.D.; Folkman, J. Reduction of smooth muscle hyperplasia in vein grafts in athymic rats. Lab. Investig. 1991, 65, 15–22. [Google Scholar] [PubMed]
- McClure, M.J.; Olson, L.C.; Cohen, D.J.; Huang, Y.C.; Zhang, S.; Nguyen, T.; Boyan, B.D.; Schwartz, Z. RNU (Foxn1 (RNU)-Nude) Rats Demonstrate an Improved Ability to Regenerate Muscle in a Volumetric Muscle Injury Compared to Sprague Dawley Rats. Bioengineering 2021, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.D.; Gupta, R.; Sefton, M.V. Chimeric vessel tissue engineering driven by endothelialized modules in immunosuppressed Sprague-Dawley rats. Tissue Eng. Part A 2011, 17, 151–160. [Google Scholar] [CrossRef]
- Deng, J.B.; Yu, D.M.; Wu, P.; Li, M.S. The tracing study of developing entorhino-hippocampal pathway. Int. J. Dev. Neurosci. 2007, 25, 251–258. [Google Scholar] [CrossRef]
- Deng, J.B.; Yu, D.M.; Li, M.S. Formation of the entorhino-hippocampal pathway: A tracing study in vitro and in vivo. Neurosci. Bull. 2006, 22, 305–314. [Google Scholar] [PubMed]
- Henriksson, H.B.; Svanvik, T.; Jonsson, M.; Hagman, M.; Horn, M.; Lindahl, A.; Brisby, H. Transplantation of human mesenchymal stems cells into intervertebral discs in a xenogeneic porcine model. Spine 2009, 34, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Iwashina, T.; Mochida, J.; Sakai, D.; Yamamoto, Y.; Miyazaki, T.; Ando, K.; Hotta, T. Feasibility of using a human nucleus pulposus cell line as a cell source in cell transplantation therapy for intervertebral disc degeneration. Spine 2006, 31, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Small animal models of xenotransplantation. Methods Mol. Biol. 2012, 885, 125–153. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.; Gagnon, J.; Barclay, A.N.; Williams, A.F. Purification, chain separation and sequence of the MRC OX-8 antigen, a marker of rat cytotoxic T lymphocytes. EMBO J. 1985, 4, 2539–2545. [Google Scholar] [CrossRef] [PubMed]
- Saalmuller, A.; Reddehase, M.J.; Buhring, H.J.; Jonjic, S.; Koszinowski, U.H. Simultaneous expression of CD4 and CD8 antigens by a substantial proportion of resting porcine T lymphocytes. Eur. J. Immunol. 1987, 17, 1297–1301. [Google Scholar] [CrossRef] [PubMed]
- Bradl, M.; Bauer, J.; Flugel, A.; Wekerle, H.; Lassmann, H. Complementary contribution of CD4 and CD8 T lymphocytes to T-cell infiltration of the intact and the degenerative spinal cord. Am. J. Pathol. 2005, 166, 1441–1450. [Google Scholar] [CrossRef]
- Bernhardsson, M.; Dietrich-Zagonel, F.; Tatting, L.; Eliasson, P.; Aspenberg, P. Depletion of cytotoxic (CD8+) T cells impairs implant fixation in rat cancellous bone. J. Orthop. Res. 2019, 37, 805–811. [Google Scholar] [CrossRef]
- Kaur, G.; Wright, K.; Mital, P.; Hibler, T.; Miranda, J.M.; Thompson, L.A.; Halley, K.; Dufour, J.M. Neonatal Pig Sertoli Cells Survive Xenotransplantation by Creating an Immune Modulatory Environment Involving CD4 and CD8 Regulatory T Cells. Cell Transplant. 2020, 29, 963689720947102. [Google Scholar] [CrossRef] [PubMed]
- Veld, P.I.; Pavlovic, D.; Bogdani, M.; Pipeleers-Marichal, M.; Pipeleers, D. Xenotransplantation of purified pre-natal porcine beta cells in mice normalizes diabetes when a short anti-CD4-CD8 antibody treatment is combined with transient insulin injections. Xenotransplantation 2006, 13, 415–422. [Google Scholar] [CrossRef]
