Measuring Bone Healing: Parameters and Scores in Comparison
Abstract
:1. Introduction
- The histological bone healing score correlates better with the biomechanical properties of the bone defect than the bone fraction in the defect.
- a.
- Bending stiffness is more appropriate than maximum fracture strength.
- b.
- Osteocalcin expression correlates with BMD.
- The radiological and histological analyses correlate with the biomechanical properties of the bone defect.
- Histological analysis is at least equivalent to radiological analysis in this regard.
2. Materials and Methods
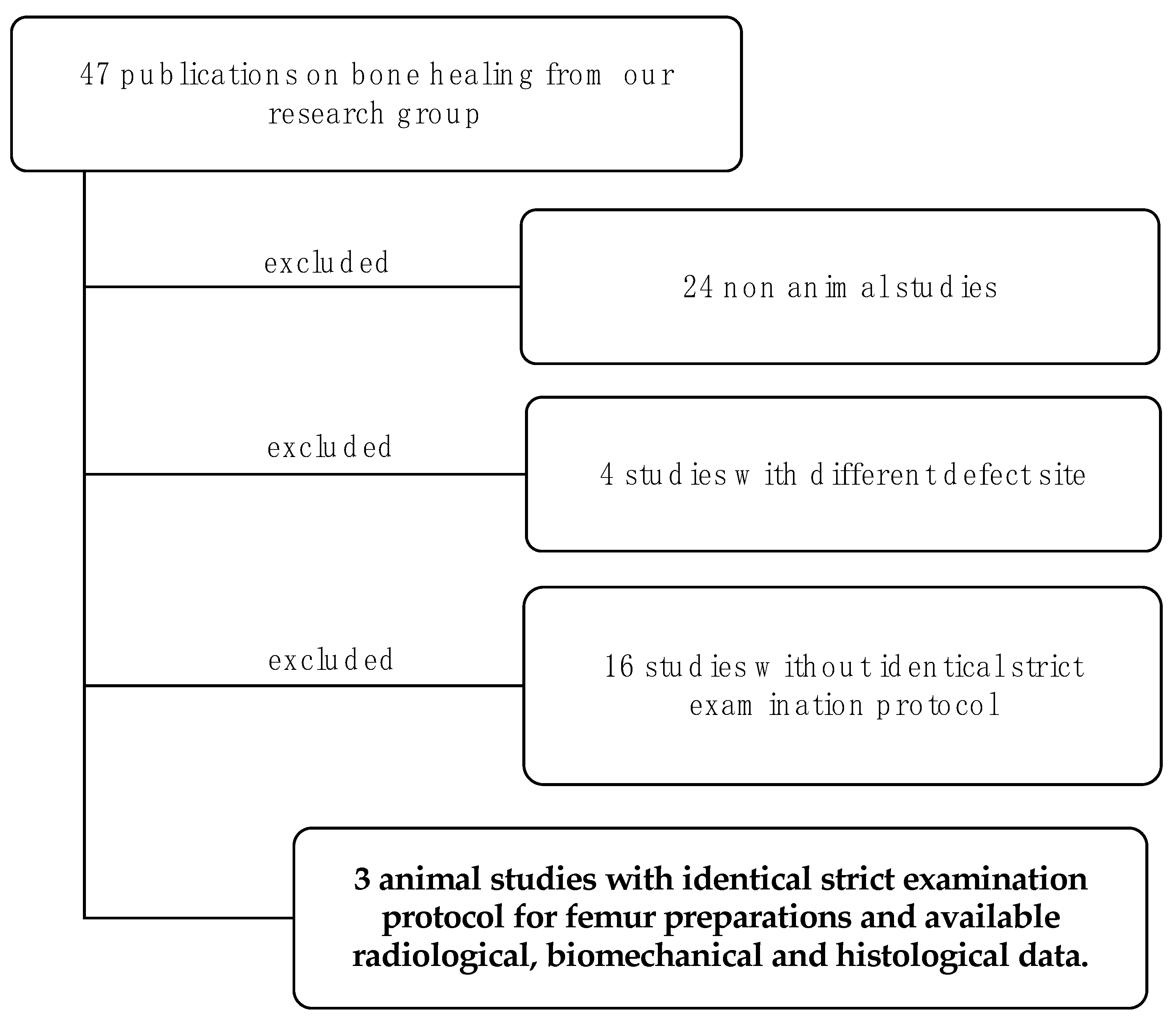
2.1. Study Selection and Overview
2.2. Post-Processing
2.2.1. Histology
2.2.2. µ-CT
2.3. Bone Healing Score
2.4. Statistics
3. Results
3.1. Comparison of Two- and Three-Dimensional Analysis Using InVesalius to Calculate BV/TV
3.2. Correlation Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanakaris, N.K.; Harwood, P.J.; Mujica-Mota, R.; Mohrir, G.; Chloros, G.; Giannoudis, P.V. Treatment of tibial bone defects: Pilot analysis of direct medical costs between distraction osteogenesis with an Ilizarov frame and the Masquelet technique. Eur. J. Trauma Emerg. Surg. 2023, 49, 951–964. [Google Scholar] [CrossRef]
- Liodakis, E.; Giannoudis, V.P.; Sehmisch, S.; Jha, A.; Giannoudis, P.V. Bone defect treatment: Does the type and properties of the spacer affect the induction of Masquelet membrane? Evidence today. Eur. J. Trauma Emerg. Surg. 2022, 48, 4403–4424. [Google Scholar] [CrossRef]
- Henrich, D. Focus on bone healing: New strategies for improvement of bone healing. Eur. J. Trauma Emerg. Surg. 2020, 46, 229–230. [Google Scholar] [CrossRef]
- Verboket, R.D.; Sohling, N.; Heilani, M.; Fremdling, C.; Schaible, A.; Schroder, K.; Brune, J.C.; Marzi, I.; Henrich, D. The Induced Membrane Technique-The Filling Matters: Evaluation of Different Forms of Membrane Filling with and without Bone Marrow Mononuclear Cells (BMC) in Large Femoral Bone Defects in Rats. Biomedicines 2022, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Sohling, N.; Ondreka, M.; Kontradowitz, K.; Reichel, T.; Marzi, I.; Henrich, D. Early Immune Response in Foreign Body Reaction Is Implant/Material Specific. Materials 2022, 15, 2195. [Google Scholar] [CrossRef] [PubMed]
- Mosaddad, S.A.; Yazdanian, M.; Tebyanian, H.; Tahmasebi, E.; Yazdanian, A.; Seifalian, A.; Tavakolizadeh, M. Fabrication and properties of developed collagen/strontium-doped Bioglass scaffolds for bone tissue engineering. J. Mater. Res. Technol. 2020, 9, 14799–14817. [Google Scholar] [CrossRef]
