Carrot Discard as a Promising Feedstock to Produce 2,3-Butanediol by Fermentation with P. polymyxa DSM 365
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Enzymatic Hydrolysate of Carrot Discard
2.3. Microorganism and Inoculum
2.4. Fermentation Assays
2.4.1. Semi-Defined Fermentation Media
2.4.2. Carrot Discard (CD) Enzymatic Hydrolysate-Based Fermentation Medium
2.5. Analytical Methods
2.6. Data Analysis
3. Results and Discussion
3.1. BDO Production from Semi-Defined Media: Influence of Pre-Culture and Substrate
3.1.1. Tolerance of P. polymyxa to Simple Sugars
3.1.2. Influence of Mixed Sugars in 2,3-Butanediol Production
3.2. BDO Production from Enzymatic Hydrolysate of Carrot Discard
3.2.1. Influence of Stirring
3.2.2. Influence of the Presence of Tryptone
3.3. By-Product Formation: Ethanol and Acetoin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Okonkwo, C.C.; Ujor, V.; Ezeji, T.C. Production of 2,3-Butanediol from non-detoxified wheat straw hydrolysate: Impact of microbial inhibitors on Paenibacillus polymyxa DSM 365. Ind. Crops Prod. 2021, 159, 113047. [Google Scholar] [CrossRef]
- Hong, E.; Kim, D.; Kim, J.; Kim, J.; Yoon, S.; Rhie, S.; Ha, S.; Ryu, Y. Optimization of alkaline pretreatment on corn stover for enhanced production of 1.3-propanediol and 2,3-butanediol by Klebsiella pneumoniae AJ4. Biomass Bioenergy 2015, 77, 177–185. [Google Scholar] [CrossRef]
- Xie, S.; Li, Z.; Zhu, G.; Song, W.; Yi, C. Cleaner production and downstream processing of bio-based 2,3-butanediol: A review. J. Clean. Prod. 2022, 343, 131033. [Google Scholar] [CrossRef]
- Hazeena, S.H.; Nair Salini, C.; Sindhu, R.; Pandey, A.; Binod, P. Simultaneous saccharification and fermentation of oil palm front for the production of 2,3-butanediol. Bioresour. Technol. 2019, 278, 145–149. [Google Scholar] [CrossRef]
- Maina, S.; Prabhu, A.A.; Vivek, N.; Vlysidis, A.; Koutinas, A.; Kumar, V. Prospects on bio-based 2,3-butanediol and acetoin production: Recent progress and advances. Biotechnol. Adv. 2022, 54, 107783. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Zhang, Y.; Cao, M.; Zhang, W.; Lü, C.; Yang, C.; Gao, C.; Xu, P.; Ma, C. Efficient 2,3-butanediol production from whey powder using metabolically engineered Klebsiella oxytoca. Microb. Cell Fact. 2020, 19, 162. [Google Scholar] [CrossRef]
