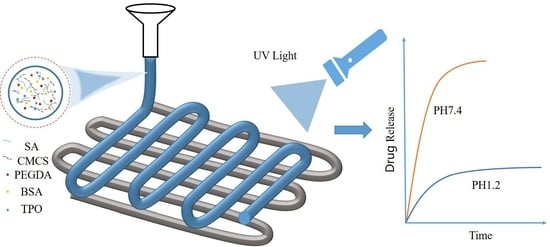
Development of pH-Responsive Polypills via Semi-Solid Extrusion 3D Printing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Printing Ink
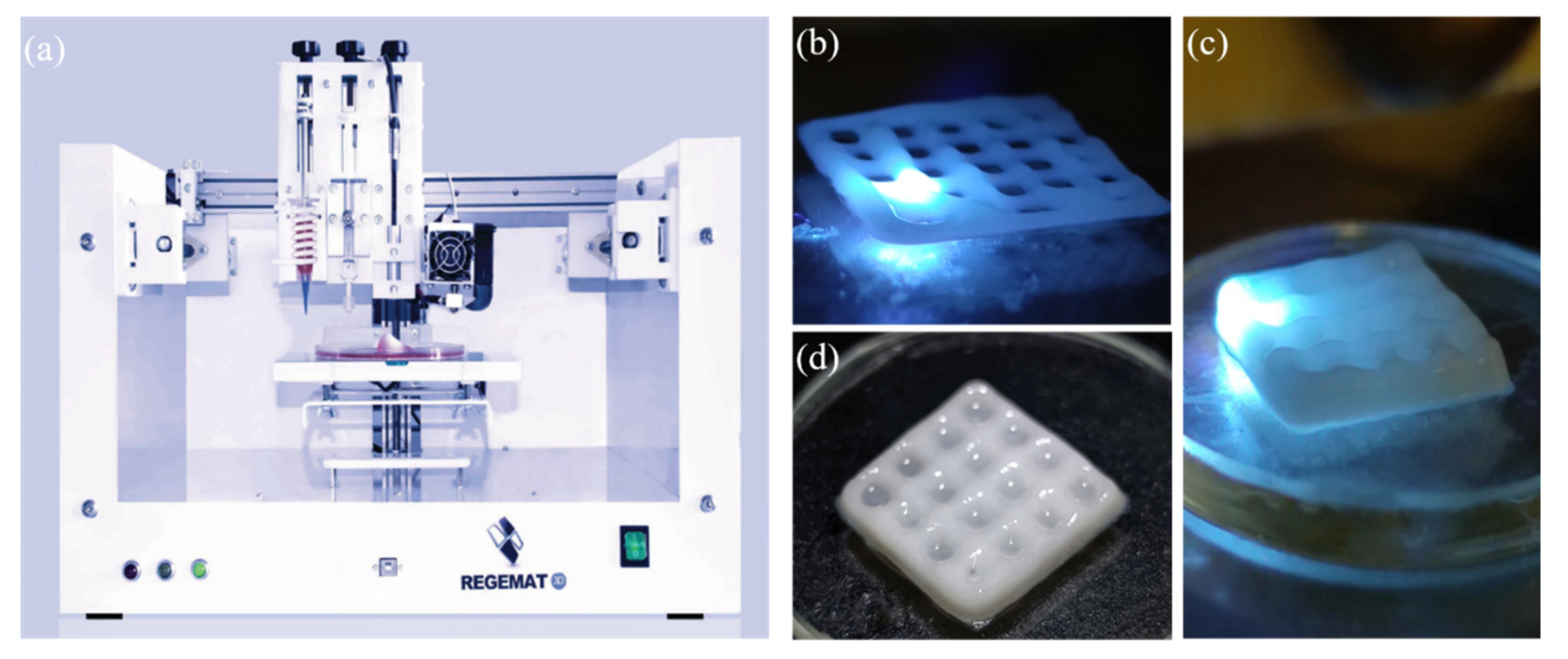
2.3. Printing of Tablets
2.4. Post-Treatment of Tablets
2.5. Characterisation of Hydrogel Properties
2.6. Preparation of Artificial Gastric and Intestinal Fluids
2.6.1. Preparation of Hydrochloric Acid Solution with a pH Value of 1.2 (Artificial Gastric Fluid)
2.6.2. Preparation of PBS Solution (Artificial Intestinal Solution) with a pH Value of 7.4
2.7. Fourier Transform Infrared Spectroscopy (FTIR)
2.8. Drug Release Testing
3. Results and Discussion
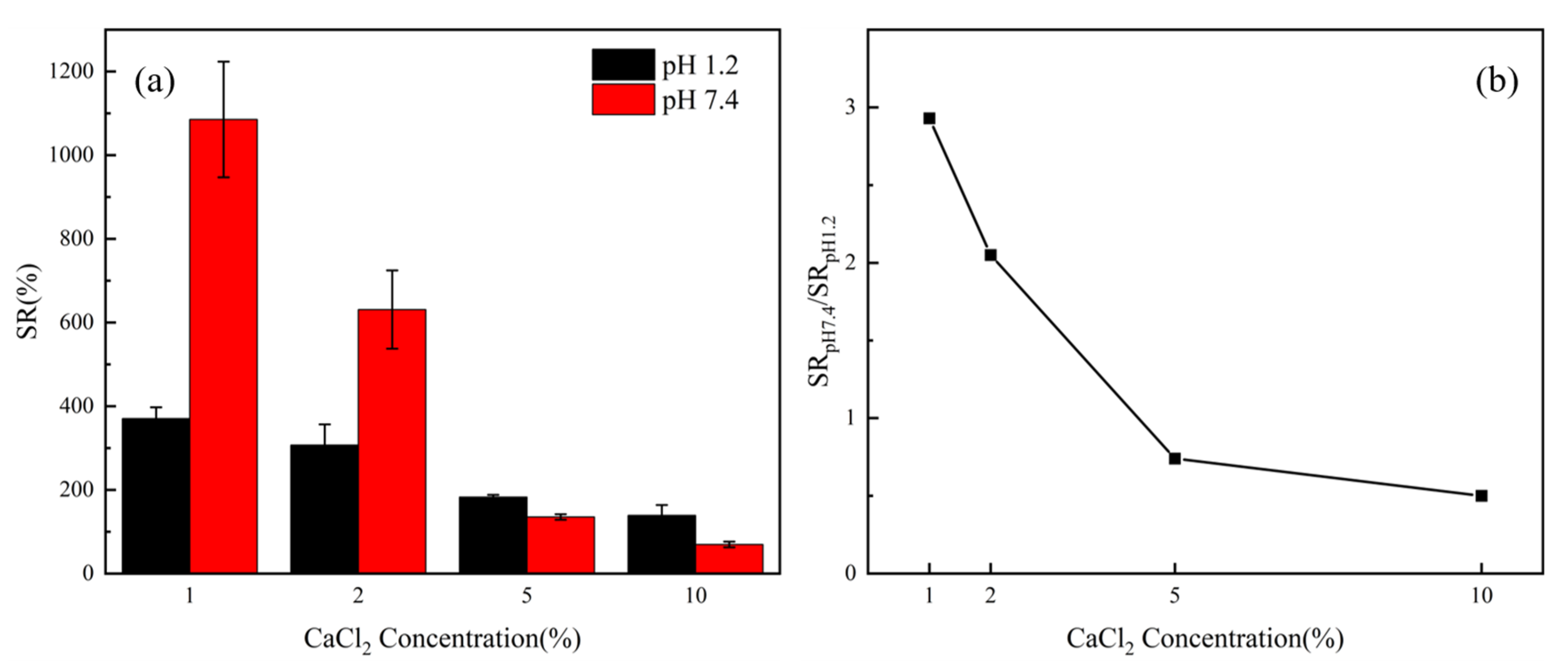
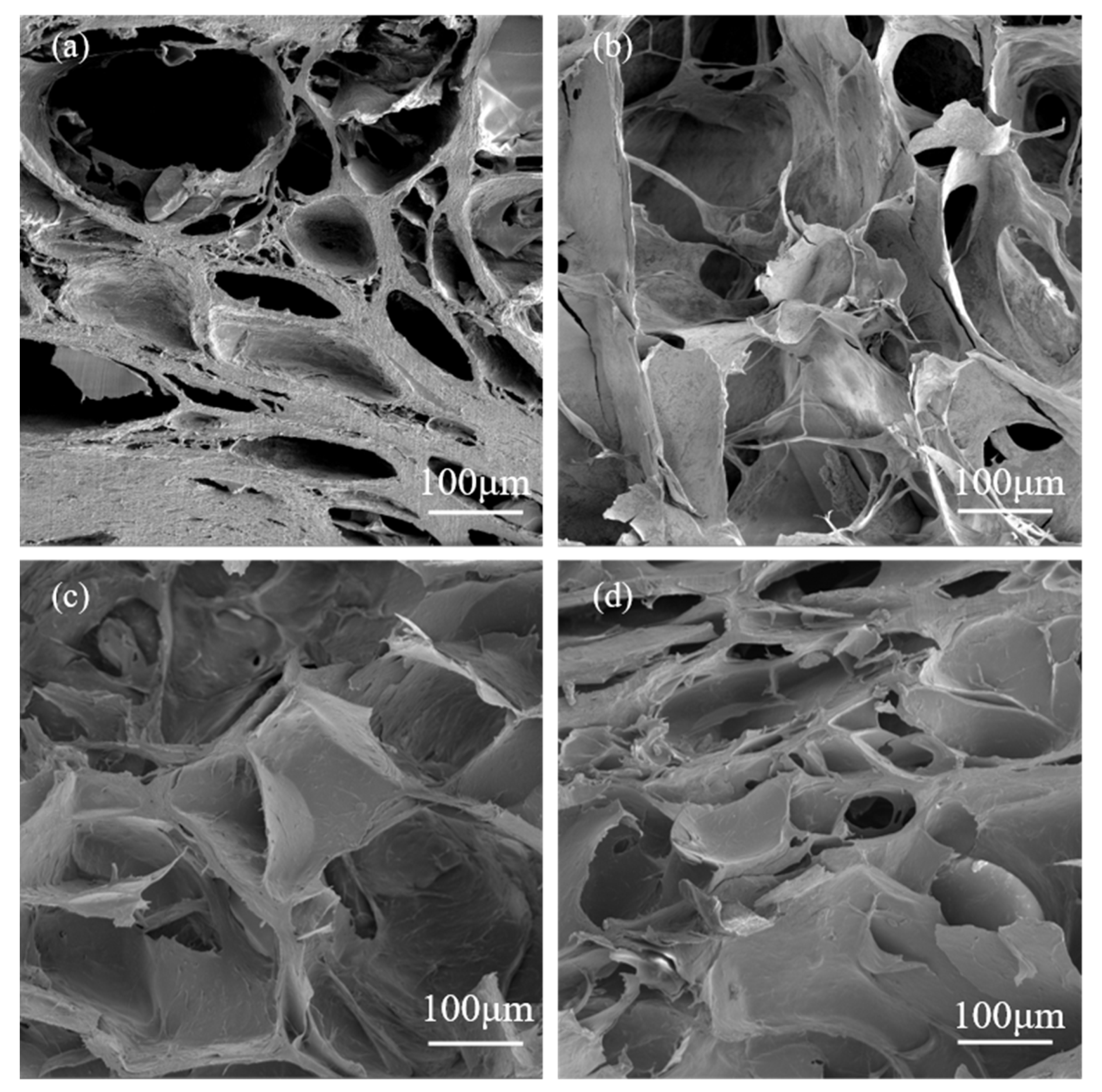
3.1. Effects of Calcium ion Concentration and Crosslinking Time on pH-Responsive Properties
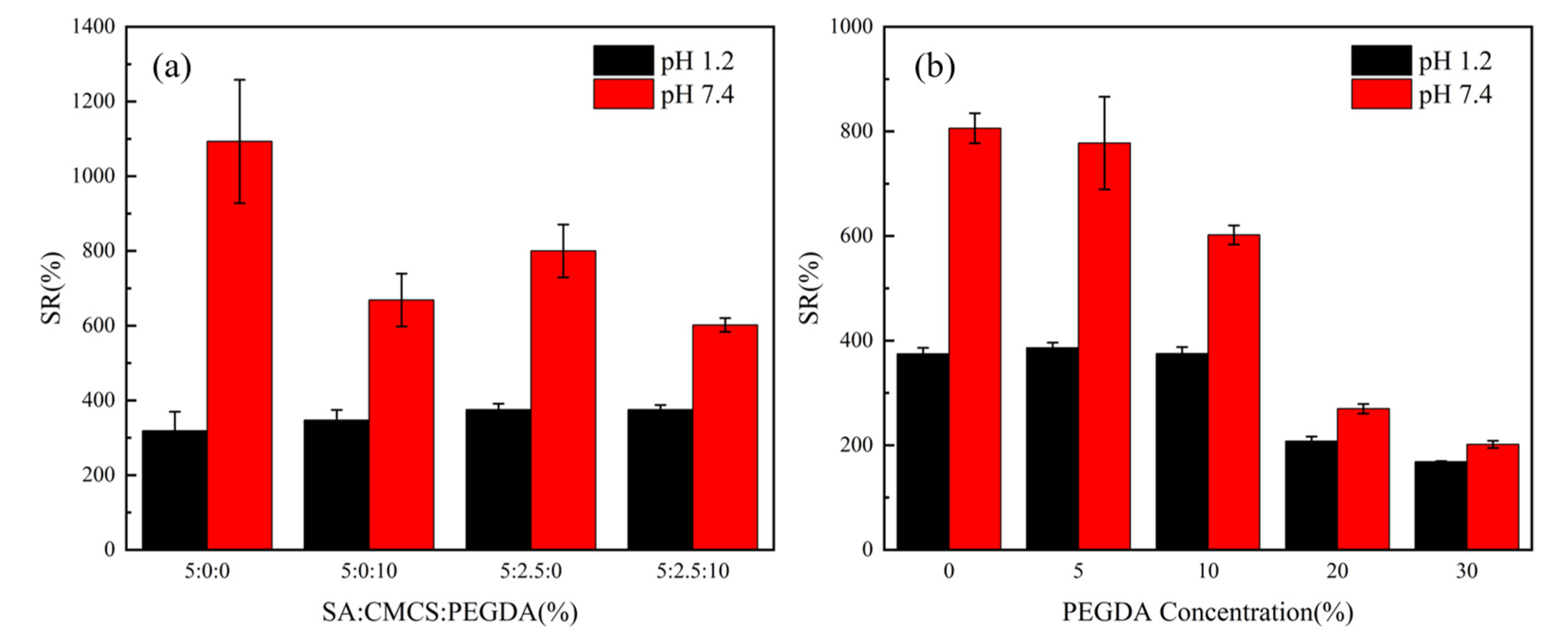
3.2. Effects of CMCS and PEGDA Concentrations on pH-Responsive Behaviours
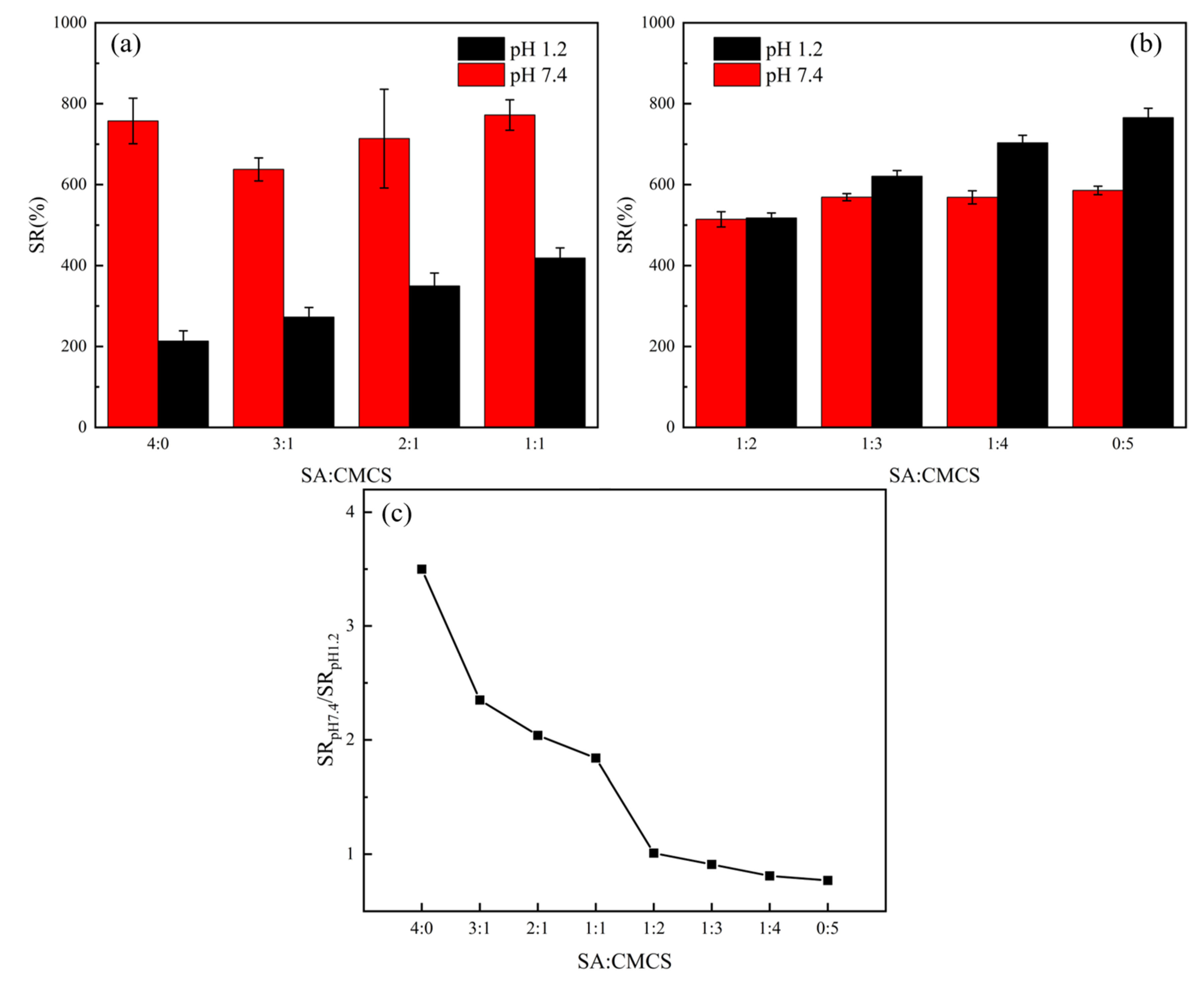
3.3. Effects of the Ratio between SA to CMCS on pH-Responsive Behaviours
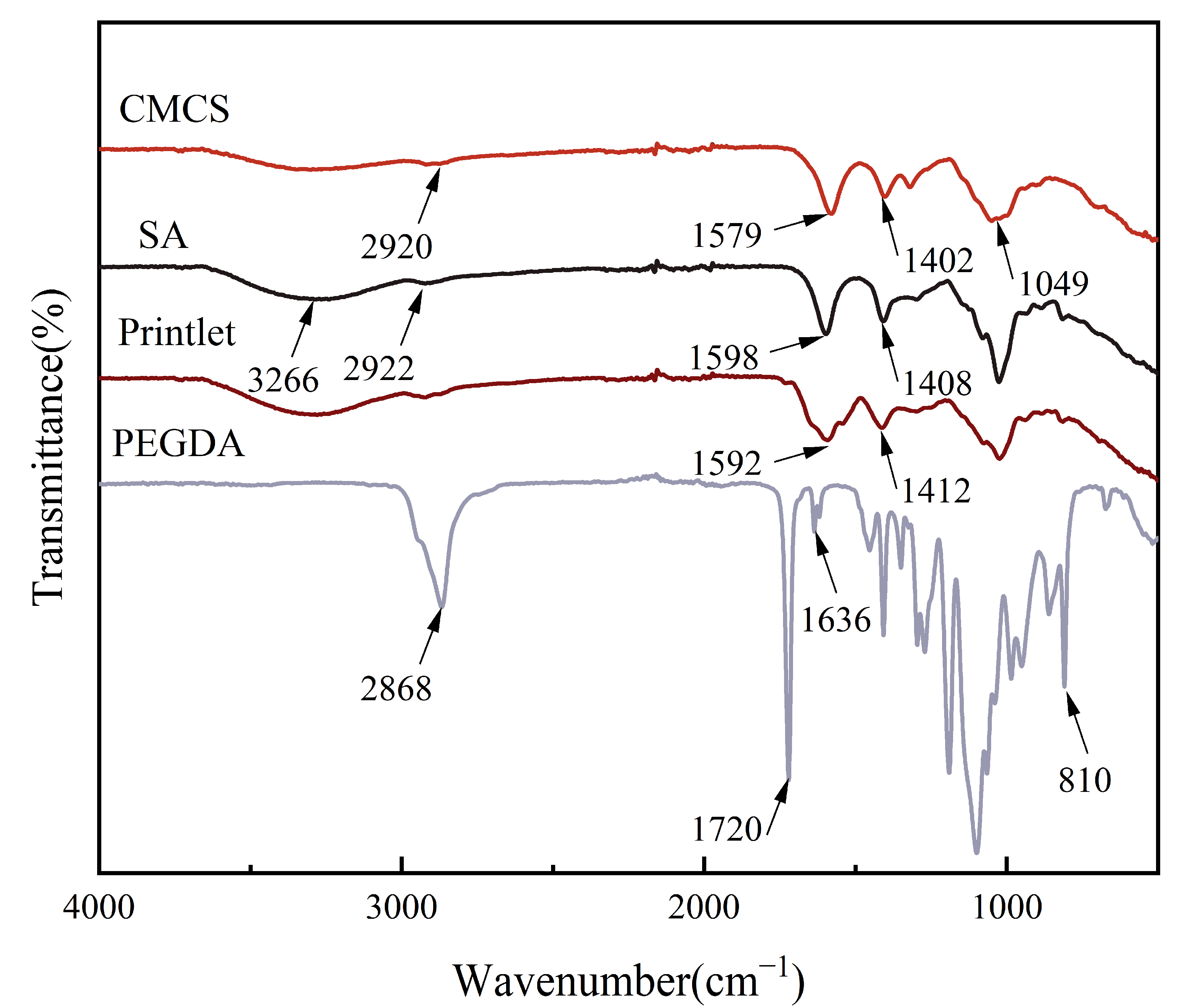
3.4. Fourier Transform Infrared Spectroscopy (FTIR)
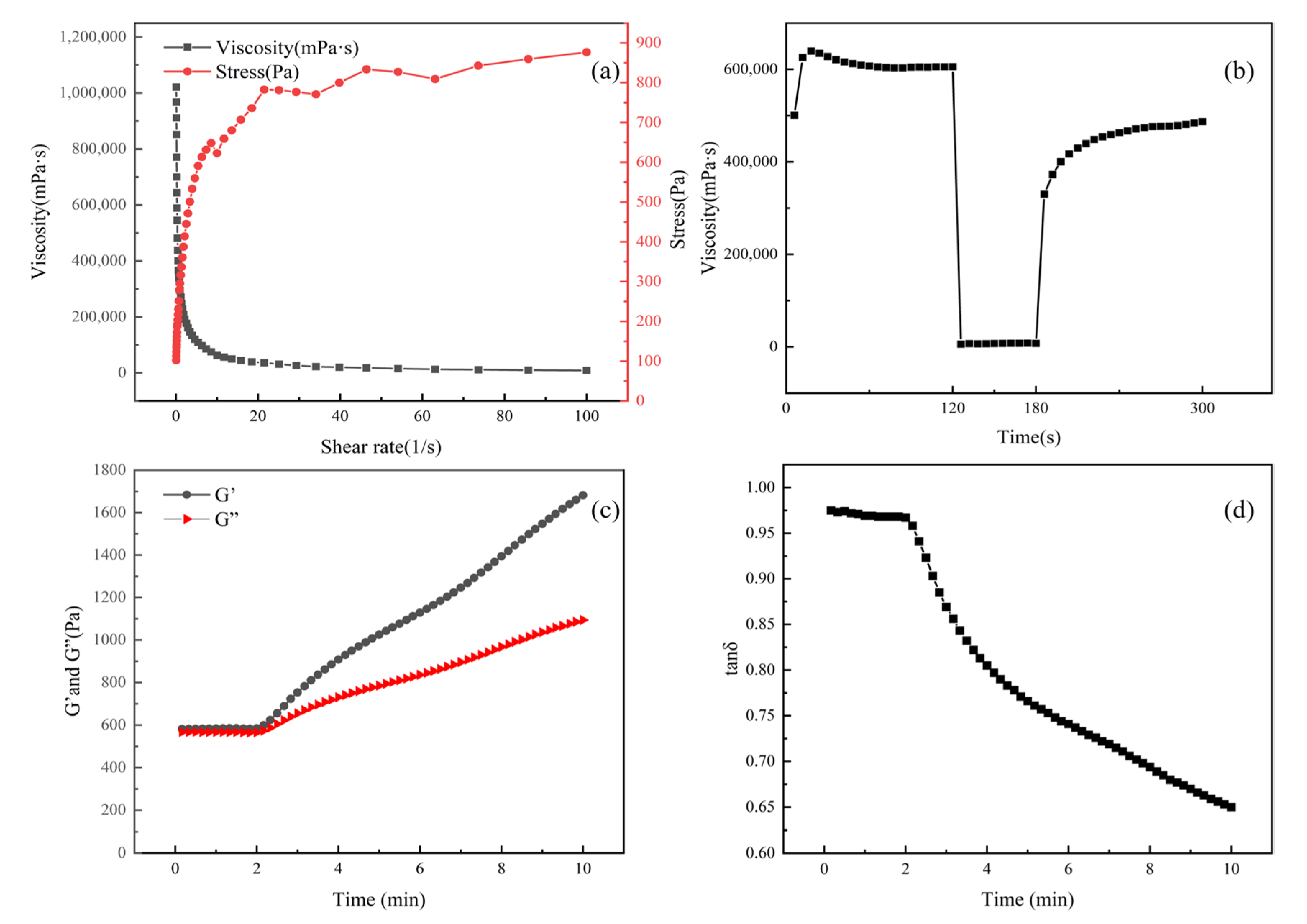
3.5. Rheological Properties
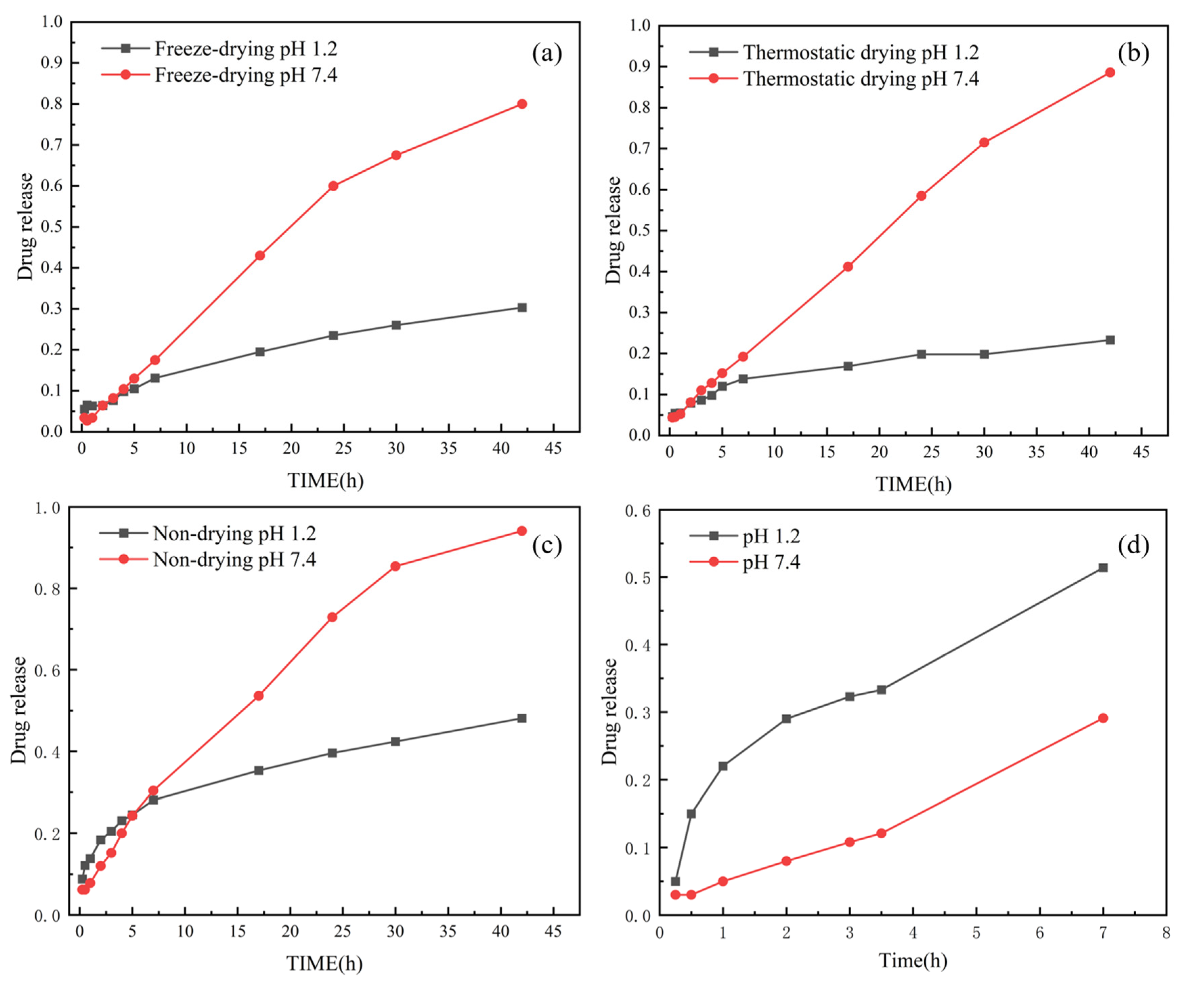
3.6. In Vitro Drug Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, W.; Ling, P.; Zhang, T. Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water-soluble drugs. J. Drug Deliv. 2013, 2013, 340315. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.C.; Isreb, A.; Forbes, R.T.; Dores, F.; Habashy, R.; Petit, J.-B.; Alhnan, M.A.; Oga, E.F. ‘Temporary Plasticiser’: A novel solution to fabricate 3D printed patient-centred cardiovascular ‘Polypill’ architectures. Eur. J. Pharm. Biopharm. 2019, 135, 94–103. [Google Scholar] [CrossRef]