- Zhao, B.; Xia, J.J.; Wang, L.M.; Gao, C.; Li, J.L.; Liu, J.Y.; Cheng, Q.J.; Dai, C.; Ma, Q.L.; Qi, Z.Q.; et al. Immunosuppressive effect of arsenic trioxide on islet xenotransplantation prolongs xenograft survival in mice. Cell Death Dis. 2018, 9, 408. [Google Scholar] [CrossRef]
- Alhaj Hussen, K.; Michonneau, D.; Biajoux, V.; Keita, S.; Dubouchet, L.; Nelson, E.; Setterblad, N.; Le Buanec, H.; Bouaziz, J.D.; Guimiot, F.; et al. CD4(+)CD8(+) T-Lymphocytes in Xenogeneic and Human Graft-versus-Host Disease. Front. Immunol. 2020, 11, 579776. [Google Scholar] [CrossRef]
- O’Connell, P.J.; Yi, S.; Carrington, E.M.; Lew, A.M. Role of regulatory T cells in xenotransplantation. Curr. Opin. Organ. Transplant. 2010, 15, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Satzer, D.; Miller, C.; Maxon, J.; Voth, J.; DiBartolomeo, C.; Mahoney, R.; Dutton, J.R.; Low, W.C.; Parr, A.M. T cell deficiency in spinal cord injury: Altered locomotor recovery and whole-genome transcriptional analysis. BMC Neurosci. 2015, 16, 74. [Google Scholar] [CrossRef]
- Newson, J.; Stables, M.; Karra, E.; Arce-Vargas, F.; Quezada, S.; Motwani, M.; Mack, M.; Yona, S.; Audzevich, T.; Gilroy, D.W. Resolution of acute inflammation bridges the gap between innate and adaptive immunity. Blood 2014, 124, 1748–1764. [Google Scholar] [CrossRef] [PubMed]
- Silver, I.A. Tissue PO2 changes in acute inflammation. Adv. Exp. Med. Biol. 1977, 94, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Kataranovski, M.; Magic, Z.; Pejnovic, N. Early inflammatory cytokine and acute phase protein response under the stress of thermal injury in rats. Physiol. Res. 1999, 48, 473–482. [Google Scholar] [PubMed]
- Smith, L.L. Acute inflammation: The underlying mechanism in delayed onset muscle soreness? Med. Sci. Sports Exerc. 1991, 23, 542–551. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Später, T.; Kaneda, G.; Chavez, M.; Sheyn, J.; Wechsler, J.; Yu, V.; Del Rio, P.; Huang, D.; Metzger, M.; Tawackoli, W.; et al. Retention of Human iPSC-Derived or Primary Cells Following Xenotransplantation into Rat Immune-Privileged Sites. Bioengineering 2023, 10, 1049. https://doi.org/10.3390/bioengineering10091049
Später T, Kaneda G, Chavez M, Sheyn J, Wechsler J, Yu V, Del Rio P, Huang D, Metzger M, Tawackoli W, et al. Retention of Human iPSC-Derived or Primary Cells Following Xenotransplantation into Rat Immune-Privileged Sites. Bioengineering. 2023; 10(9):1049. https://doi.org/10.3390/bioengineering10091049
Chicago/Turabian StyleSpäter, Thomas, Giselle Kaneda, Melissa Chavez, Julia Sheyn, Jacob Wechsler, Victoria Yu, Patricia Del Rio, Dave Huang, Melodie Metzger, Wafa Tawackoli, and et al. 2023. "Retention of Human iPSC-Derived or Primary Cells Following Xenotransplantation into Rat Immune-Privileged Sites" Bioengineering 10, no. 9: 1049. https://doi.org/10.3390/bioengineering10091049
APA StyleSpäter, T., Kaneda, G., Chavez, M., Sheyn, J., Wechsler, J., Yu, V., Del Rio, P., Huang, D., Metzger, M., Tawackoli, W., & Sheyn, D. (2023). Retention of Human iPSC-Derived or Primary Cells Following Xenotransplantation into Rat Immune-Privileged Sites. Bioengineering, 10(9), 1049. https://doi.org/10.3390/bioengineering10091049