- Jeevitha, P.; Ranjitha, J.; Anand, M.; Mahboob, S.; Vijayalakshmi, S. Production of 2-3-butanediol from various microorganisms, in Valorization of Biomass and Bioproducts. Org. Acids Biofuels 2023, 12, 223–239. [Google Scholar] [CrossRef]
- Jiang, L.-Q.; Fang, Z.; Zhao, Z.-L.; He, F.; Li, H.-B. 2,3-butanediol and acetoin production from enzymatic hydrolysate of ionic liquid-pretreated cellulose by Paenibacillus polymyxa. BioResources 2015, 10, 1318–1329. [Google Scholar] [CrossRef]
- Hakizimana, O.; Matabaro, E.; Lee, B.H. The current strategies and parameters for the enhanced microbial production of 2,3-butanediol. Biotechnol. Reports 2020, 25, e00397. [Google Scholar] [CrossRef]
- Kanno, M.; Carroll, A.L.; Atsumi, S. Global metabolic rewiring for improved CO2 fixation and chemical production in cyanobacteria. Nat. Commun. 2017, 8, 14724. [Google Scholar] [CrossRef]
- Ma, K.; He, M.; You, H.; Pan, L.; Wang, Z.; Wang, Y.; Hu, G.; Cui, Y.; Maeda, T. Improvement of (R,R)-2,3-butanediol production from corn stover hydrolysate by cell recycling continuous fermentation. Chem. Eng. J. 2018, 332, 361–369. [Google Scholar] [CrossRef]
- Yuan, J.; He, Y.-Z.; Guo, Z.-W.; Gao, H.-F.; Chen, F.-B.; Li, L.-Z.; Li, Y.-Y.; Zhang, L.-Y. Utilization of Sweet Sorghum Juice for Efficient 2,3-Butanediol Production by Serratia marcescens H30. BioResources 2017, 12, 4926–4942. [Google Scholar] [CrossRef]
- Narisetty, V.; Narisetty, S.; Jacob, S.; Kumar, D.; Leeke, G.A.; Chandel, A.K.; Singh, V.; Srivastava, V.C.; Kumar, V. Biological production and recovery of 2,3-butanediol using arabinose from sugar beet pulp by Enterobacter ludwigii. Renew. Energy 2022, 191, 394–404. [Google Scholar] [CrossRef]
- Cortivo, P.R.D.; Machado, J.; Hickert, L.R.; Rossi, D.M.; Ayub, M.A.Z. Production of 2,3-butanediol by Klebsiella pneumoniae BLh-1 and Pantoea agglomerans BL1 cultivated in acid and enzymatic hydrolysates of soybean hull. Biotechnol. Prog. 2019, 35, e2793. [Google Scholar] [CrossRef] [PubMed]
- Tinôco, D.; de Castro, A.M.; Seldin, L.; Freire, D.M.G. Production of (2R,3R)-butanediol by Paenibacillus polymyxa PM 3605 from crude glycerol supplemented with sugarcane molasses. Process Biochem. 2021, 106, 88–95. [Google Scholar] [CrossRef]