- Roy, A.; Naik, N.; Srinath Reddy, K. Strengths and Limitations of Using the Polypill in Cardiovascular Prevention. Curr. Cardiol. Rep. 2017, 19, 45. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Aimetti, A.A.; Langer, R.; Gu, Z. Bioresponsive materials. Nat. Rev. Mater. 2016, 2, 16075. [Google Scholar] [CrossRef]
- Liu, L.; Yao, W.; Rao, Y.; Lu, X.; Gao, J. pH-Responsive carriers for oral drug delivery: Challenges and opportunities of current platforms. Drug Deliv. 2017, 24, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Agüero, L.; Zaldivar-Silva, D.; Peña, L.; Dias, M.L. Alginate microparticles as oral colon drug delivery device: A review. Carbohydr. Polym. 2017, 168, 32–43. [Google Scholar] [CrossRef]
- Hariyadi, D.M.; Lin, S.C.; Wang, Y.; Bostrom, T.; Turner, M.S.; Bhandari, B.; Coombes, A.G. Diffusion loading and drug delivery characteristics of alginate gel microparticles produced by a novel impinging aerosols method. J. Drug Target. 2010, 18, 831–841. [Google Scholar] [CrossRef]
- Mirtic, J.; Ilas, J.; Kristl, J. Influence of different classes of crosslinkers on alginate polyelectrolyte nanoparticle formation, thermodynamics and characteristics. Carbohydr. Polym. 2018, 181, 93–102. [Google Scholar] [CrossRef]
- Agulhon, P.; Robitzer, M.; David, L.; Quignard, F. Structural regime identification in ionotropic alginate gels: Influence of the cation nature and alginate structure. Biomacromolecules 2012, 13, 215–220. [Google Scholar] [CrossRef]