- Nilsson, B.; Ekdahl, K.N.; Mollnes, T.E.; Lambris, J.D. The role of complement in biomaterial-induced inflammation. Mol. Immunol. 2007, 44, 82–94. [Google Scholar] [CrossRef]
- Boehler, R.M.; Graham, J.G.; Shea, L.D. Tissue engineering tools for modulation of the immune response. Biotechniques 2011, 51, 239–240, 242, 244 passim. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, S.; Miramini, S.; Edwards, G.; Rotne, R.; Xu, J.; Ebeling, P.; Zhang, L. The investigation of bone fracture healing under intramembranous and endochondral ossification. Bone Rep. 2021, 14, 100740. [Google Scholar] [CrossRef]
- Peric, M.; Dumic-Cule, I.; Grcevic, D.; Matijasic, M.; Verbanac, D.; Paul, R.; Grgurevic, L.; Trkulja, V.; Bagi, C.M.; Vukicevic, S. The rational use of animal models in the evaluation of novel bone regenerative therapies. Bone 2015, 70, 73–86. [Google Scholar] [CrossRef]
- Verboket, R.D.; Irrle, T.; Busche, Y.; Schaible, A.; Schroder, K.; Brune, J.C.; Marzi, I.; Nau, C.; Henrich, D. Fibrous Demineralized Bone Matrix (DBM) Improves Bone Marrow Mononuclear Cell (BMC)-Supported Bone Healing in Large Femoral Bone Defects in Rats. Cells 2021, 10, 1249. [Google Scholar] [CrossRef]
- Verboket, R.D.; Leiblein, M.; Janko, M.; Schaible, A.; Brune, J.C.; Schroder, K.; Heilani, M.; Fremdling, C.; Busche, Y.; Irrle, T.; et al. From two stages to one: Acceleration of the induced membrane (Masquelet) technique using human acellular dermis for the treatment of non-infectious large bone defects. Eur. J. Trauma Emerg. Surg. 2020, 46, 317–327. [Google Scholar] [CrossRef]
- Martinez-de la Cruz, G.; Yamauchi, K.; Odajima, K.; Kataoka, Y.; Nogami, S.; Takahashi, T. Histologic and histomorphometric evaluation of the bone regeneration following cortical bone repositioning in a rabbit mandible. Clin. Implant. Dent. Relat. Res. 2019, 21, 613–620. [Google Scholar] [CrossRef]
- Schatzlein, E.; Kicker, C.; Sohling, N.; Ritz, U.; Neijhoft, J.; Henrich, D.; Frank, J.; Marzi, I.; Blaeser, A. 3D-Printed PLA-Bioglass Scaffolds with Controllable Calcium Release and MSC Adhesion for Bone Tissue Engineering. Polymers 2022, 14, 2389. [Google Scholar] [CrossRef]
- Sohling, N.; Neijhoft, J.; Nienhaus, V.; Acker, V.; Harbig, J.; Menz, F.; Ochs, J.; Verboket, R.D.; Ritz, U.; Blaeser, A.; et al. 3D-Printing of Hierarchically Designed and Osteoconductive Bone Tissue Engineering Scaffolds. Materials 2020, 13, 1836. [Google Scholar] [CrossRef] [PubMed]
- Guda, T.; Labella, C.; Chan, R.; Hale, R. Quality of bone healing: Perspectives and assessment techniques. Wound Repair. Regen. 2014, 22 (Suppl. S1), 39–49. [Google Scholar] [CrossRef]