- Hazeena, S.H.; Pandey, A.; Binod, P. Evaluation of oil palm front hydrolysate as a novel substrate for 2,3-butanediol production using a novel isolate Enterobacter cloacae SG1. Renew. Energy 2016, 98, 216–220. [Google Scholar] [CrossRef]
- Sikora, B.; Kubik, C.; Kalinowska, H.; Gromek, E.; Białkowska, A.; Jędrzejczak-Krzepkowska, M.; Schüett, F.; Turkiewicz, M. Application of byproducts from food processing for production of 2,3-butanediol using Bacillus amyloliquefaciens TUL 308. Prep. Biochem. Biotechnol. 2016, 46, 610–619. [Google Scholar] [CrossRef]
- Wong, C.L.; Huang, C.C.; Lu, W.B.; Chen, W.M.; Chang, J.S. Producing 2,3-butanediol from agricultural waste using an indigenous Klebsiella sp. Zmd30 strain. Biochem. Eng. J. 2012, 69, 32–40. [Google Scholar] [CrossRef]
- Liakou, V.; Pateraki, C.; Palaiogeorgou, A.M.; Kopsahelis, N.; Machado de Castro, A.; Guimarães Freire, D.M.; Nychas, G.J.E.; Papanikolaou, S.; Koutinas, A. Valorisation of fruit and vegetable waste from open markets for the production of 2,3-butanediol. Food Bioprod. Process. 2018, 108, 27–36. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Esparza, I.; Jiménez-Moreno, N.; Bimbela, F.; Ancín-Azpilicueta, C.; Gandía, L.M. Fruit and vegetable waste management: Conventional and emerging approaches. J. Environ. Manag. 2020, 265, 110510. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Garita-Cambronero, J.; Paniagua-García, A.I.; Díez-Antolínez, R. Tomato Waste from Processing Industries as a Feedstock for Biofuel Production. Bioenergy Res. 2019, 12, 1000–1011. [Google Scholar] [CrossRef]
- Encalada, A.M.I.; Pérez, C.D.; Flores, S.K.; Rossetti, L.; Fissore, E.N.; Rojas, A.M. Antioxidant pectin enriched fractions obtained from discarded carrots (Daucus carota L.) by ultrasound-enzyme assisted extraction. Food Chem. 2019, 289, 453–460. [Google Scholar] [CrossRef]
- MAPA Ministry of Agriculture, Fisheries and Food. Anuario de Estadística. Available online: www.mapa.gob.es/es/estadistica/temas/publicaciones/anuario-de-estadistica/default.aspx (accessed on 2 September 2022).
- Ramos-Andrés, M.; Aguilera-Torre, B.; García-Serna, J. Hydrothermal production of high-molecular weight hemicellulose-pectin, free sugars and residual cellulose pulp from discarded carrots. J. Clean. Prod. 2021, 290, 125179. [Google Scholar] [CrossRef]