- Hariyadi, D.M.; Islam, N. Current Status of Alginate in Drug Delivery. Adv. Pharm. Pharm. Sci. 2020, 2020, 8886095. [Google Scholar] [CrossRef]
- Treenate, P.; Monvisade, P. In vitro drug release profiles of pH-sensitive hydroxyethylacryl chitosan/sodium alginate hydrogels using paracetamol as a soluble model drug. Int. J. Biol. Macromol. 2017, 99, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Ganguly, S. Alginate-chitosan composite hydrogel film with macrovoids in the inner layer for biomedical applications. J. Appl. Polym. Sci. 2019, 136. [Google Scholar] [CrossRef]
- Chen, S.C.; Wu, Y.C.; Mi, F.L.; Lin, Y.H.; Yu, L.C.; Sung, H.W. A novel pH-sensitive hydrogel composed of N,O-carboxymethyl chitosan and alginate cross-linked by genipin for protein drug delivery. J. Control. Release 2004, 96, 285–300. [Google Scholar] [CrossRef]
- Risbud, M.V.; Hardikar, A.A.; Bhat, S.V.; Bhonde, R.R. pH-sensitive freeze-dried chitosan–polyvinyl pyrrolidone hydrogels as controlled release system for antibiotic delivery. J. Control. Release 2000, 68, 23–30. [Google Scholar] [CrossRef]
- Nakatsuka, S.; Andrady, A.L. Permeability of vitamin B-12 in chitosan membranes. Effect of crosslinking and blending with poly(vinyl alcohol) on permeability. J. Appl. Polym. Sci. 1992, 44, 17–28. [Google Scholar] [CrossRef]
- Thacharodi, D.; Rao, K.P. Propranolol hydrochloride release behaviour of crosslinked chitosan membranes. J. Chem. Technol. Biotechnol. 1993, 58, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Illum, L. Chitosan and its use as a pharmaceutical excipient. Pharm. Res. 1998, 15, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Seidi, F.; Khodadadi Yazdi, M.; Jouyandeh, M.; Dominic, M.; Naeim, H.; Nezhad, M.N.; Bagheri, B.; Habibzadeh, S.; Zarrintaj, P.; Saeb, M.R.; et al. Chitosan-based blends for biomedical applications. Int. J. Biol. Macromol. 