- Tawonsawatruk, T.; Hamilton, D.F.; Simpson, A.H. Validation of the use of radiographic fracture-healing scores in a small animal model. J. Orthop. Res. 2014, 32, 1117–1119. [Google Scholar] [CrossRef] [PubMed]
- Bagi, C.M.; Hanson, N.; Andresen, C.; Pero, R.; Lariviere, R.; Turner, C.H.; Laib, A. The use of micro-CT to evaluate cortical bone geometry and strength in nude rats: Correlation with mechanical testing, pQCT and DXA. Bone 2006, 38, 136–144. [Google Scholar] [CrossRef]
- Brunauer, A.; Verboket, R.D.; Kainz, D.M.; von Stetten, F.; Fruh, S.M. Rapid Detection of Pathogens in Wound Exudate via Nucleic Acid Lateral Flow Immunoassay. Biosensors 2021, 11, 74. [Google Scholar] [CrossRef]
- InVesalius. Available online: https://invesalius.github.io/ (accessed on 13 July 2023).
- Kim, Y.; Brodt, M.D.; Tang, S.Y.; Silva, M.J. MicroCT for Scanning and Analysis of Mouse Bones. Methods Mol. Biol. 2021, 2230, 169–198. [Google Scholar] [CrossRef] [PubMed]
- Leiblein, M.; Winkenbach, A.; Koch, E.; Schaible, A.; Buchner, H.; Marzi, I.; Henrich, D.; Nau, C. Impact of scaffold granule size use in Masquelet technique on periosteal reaction: A study in rat femur critical size bone defect model. Eur. J. Trauma Emerg. Surg. 2022, 48, 679–687. [Google Scholar] [CrossRef]
- Nau, C.; Henrich, D.; Seebach, C.; Schroder, K.; Fitzsimmons, S.J.; Hankel, S.; Barker, J.H.; Marzi, I.; Frank, J. Treatment of Large Bone Defects with a Vascularized Periosteal Flap in Combination with Biodegradable Scaffold Seeded with Bone Marrow-Derived Mononuclear Cells: An Experimental Study in Rats. Tissue Eng. Part A 2016, 22, 133–141. [Google Scholar] [CrossRef]
- Janko, M.; Sahm, J.; Schaible, A.; Brune, J.C.; Bellen, M.; Schroder, K.; Seebach, C.; Marzi, I.; Henrich, D. Comparison of three different types of scaffolds preseeded with human bone marrow mononuclear cells on the bone healing in a femoral critical size defect model of the athymic rat. J. Tissue Eng. Regen. Med. 2018, 12, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Stormann, P.; Kupsch, J.; Kontradowitz, K.; Leiblein, M.; Verboket, R.; Seebach, C.; Marzi, I.; Henrich, D.; Nau, C. Cultivation of EPC and co-cultivation with MSC on beta-TCP granules in vitro is feasible without fibronectin coating but influenced by scaffolds’ design. Eur. J. Trauma Emerg. Surg. 2019, 45, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Bruker-Mikro-CT, C. Bone Mineral Density (BMD) and Tissue Mineral Density (TMD) Calibration and Measurement by Micro-CT Using Bruker-MicroCT. Bruker Method Note 2010, 1–30. Available online: www.bruker.com (accessed on 2 July 2023).