- Clementz, A.; Torresi, P.A.; Molli, J.S.; Cardell, D.; Mammarella, E.; Yori, J.C. Novel method for valorization of by-products from carrot discards. LWT 2019, 100, 374–380. [Google Scholar] [CrossRef]
- Häßler, T.; Schieder, D.; Pfaller, R.; Faulstich, M.; Sieber, V. Enhanced fed-batch fermentation of 2,3-butanediol by Paenibacillus polymyxa DSM 365. Bioresour. Technol. 2012, 124, 237–244. [Google Scholar] [CrossRef]
- Okonkwo, C.C.; Ujor, V.C.; Mishra, P.K.; Ezeji, T.C. Process Development for Enhanced 2,3-Butanediol Production by Paenibacillus polymyxa DSM 365. Fermentation 2017, 3, 18. [Google Scholar] [CrossRef]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass; Jan, Rep. No. TP-510-42619; National Renewable Energy Laboratory: Golden, CO, USA, 2005. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of ash in biomass. In National Renewable Energy Laboratory; Jan, Rep. No. TP-510-42622; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of structural carbohydrates and lignin in biomass. In National Renewable Energy Laboratory; Jan, Rep. No. TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2011. [Google Scholar]
- Qin, J.; Xiao, Z.; Ma, C.; Xie, N.; Liu, P.; Xu, P. Production of 2,3-Butanediol by Klebsiella Pneumoniae Using Glucose and Ammonium Phosphate. Chin. J. Chem. Eng. 2006, 14, 132–136. [Google Scholar] [CrossRef]
- Sun, L.H.; Wang, X.D.; Dai, J.Y.; Xiu, Z.L. Microbial production of 2,3-butanediol from Jerusalem artichoke tubers by Klebsiella pneumoniae. Appl. Microbiol. Biotechnol. 2009, 82, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Erian, A.M.; Freitag, P.; Gibish, M.; Pflügl, S. High rate 2,3-butanediol production with Vibrio natriegens. Bioresour. Technol. 2020, 10, 100408. [Google Scholar] [CrossRef]
- Santos, D.d.A.; Cassan, L.P.; Lucas, S.C.O.; Romão, L.P.C.; Porto, A.L.M. Butanediol production from glycerol and glucose by Serratia marcescens isolated from tropical pat soil. Biocatal. Agric. Biotechnol. 2020, 26, 101615. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Bae, J.-M.; Kim, S.-J. Enantiopure meso-2,3-butanediol production by metabolically engineered Saccharomyces cerevisiae expressing 2,3-BDO dehydrogenase from Klebsiella oxytoca. J. Biotechnol. 