2021, 183, 1818–1850. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, F.; Cao, H.; Li, L.; He, N.; Han, X. Design and Fused Deposition Modeling of Triply Periodic Minimal Surface Scaffolds with Channels and Hydrogel for Breast Reconstruction. Int. J. Bioprinting. 2023, 9, 2. [Google Scholar] [CrossRef]
- Patel, S.K.; Khoder, M.; Peak, M.; Alhnan, M.A. Controlling drug release with additive manufacturing-based solutions. Adv. Drug Deliv. Rev. 2021, 174, 369–386. [Google Scholar] [CrossRef]
- Ling, L.; Zhu, X.; Yang, H.; Liang, B.; Yuan, L.; Hu, Y.; Chen, F.; Han, X. Phase-Field Model for Drug Release of Water-Swellable Filaments for Fused Filament Fabrication. Mol. Pharm. 2022, 19, 2854–2867. [Google Scholar]
- Seoane-Viano, I.; Januskaite, P.; Alvarez-Lorenzo, C.; Basit, A.W.; Goyanes, A. Semi-solid extrusion 3D printing in drug delivery and biomedicine: Personalised solutions for healthcare challenges. J. Control. Release 2021, 332, 367–389. [Google Scholar] [CrossRef] [PubMed]
- Falcone, G.; Mazzei, P.; Piccolo, A.; Esposito, T.; Mencherini, T.; Aquino, R.P.; Del Gaudio, P.; Russo, P. Advanced printable hydrogels from pre-crosslinked alginate as a new tool in semi solid extrusion 3D printing process. Carbohydr. Polym. 2022, 276, 118746. [Google Scholar] [CrossRef]
- Goh, W.J.; Tan, S.X.; Pastorin, G.; Ho, P.C.L.; Hu, J.; Lim, S.H. 3D printing of four-in-one oral polypill with multiple release profiles for personalized delivery of caffeine and vitamin B analogues. Int. J. Pharm. 2021, 598, 120360. [Google Scholar] [CrossRef]