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Muller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- ImageJ. Available online: https://imagej.nih.gov/ij/ (accessed on 18 July 2023).
- Han, Z.; Bhavsar, M.; Leppik, L.; Oliveira, K.M.C.; Barker, J.H. Histological Scoring Method to Assess Bone Healing in Critical Size Bone Defect Models. Tissue Eng. Part C Methods 2018, 24, 272–279. [Google Scholar] [CrossRef]
- Evans, J.D. Straightforward Statistics for the Behavioral Sciences; Brooks/Cole Pub. Co.: Pacific Grove, CA, USA, 1996; ISBN 0534231004/9780534231002. [Google Scholar]
- Janko, M.; Dietz, K.; Rachor, J.; Sahm, J.; Schroder, K.; Schaible, A.; Nau, C.; Seebach, C.; Marzi, I.; Henrich, D. Improvement of Bone Healing by Neutralization of microRNA-335-5p, but not by Neutralization of microRNA-92A in Bone Marrow Mononuclear Cells Transplanted into a Large Femur Defect of the Rat. Tissue Eng. Part A 2019, 25, 55–68. [Google Scholar] [CrossRef]
- Janko, M.; Pollinger, S.; Schaible, A.; Bellen, M.; Schroder, K.; Heilani, M.; Fremdling, C.; Marzi, I.; Nau, C.; Henrich, D.; et al. Determination of the effective dose of bone marrow mononuclear cell therapy for bone healing in vivo. Eur. J. Trauma Emerg. Surg. 2020, 46, 265–276. [Google Scholar] [CrossRef]
- Gessmann, J.; Rosteius, T.; Baecker, H.; Sivalingam, K.; Peter, E.; Schildhauer, T.A.; Koller, M. Is the bioactivity of induced membranes time dependent? Eur. J. Trauma Emerg. Surg. 2022, 48, 3051–3061. [Google Scholar] [CrossRef]
- Sohling, N.; Heilani, M.; Fremdling, C.; Schaible, A.; Schroder, K.; Brune, J.C.; Eras, V.; Nau, C.; Marzi, I.; Henrich, D.; et al. One Stage Masquelets Technique: Evaluation of Different Forms of Membrane Filling with and without Bone Marrow Mononuclear Cells (BMC) in Large Femoral Bone Defects in Rats. Cells 2023, 12, 1289. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A.; Lane, J.M.; Burstein, A.H.; Kopman, C.R.; Vigorita, V.J. The healing of segmental bone defects induced by demineralized bone matrix. A radiographic and biomechanical study. J. Bone Jt. Surg. Am. 1984, 66, 274–279. [Google Scholar] [CrossRef]
- Guerkov, H.H.; Lohmann, C.H.; Liu, Y.; Dean, D.D.; Simon, B.J.; Heckman, J.D.; Schwartz, Z.; Boyan, B.D. Pulsed electromagnetic fields increase growth factor release by nonunion cells. Clin. Orthop. Relat. Res. 2001, 384, 26–279. [Google Scholar] [CrossRef]
- Reed, A.A.; Joyner, C.J.; Brownlow, H.C.; Simpson, A.H. Human atrophic fracture non-unions are not avascular. J. Orthop. Res. 2002, 20, 593–599. [Google Scholar] [CrossRef]
- Komori, T. Functions of Osteocalcin in Bone, Pancreas, Testis, and Muscle. Int. J. Mol. Sci. 2020, 21, 7513. [Google Scholar] [CrossRef] [PubMed]
- Berezovska, O.; Yildirim, G.; Budell, W.C.; Yagerman, S.; Pidhaynyy, B.; Bastien, C.; van der Meulen, M.C.H.; Dowd, T.L. Osteocalcin affects bone mineral and mechanical properties in female mice. Bone 2019, 128, 115031. [Google Scholar] [CrossRef] [PubMed]
- Ingram, R.T.; Clarke, B.L.; Fisher, L.W.; Fitzpatrick, L.A. Distribution of noncollagenous proteins in the matrix of adult human bone: Evidence of anatomic and functional heterogeneity. J. Bone Miner. Res. 1993, 8, 1019–1029. [Google Scholar] [CrossRef]
- Hauschka, P.V.; Wians, F.H., Jr. Osteocalcin-hydroxyapatite interaction in the extracellular organic matrix of bone. Anat. Rec. 1989, 224, 180–188. [Google Scholar] [CrossRef]