2022, 354, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schilling, C.; Ciccone, R.; Sieber, V.; Schmid, J. Engineering of the 2,3-butanediol pathway of Paenibacillus polymyza DSM 365. Metab. Eng. 2020, 61, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kim, T.; Woo, H.M.; Lee, J.; Kim, Y.; Um, Y. Enhanced 2,3-Butanediol Production by Optimizing Fermentation Conditions and Engineering Klebsiella oxytoca M1 through Overexpression of Acetoin Reductase. PLoS ONE 2015, 10, e0138109. [Google Scholar] [CrossRef] [PubMed]
- Maina, S.; Schneider, R.; Alexandri, M.; Papapostolou, H.; Nychas, G.J.; Koutinas, A.; Venus, J. Volumetric oxygen transfer coefficient as fermentation control parameter to manipulate the production of either acetoin or D-2,3-butanediol using bakery waste. Bioresour. Technol. 2021, 335, 125155. [Google Scholar] [CrossRef]
- Park, J.M.; Song, H.; Lee, H.J.; Seung, D. In silico aided metabolic engineering of Klebsiella oxytoca and fermentation optimization for enhanced 2,3-butanediol production. J. Ind. Microbiol. Biotechnol. 2013, 40, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chu, H.; Gao, C.; Tao, F.; Zhou, Z.; Li, K.; Li, L.; Ma, C.; Xu, P. Systematic metabolic engineering of Escherichia coli for high-yield production of fuel bio-chemical 2,3-butanediol. Metab. Eng. 2014, 23, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.J.; Huang, H.; Du, J.; Zhu, J.G.; Ren, L.J.; Hu, N.; Li, S. Enhanced 2,3-butanediol production by Klebsiella oxytoca using a two-stage agitation speed control strategy. Bioresour. Technol. 2009, 100, 3410–3414. [Google Scholar] [CrossRef]
- Chan, S.; Jantama, S.S.; Kanchanatawee, S.; Jantama, K. Process Optimization on Micro-Aeration Supply for High Production Yield of 2,3-Butanediol from Maltodextrin by Metabolically-Engineered Klebsiella oxytoca. PLoS ONE 2016, 11, e0161503. [Google Scholar] [CrossRef]
- Białkowska, A.M.; Jędrzejczak-Krzepkowska, M.; Gromek, E.; Krysiak, J.; Sikora, B.; Kalinowska, H.; Kubik, C.; Schütt, F.; Turkiewicz, M. Effects of genetic modifications and fermentation conditions on 2,3-butanediol production by alkaliphilic Bacillus subtilis. Appl. Microbiol. Biotechnol. 2016, 100, 2663–2676. [Google Scholar] [CrossRef]