- Hiller, A.; Borchers, K.; Tovar, G.E.M.; Southan, A. Impact of intermediate UV curing and yield stress of 3D printed poly(ethylene glycol) diacrylate hydrogels on interlayer connectivity and maximum build height. Addit. Manuf. 2017, 18, 136–144. [Google Scholar] [CrossRef]
- Shi, L.; Carstensen, H.; Hölzl, K.; Lunzer, M.; Li, H.; Hilborn, J.; Ovsianikov, A.; Ossipov, D.A. Dynamic Coordination Chemistry Enables Free Directional Printing of Biopolymer Hydrogel. Chem. Mater. 2017, 29, 5816–5823. [Google Scholar] [CrossRef]
- Macleod; Graeme, S.; Collett, J.H.; Fell, J.T. The Potential Use of Mixed Films of Pectin, Chitosan and Hpmc for Bimodal Drug Release. J. Control. Release 1999, 58, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Fu, H.; Hou, Z.; Si, Y.; Shan, W.; Yang, Y. Three-Dimensional Printing of Gastro-Floating Tablets Using Polyethylene Glycol Diacrylate-Based Photocurable Printing Material. Int. J. Pharm. 2021, 603, 120674. [Google Scholar] [CrossRef]
- Real, J.P.; Barberis, M.E.; Camacho, N.M.; Sánchez Bruni, S.; Palma, S.D. Design of novel oral ricobendazole formulation applying melting solidification printing process (MESO-PP): An innovative solvent-free alternative method for 3D printing using a simplified concept and low temperature. Int. J. Pharm. 2020, 587, 119653. [Google Scholar] [CrossRef]
- Jing, H.; Huang, X.; Du, X.; Mo, L.; Ma, C.; Wang, H. Facile synthesis of pH-responsive sodium alginate/carboxymethyl chitosan hydrogel beads promoted by hydrogen bond. Carbohydr. Polym. 2022, 278, 118993. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, R.; Zhang, W.; Song, X.; Xu, L.; Zhao, Y.; Shang, L.; Zhang, J. Preparation of carboxylmethylchitosan and alginate blend membrane for diffusion-controlled release of diclofenac diethylamine. J. Mater. Sci. Technol. 2021, 63, 210–215. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Ramos, V.; Stanic, V.; Bruno, D.; Mattioli-Belmonte, M.; Tosi, G.; Giardino, R. Osteogenesis promoted by calcium phosphate N,N-dicarboxymethyl chitosan. Carbohydr. Polym. 1998, 36, 267–276. [Google Scholar] [CrossRef]
- Fan, L.-H.; Pan, X.-R.; Zhou, Y.; Chen, L.-Y.; Xie, W.-G.; Long, Z.-H.; Zheng, H. Preparation and characterization of crosslinked carboxymethyl chitosan–oxidized sodium alginate hydrogels. J. Appl. Polym. Sci. 2011, 122, 2331–2337. [Google Scholar] [CrossRef]
- Adamov, I.; Stanojević, G.; Medarević, D.; Ivković, B.; Kočović, D.; Mirković, D.; Ibrić, S. Formulation and characterization of immediate-release oral dosage forms with zolpidem tartrate fabricated by digital light processing (DLP) 3D printing technique. Int. J. Pharm. 2022, 624, 122046. [Google Scholar] [CrossRef] [PubMed]
- Zidan, A.; Alayoubi, A.; Asfari, S.; Coburn, J.; Ghammraoui, B.; Aqueel, S.; Cruz, C.N.; Ashraf, M. Development of mechanistic models to identify critical formulation and process variables of pastes for 3D printing of modified release tablets. Int. J. Pharm. 2019, 555, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Conceição, J.; Farto-Vaamonde, X.; Goyanes, A.; Adeoye, O.; Concheiro, A.; Cabral-Marques, H.; Sousa Lobo, J.M.; Alvarez-Lorenzo, C. Hydroxypropyl-β-cyclodextrin-based fast dissolving carbamazepine printlets prepared by semisolid extrusion 3D printing. Carbohydr. Polym. 2019, 221, 55–62. [Google Scholar] [CrossRef]