- Aydin, A.; Halici, Z.; Albayrak, A.; Polat, B.; Karakus, E.; Yildirim, O.S.; Bayir, Y.; Cadirci, E.; Ayan, A.K.; Aksakal, A.M. Treatment with Carnitine Enhances Bone Fracture Healing under Osteoporotic and/or Inflammatory Conditions. Basic. Clin. Pharmacol. Toxicol. 2015, 117, 173–179. [Google Scholar] [CrossRef]
- Chow, D.H.; Leung, K.S.; Qin, L.; Leung, A.H.; Cheung, W.H. Low-magnitude high-frequency vibration (LMHFV) enhances bone remodeling in osteoporotic rat femoral fracture healing. J. Orthop. Res. 2011, 29, 746–752. [Google Scholar] [CrossRef]
- Ekeuku, S.O.; Chong, P.N.; Chan, H.K.; Mohamed, N.; Froemming, G.R.A.; Okechukwu, P.N. Spirulina supplementation improves bone structural strength and stiffness in streptozocin-induced diabetic rats. J. Tradit. Complement. Med. 2022, 12, 225–234. [Google Scholar] [CrossRef]
- Waarsing, J.H.; Day, J.S.; Weinans, H. An improved segmentation method for in vivo microCT imaging. J. Bone Miner. Res. 2004, 19, 1640–1650. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, R.D.; Stroup, G.B.; Badger, A.M.; Koller, B.; Levin, J.M.; Coatney, R.W.; Dodds, R.A.; Liang, X.; Lark, M.W.; Gowen, M. Applications of micro-CT and MR microscopy to study pre-clinical models of osteoporosis and osteoarthritis. Technol. Health Care 1998, 6, 361–372. [Google Scholar] [CrossRef]
- Bonnet, N.; Laroche, N.; Vico, L.; Dolleans, E.; Courteix, D.; Benhamou, C.L. Assessment of trabecular bone microarchitecture by two different x-ray microcomputed tomographs: A comparative study of the rat distal tibia using Skyscan and Scanco devices. Med. Phys. 2009, 36, 1286–1297. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Zhou, X.; Sun, J.; Zhu, B.; Duan, C.; Chen, P.; Guo, X.; Zhang, T.; Guo, H. A New Self-Healing Hydrogel Containing hucMSC-Derived Exosomes Promotes Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 564731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hao, Z.; Wang, P.; Xia, Y.; Wu, J.; Xia, D.; Fang, S.; Xu, S. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1alpha-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2019, 52, e12570. [Google Scholar] [CrossRef]
- Kamenaga, T.; Kuroda, Y.; Nagai, K.; Tsubosaka, M.; Takashima, Y.; Kikuchi, K.; Fujita, M.; Ikuta, K.; Anjiki, K.; Maeda, T.; et al. Cryopreserved human adipose-derived stromal vascular fraction maintains fracture healing potential via angiogenesis and osteogenesis in an immunodeficient rat model. Stem Cell Res. Ther. 2021, 12, 110. [Google Scholar] [CrossRef]
- Rosales Rocabado, J.M.; Kaku, M.; Nozaki, K.; Ida, T.; Kitami, M.; Aoyagi, Y.; Uoshima, K. A multi-factorial analysis of bone morphology and fracture strength of rat femur in response to ovariectomy. J. Orthop. Surg. Res. 2018, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Guler Okyay, A.; Kavak, S.; Turktas, U.; Alkis, I.; Guner, S.; Aksakal, B. Biomechanical effects of menopause in ovariectomized rats’ femurs. Gynecol. Endocrinol. 2014, 30, 62–65. [Google Scholar] [CrossRef]
- Seifert, L.B.; Mainka, T.; Herrera-Vizcaino, C.; Verboket, R.; Sader, R. Orbital floor fractures: Epidemiology and outcomes of 1594 reconstructions. Eur. J. Trauma Emerg. Surg. 2022, 48, 1427–1436. [Google Scholar] [CrossRef]