- Rehman, S.; Khairul Islam, M.; Khalid Khanzada, N.; Kyoungjin An, A.; Chaiprapat, S.; Leu, S.Y. Whole sugar 2,3-butanediol fermentation for oil palm empty fruit bunches biorefinery by a newly isolated Klebsiella pneumoniae PM2. Bioresour. Technol. 2021, 333, 125206. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Islam, M.K.; Khanzada, N.K.; Zhuang, H.; Wang, H.; Chaiprapat, S.; Leu, S.Y. Sustainability index accounting food and carbon benefits on circular 2,3-butanediol biorefinery with oil palm empty fruit bunches. Appl. Energy 2021, 303, 117667. [Google Scholar] [CrossRef]
- OHair, J.; Jin, Q.; Yu, D.; Wu, J.; Wang, H.; Zhou, S.; Huang, H. Non-sterile fermentation of food waste using thermophilic and alkaliphilic Bacillus licheniformis YNP5-TSU for 2,3-butanediol production. Waste Manag. 2021, 120, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Amiri, H. Recent innovations for reviving the ABE fermentation for production of butanol as a drop-in liquid biofuel. Biofuel Res. J. 2020, 7, 1256–1266. [Google Scholar] [CrossRef]
- Blomqvist, K.; Nikkola, M.; Lehtovaara, P.; Suihko, M.L.; Airaksinen, U.; Straby, K.B.; Knowles, J.K.C.; Penttila, M.E. Characterization of the genes of the 2,3-butanediol operons from Klebsiella terrigena and Enterobacter aerogenes. J. Bacteriol. 1993, 175, 1392–1404. [Google Scholar] [CrossRef]

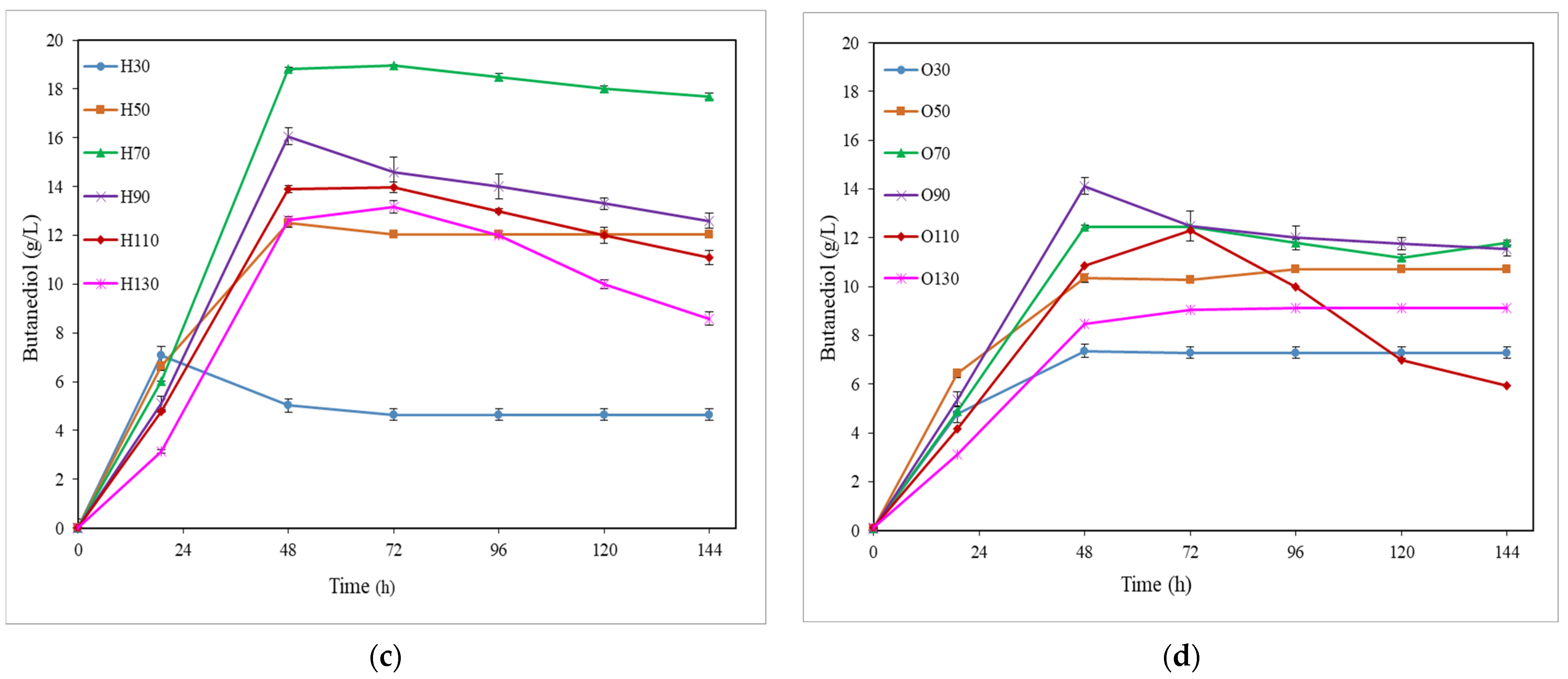



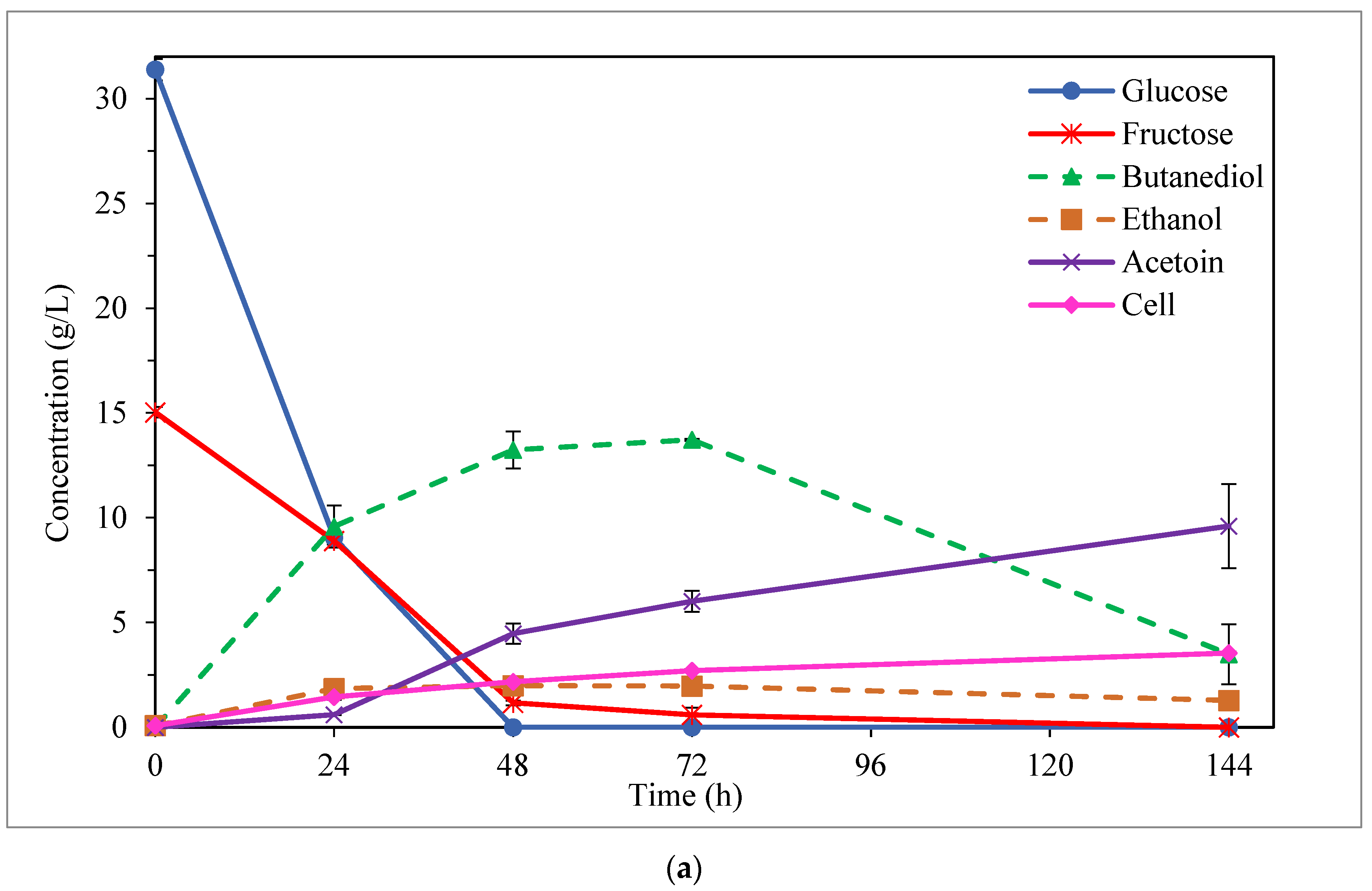
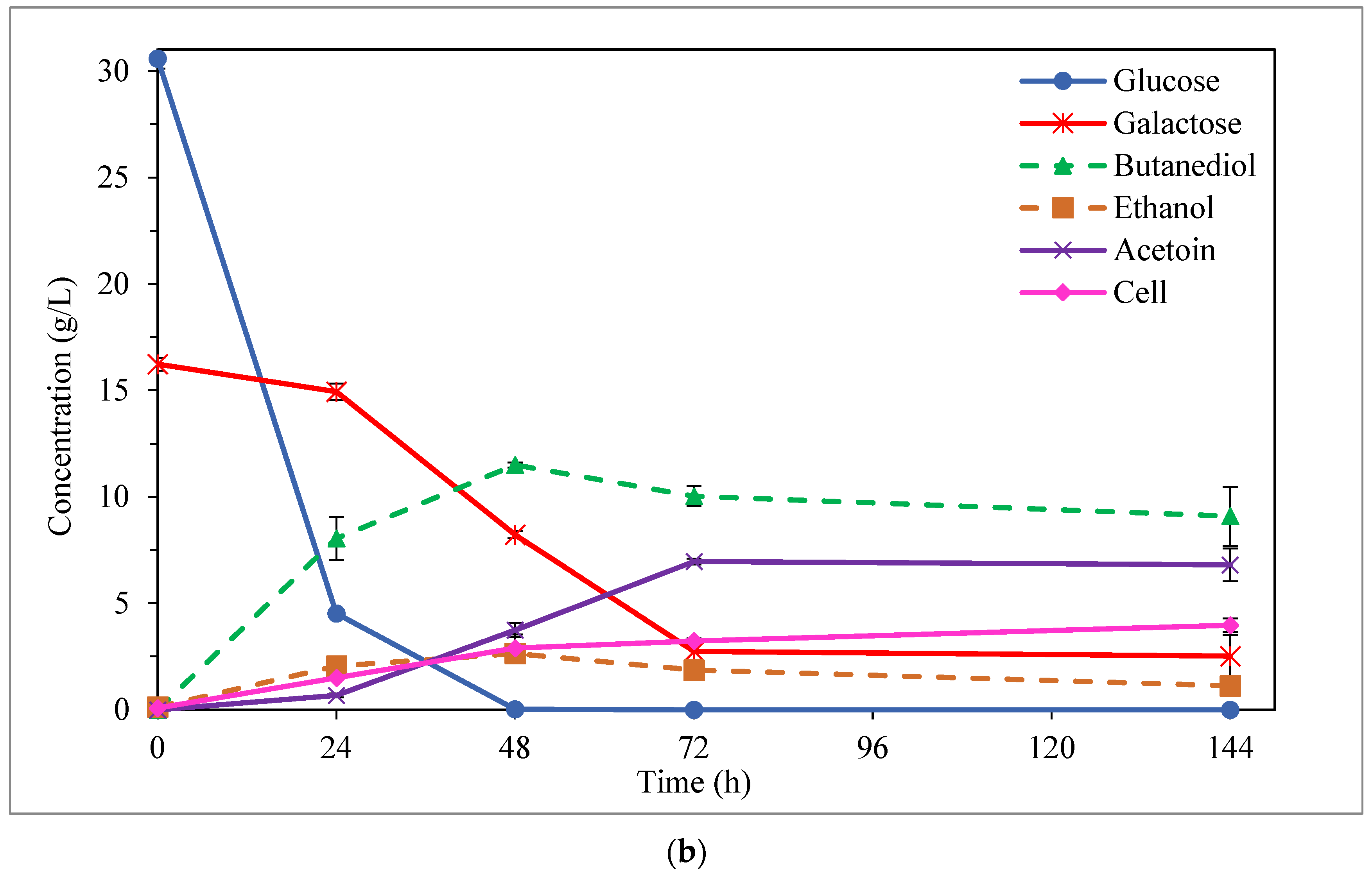

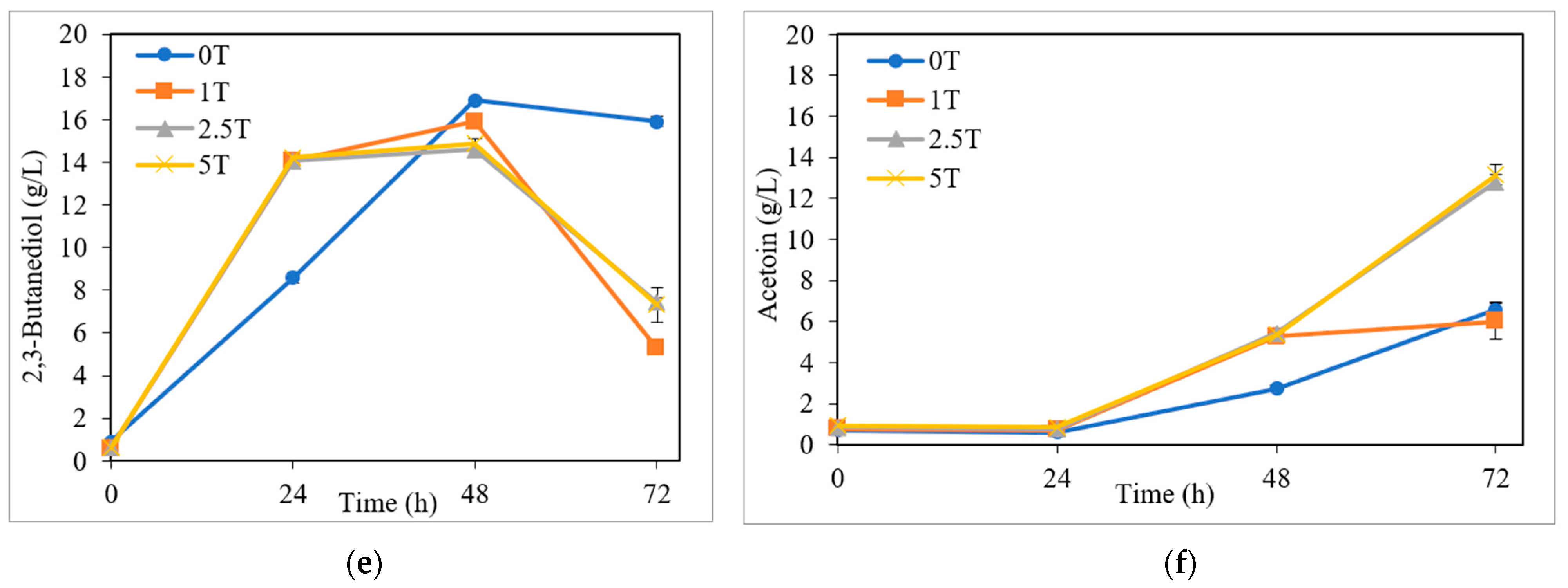
| Initial Sugar Conc. (g/L) | Fermentation Medium | Time (h) | Sugar Uptake (%) | BDO (g/L) | Ethanol (g/L) | Acetoin (g/L) | Cell (g/L) | YBDO/sugars (g/g) | PBDO (g/L·h) |
|---|---|---|---|---|---|---|---|---|---|
| 30 G | H | 24 | 86.0 (100) | 7.1 ± 0.3 | 0.9 ± 0.0 | 1.6 ± 0.1 | 1.6 ± 0.1 | 0.35 | 0.37 |
| O | 48 | 100 (100) | 7.4 ± 0.4 | 1.0 ± 0.0 | 3.5 ± 0.1 | 2.7 ± 0.0 | 0.27 | 0.15 | |
| 50 G | H | 48 | 100 (100) | 12.5 ± 0.2 | 2.1 ± 0.1 | 2.8 ± 0.2 | 3.7 ± 0.3 | 0.32 | 0.26 |
| O | 48 | 78.7 (86.0) | 10.3 ± 0.3 | 1.2 ± 0.1 | 3.5 ± 0.6 | 2.8 ± 0.5 | 0.30 | 0.22 | |
| 70 G | H | 72 | 93.3 (93.3) | 19.0 ± 0.0 | 2.4 ± 0.0 | 1.9 ± 0.2 | 3.4 ± 0.4 | 0.37 | 0.26 |
| O | 48 | 57.5 (57.5) | 12.4 ± 0.3 | 0.7 ± 0.0 | 2.5 ± 0.0 | 3.2 ± 0.1 | 0.35 | 0.26 | |