- Bom, S.; Ribeiro, R.; Ribeiro, H.M.; Santos, C.; Marto, J. On the progress of hydrogel-based 3D printing: Correlating rheological properties with printing behaviour. Int. J. Pharm. 2022, 615, 121506. [Google Scholar] [CrossRef]
- Müller, A.; Ni, Z.; Hessler, N.; Wesarg, F.; Müller, F.A.; Kralisch, D.; Fischer, D. The Biopolymer Bacterial Nanocellulose as Drug Delivery System: Investigation of Drug Loading and Release using the Model Protein Albumin. J. Pharm. Sci. 2013, 102, 579–592. [Google Scholar] [CrossRef]
- Conzatti, G.; Faucon, D.; Castel, M.; Ayadi, F.; Cavalie, S.; Tourrette, A. Alginate/chitosan polyelectrolyte complexes: A comparative study of the influence of the drying step on physicochemical properties. Carbohydr. Polym. 2017, 172, 142–151. [Google Scholar] [CrossRef]
- Noyes, A.A.; Whitney, W.R. The Rate of Solution of Solid Substances in Their Own Solutions. J. Am. Chem. Soc. 1897, 19, 930–934. [Google Scholar] [CrossRef]
- Zhang, B.; Teoh, X.Y.; Yan, J.; Gleadall, A.; Belton, P.; Bibb, R.; Qi, S. Development of combi-pills using the coupling of semi-solid syringe extrusion 3D printing with fused deposition modelling. Int. J. Pharm. 2022, 625, 122140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Nasereddin, J.; McDonagh, T.; von Zeppelin, D.; Gleadall, A.; Alqahtani, F.; Bibb, R.; Belton, P.; Qi, S. Effects of porosity on drug release kinetics of swellable and erodible porous pharmaceutical solid dosage forms fabricated by hot melt droplet deposition 3D printing. Int. J. Pharm. 2021, 604, 120626. [Google Scholar] [CrossRef] [PubMed]