- Taes, Y.; Lapauw, B.; Griet, V.; De Bacquer, D.; Goemaere, S.; Zmierczak, H.; Kaufman, J.M. Prevalent fractures are related to cortical bone geometry in young healthy men at age of peak bone mass. J. Bone Miner. Res. 2010, 25, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Mittra, E.; Rubin, C.; Gruber, B.; Qin, Y.X. Evaluation of trabecular mechanical and microstructural properties in human calcaneal bone of advanced age using mechanical testing, microCT, and DXA. J. Biomech. 2008, 41, 368–375. [Google Scholar] [CrossRef] [PubMed]

| Study | Number of Examined Bones |
|---|---|
| 20 |
| 33 |
| 75 |
| p-Value | rho | Effect Strength | |
|---|---|---|---|
| histological vs. radiological | |||
| bone fraction (Movat pentachrome) vs. BMD | 0.0000 | −0.6268 | strong |
| bone fraction (osteocalcin) vs. BV/TV (InVesalius + ImageJ) | 0.0000 | 0.4942 | moderate |
| bone healing score vs. BV/TV (ImageJ) | 0.0000 | −0.4570 | moderate |
| bone fraction (Movat pentachrome) vs. BV/TV (ImageJ) | 0.0000 | −0.4907 | moderate |
| bone fraction (Movat pentachrome) vs. BV/TV (InVesalius + ImageJ) | 0.1826 | −0.1297 | weak |
| bone healing score vs. BV/TV (InVesalius + ImageJ) | 0.3839 | −0.0788 | weak |
| bone fraction (osteocalcin) vs. BV/TV (ImageJ) | 0.0656 | 0.1667 | weak |
| bone fraction (osteocalcin) vs. BMD | 0.0012 | 0.2941 | weak |
| histological vs. biomechanical | |||
| bone fraction (Movat pentachrome) vs. bending stiffness | 0.0000 | 0.6339 | strong |
| bone healing score vs. bending stiffness | 0.0000 | 0.6253 | strong |
| bone fraction (Movat pentachrome) vs. breaking load | 0.0000 | 0.5713 | moderate |
| bone healing score vs. breaking load | 0.0000 | 0.5642 | moderate |
| bone fraction (osteocalcin) vs. breaking load | 0.0093 | 0.2482 | weak |
| bone fraction (osteocalcin) vs. bending stiffness | 0.0065 | 0.2593 | weak |
| biomechanical vs. radiological | |||
| bending stiffness vs. BV/TV (ImageJ) | 0.0000 | −0.4121 | moderate |
| bending stiffness vs. BMD | 0.0000 | −0.4762 | moderate |
| breaking load vs. BMD | 0.0000 | −0.4164 | moderate |
| bending stiffness vs. BV/TV (InVesalius + ImageJ) | 0.0238 | 0.2144 | weak |
| breaking load vs. BV/TV (InVesalius + ImageJ) | 0.0025 | 0.2848 | weak |
| breaking load vs. BV/TV (ImageJ) | 0.0001 | −0.3675 | weak |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Söhling, N.; Von Jan, O.; Janko, M.; Nau, C.; Ritz, U.; Marzi, I.; Henrich, D.; Verboket, R.D. Measuring Bone Healing: Parameters and Scores in Comparison. Bioengineering 2023, 10, 1011. https://doi.org/10.3390/bioengineering10091011
Söhling N, Von Jan O, Janko M, Nau C, Ritz U, Marzi I, Henrich D, Verboket RD. Measuring Bone Healing: Parameters and Scores in Comparison. Bioengineering. 2023; 10(9):1011. https://doi.org/10.3390/bioengineering10091011
Chicago/Turabian StyleSöhling, Nicolas, Olivia Von Jan, Maren Janko, Christoph Nau, Ulrike Ritz, Ingo Marzi, Dirk Henrich, and René D. Verboket. 2023. "Measuring Bone Healing: Parameters and Scores in Comparison" Bioengineering 10, no. 9: 1011. https://doi.org/10.3390/bioengineering10091011
APA StyleSöhling, N., Von Jan, O., Janko, M., Nau, C., Ritz, U., Marzi, I., Henrich, D., & Verboket, R. D. (2023). Measuring Bone Healing: Parameters and Scores in Comparison. Bioengineering, 10(9), 1011. https://doi.org/10.3390/bioengineering10091011