| 90 G | H | 48 | 58.3 (63.1) | 16.1 ± 0.3 | 2.2 ± 0.1 | 1.6 ± 0.3 | 2.9 ± 0.4 | 0.39 | 0.34 |
| O | 48 | 43.8 (52.7) | 14.1 ± 0.0 | 0.7 ± 0.1 | 1.8 ± 0.5 | 3.1 ± 0.3 | 0.42 | 0.29 | |
| 110 G | H | 48 | 43.7 (43.7) | 13.9 ± 0.1 | 2.2 ± 0.0 | 1.3 ± 0.1 | 1.6 ± 0.2 | 0.40 | 0.29 |
| O | 72 | 29.0 (29.0) | 12.3 ± 0.2 | 0.7 ± 0.1 | 2.0 ± 0.2 | 2.4 ± 0.3 | 0.46 | 0.17 | |
| 130 G | H | 72 | 33.2 (33.2) | 13.2 ± 0.2 | 1.3 ± 0.1 | 1.3 ± 0.0 | 4.3 ± 0.3 | 0.42 | 0.18 |
| O | 72 | 22.3 (22.3) | 9.1 ± 0.1 | 0.4 ± 0.0 | 2.5 ± 0.1 | 2.8 ± 0.2 | 0.38 | 0.13 | |
| 30 F | H | 48 | 100 (100) | 4.1 ± 0.1 | 1.9 ± 0.0 | 3.4 ± 0.1 | 1.9 ± 0.1 | 0.18 | 0.09 |
| O | 24 | 70.1 (100) | 6.3 ± 0.1 | 1.4 ± 0.0 | 1.8 ± 0.1 | 1.2 ± 0.0 | 0.36 | 0.26 | |
| 50 F | H | 48 | 93.2 (100) | 10.5 ± 0.2 | 4.0 ± 0.1 | 2.2 ± 0.2 | 3.3 ± 0.2 | 0.29 | 0.22 |
| O | 24 | 41.3 (70.9) | 5.9 ± 0.1 | 1.0 ± 0.0 | 1.8 ± 0.1 | 0.8 ± 0.0 | 0.33 | 0.25 | |
| 70 F | H | 48 | 77.2 (86.9) | 14.9 ± 0.4 | 4.3 ± 0.2 | 0.6 ± 0.0 | 2.2 ± 0.1 | 0.36 | 0.31 |
| O | 48 | 48.1 (64.8) | 6.7 ± 0.1 | 1.9 ± 0.1 | 4.5 ± 0.3 | 2.3 ± 0.1 | 0.24 | 0.14 | |
| 90 F | H | 48 | 57.1 (64.3) | 14.5 ± 0.1 | 3.6 ± 0.1 | 0.6 ± 0.1 | 2.5 ± 0.3 | 0.38 | 0.30 |
| O | 144 | 52.5 (52.5) | 8.0 ± 0.3 | 0.6 ± 0.0 | 8.1 ± 0.4 | 3.9 ± 0.2 | 0.21 | 0.06 | |
| 110 F | H | 48 | 40.3 (53.1) | 10.0 ± 0.0 | 3.1 ± 0.1 | 1.0 ± 0.1 | 1.9 ± 0.1 | 0.30 | 0.21 |
| O | 72 | 44.8 (44.8) | 9.8 ± 0.2 | 1.0 ± 0.1 | 8.6 ± 0.3 | 2.5 ± 0.3 | 0.24 | 0.14 | |
| 30 Ga | H | 24 | 64.2 (100) | 4.8 ± 0.2 | 1.3 ± 0.2 | 1.4 ± 0.2 | 1.5 ± 0.1 | 0.32 | 0.20 |
| O | 48 | 100 (100) | 8.9 ± 0.2 | 0.8 ± 0.0 | 2.6 ± 0.2 | 1.7 ± 0.0 | 0.34 | 0.19 | |
| 50 Ga | H | 48 | 100 (100) | 11.4 ± 0.4 | 2.6 ± 0.2 | 1.9 ± 0.1 | 2.7 ± 0.1 | 0.30 | 0.24 |
| O | 48 | 90.0 (90.0) | 15.3 ± 0.4 | 0.8 ± 0.1 | 1.9 ± 0.0 | 2.0 ± 0.3 | 0.38 | 0.32 | |
| 70 Ga | H | 48 | 61.6 (75.0) | 10.4 ± 0.2 | 2.5 ± 0.3 | 1.3 ± 0.0 | 2.5 ± 0.2 | 0.32 | 0.22 |
| O | 48 | 54.8 (54.8) | 11.7 ± 0.3 | 2.2 ± 0.2 | 2.7 ± 0.2 | 1.6 ± 0.1 | 0.34 | 0.24 | |
| 90 Ga | H | 48 | 33.6 (48.6) | 7.0 ± 0.1 | 1.4 ± 0.0 | 1.3 ± 0.1 | 1.5 ± 0.1 | 0.31 | 0.15 |
| O | 48 | 39.1 (44.1) | 10.9 ± 0.2 | 1.1 ± 0.1 | 1.4 ± 0.1 | 2.5 ± 0.3 | 0.37 | 0.23 | |
| G + F (40 + 20) | H | 72 | 98.7 (100) | 13.7 ± 0.1 | 2.0 ± 0.1 | 6.0 ± 0.5 | 2.7 ± 0.1 | 0.30 | 0.19 |