| Sample | PEGDA/g | H2O/g | TPO/g | SA/g | CMCS/g | BSA/g | SA/CMCS |
|---|---|---|---|---|---|---|---|
| P1 | 0(0%) | 20 | 0.1 | 1 | 0.5 | 2 | 2 |
| P2 | 1(5%) | 19 | 0.1 | 1 | 0.5 | 2 | 2 |
| P3 | 2(10%) | 18 | 0.1 | 1 | 0.5 | 2 | 2 |
| P4 | 4(20%) | 16 | 0.1 | 1 | 0.5 | 2 | 2 |
| P5 | 6(30%) | 14 | 0.1 | 1 | 0.5 | 2 | 2 |
| W1 | 0 | 20 | 0 | 1 | 0 | 2 | - |
| W2 | 2 | 18 | 0 | 1 | 0 | 2 | - |
| W3 | 0 | 20 | 0 | 1 | 0.5 | 2 | 2 |
| W4 | 2 | 18 | 0.1 | 1 | 0.5 | 2 | 2 |
| B1 | 1 | 19 | 0.1 | 1 | 1 | 2 | 1 |
| B2 | 1 | 19 | 0.1 | 1 | 0.5 | 2 | 2 |
| B3 | 1 | 19 | 0.1 | 1 | 0.33 | 2 | 3 |
| B4 | 1 | 19 | 0.1 | 1 | 0 | 2 | - |
| B5 | 1 | 19 | 0.1 | 0 | 1 | 2 | 0 |
| B6 | 1 | 19 | 0.1 | 0.25 | 1 | 2 | 0.25 |
| B7 | 1 | 19 | 0.1 | 0.33 | 1 | 2 | 0.33 |
| B8 | 1 | 19 | 0.1 | 0.5 | 1 | 2 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Li, L.; Zhu, X.; Chen, F.; Han, X. Development of pH-Responsive Polypills via Semi-Solid Extrusion 3D Printing. Bioengineering 2023, 10, 402. https://doi.org/10.3390/bioengineering10040402
Wang F, Li L, Zhu X, Chen F, Han X. Development of pH-Responsive Polypills via Semi-Solid Extrusion 3D Printing. Bioengineering. 2023; 10(4):402. https://doi.org/10.3390/bioengineering10040402
Chicago/Turabian StyleWang, Fan, Ling Li, Xiaolong Zhu, Feng Chen, and Xiaoxiao Han. 2023. "Development of pH-Responsive Polypills via Semi-Solid Extrusion 3D Printing" Bioengineering 10, no. 4: 402. https://doi.org/10.3390/bioengineering10040402
APA StyleWang, F., Li, L., Zhu, X., Chen, F., & Han, X. (2023). Development of pH-Responsive Polypills via Semi-Solid Extrusion 3D Printing. Bioengineering, 10(4), 402. https://doi.org/10.3390/bioengineering10040402