| G + Ga (40 + 20) | H | 48 | 82.5 (94.6) | 11.5 ± 0.1 | 2.6 ± 0.1 | 3.7 ± 0.3 | 2.9 ± 0.3 | 0.30 | 0.24 |
| Initial Sugar Conc. (g/L) | Fermentation Medium | Time (h) | Sugar Uptake (%) | BDO (g/L) | Ethanol (g/L) | Acetoin (g/L) | Cell (g/L) | YBDO/sugars (g/g) | PBDO (g/L·h) |
|---|---|---|---|---|---|---|---|---|---|
| Study of Stirring/Aeration | |||||||||
| 100 rpm | H | 72 | 92.2 (92.2) | 18.8 ± 0.7 | 2.5 ± 0.1 | 2.1 ± 0.0 | 2.0 ± 0.3 | 0.43 | 0.26 |
| 200 rpm | H | 48 | 88.9 (100) | 16.9 ± 0.0 | 1.8 ± 0.0 | 2.7 ± 0.2 | 2.3 ± 0.1 | 0.41 | 0.35 |
| 300 rpm | H | 24 | 89.0 (100) | 16.3 ± 0.3 | 1.5 ± 0.2 | 4.2 ± 0.0 | 0.7 ± 0.2 | 0.39 | 0.68 |
| Study of Tryptone Use in Fermentation Medium | |||||||||
| 0 T | H | 48 | 88.9 (100) | 16.9 ± 0.0 | 1.8 ± 0.0 | 2.7 ± 0.2 | 2.3 ± 0.1 | 0.41 | 0.35 |
| 1 T | H | 48 | 90.0 (98.8) | 15.9 ± 0.1 | 1.3 ± 0.0 | 5.3 ± 0.3 | 2.5 ± 0.6 | 0.37 | 0.33 |
| 2.5 T | H | 48 | 90.2 (93.7) | 14.6 ± 0.0 | 1.3 ± 0.0 | 5.4 ± 0.0 | 3.0 ± 0.6 | 0.33 | 0.30 |
| 5 T | H | 48 | 90.1 (93.2) | 14.9 ± 0.2 | 1.2 ± 0.1 | 5.3 ± 0.0 | 3.0 ± 0.2 | 0.34 | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Linares, J.C.; Mateo Martínez, A.; Coca, M.; Lucas, S.; García-Cubero, M.T. Carrot Discard as a Promising Feedstock to Produce 2,3-Butanediol by Fermentation with P. polymyxa DSM 365. Bioengineering 2023, 10, 937. https://doi.org/10.3390/bioengineering10080937
López-Linares JC, Mateo Martínez A, Coca M, Lucas S, García-Cubero MT. Carrot Discard as a Promising Feedstock to Produce 2,3-Butanediol by Fermentation with P. polymyxa DSM 365. Bioengineering. 2023; 10(8):937. https://doi.org/10.3390/bioengineering10080937
Chicago/Turabian StyleLópez-Linares, Juan Carlos, Adrián Mateo Martínez, Mónica Coca, Susana Lucas, and María Teresa García-Cubero. 2023. "Carrot Discard as a Promising Feedstock to Produce 2,3-Butanediol by Fermentation with P. polymyxa DSM 365" Bioengineering 10, no. 8: 937. https://doi.org/10.3390/bioengineering10080937
APA StyleLópez-Linares, J. C., Mateo Martínez, A., Coca, M., Lucas, S., & García-Cubero, M. T. (2023). Carrot Discard as a Promising Feedstock to Produce 2,3-Butanediol by Fermentation with P. polymyxa DSM 365. Bioengineering, 10(8), 937. https://doi.org/10.3390/bioengineering10080937







