K2S Challenge: From Undersampled K-Space to Automatic Segmentation
Abstract
1. Introduction
- Reframing image reconstruction and annotation as end-to-end tasks for an eventual clinical workflow rather than sequential steps.
- Curating a large dataset (n = 300) with 3D raw k-space data and tissue segmentations to allow training of segmentation algorithms directly from undersampled k-space, and whatever additional research objectives may emerge from having raw k-space and segmentations in the same dataset.
- Investigating whether strong image reconstructions are a prerequisite for strong tissue segmentations.
- Assessing if segmentation algorithms trained from 8× undersampled data are suitable for biomarker analysis.
2. Materials and Methods
2.1. Challenge
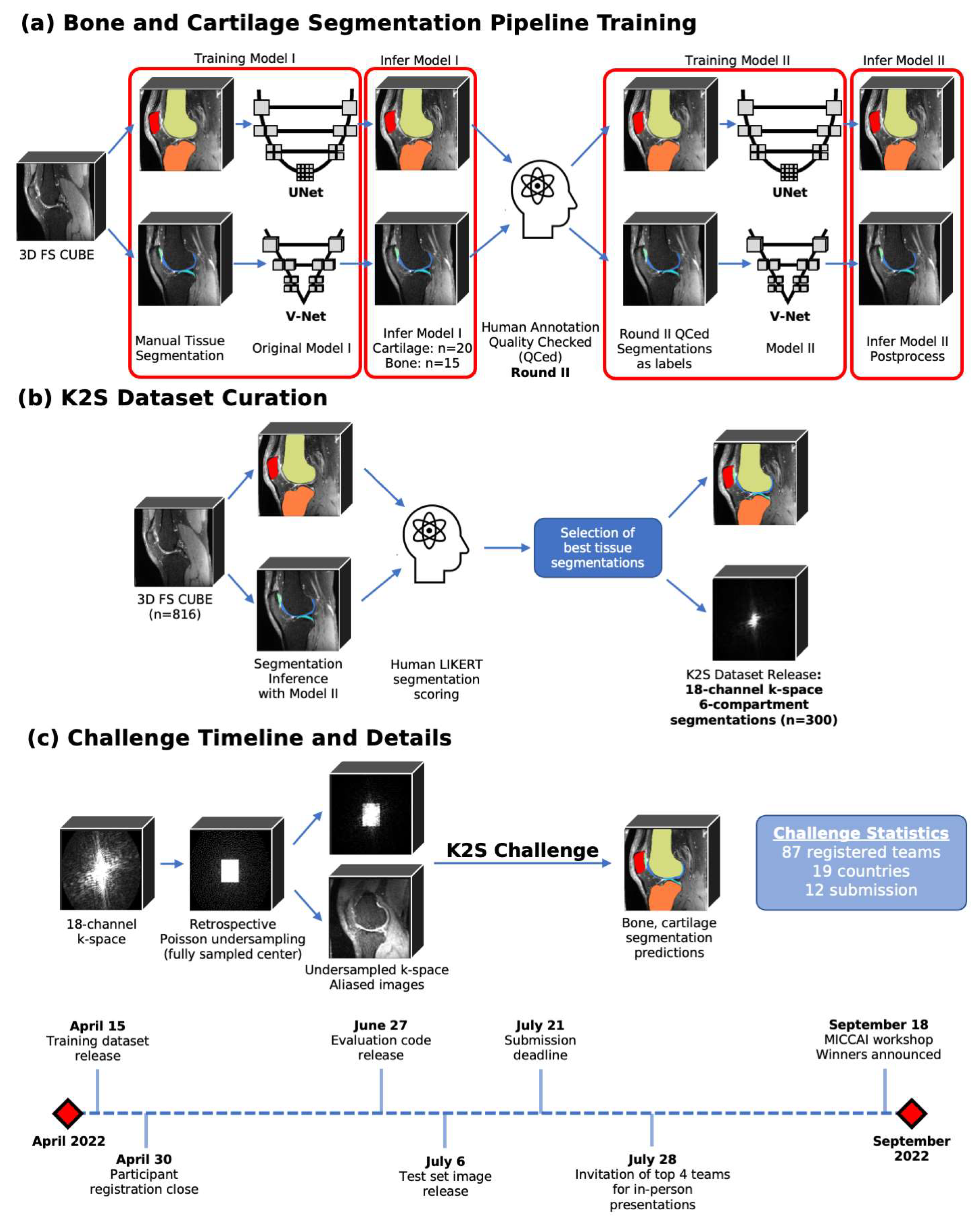
2.2. Dataset
2.2.1. Subject Eligibility and Sequence Information
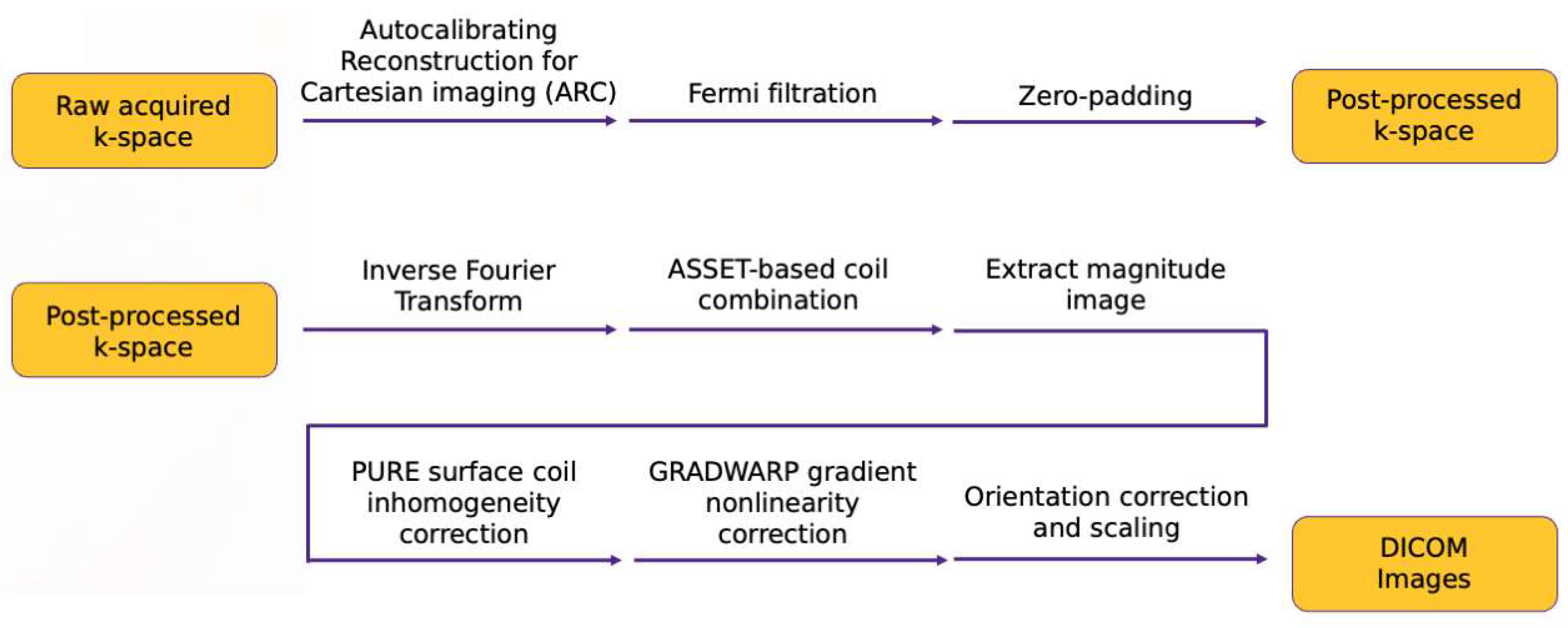
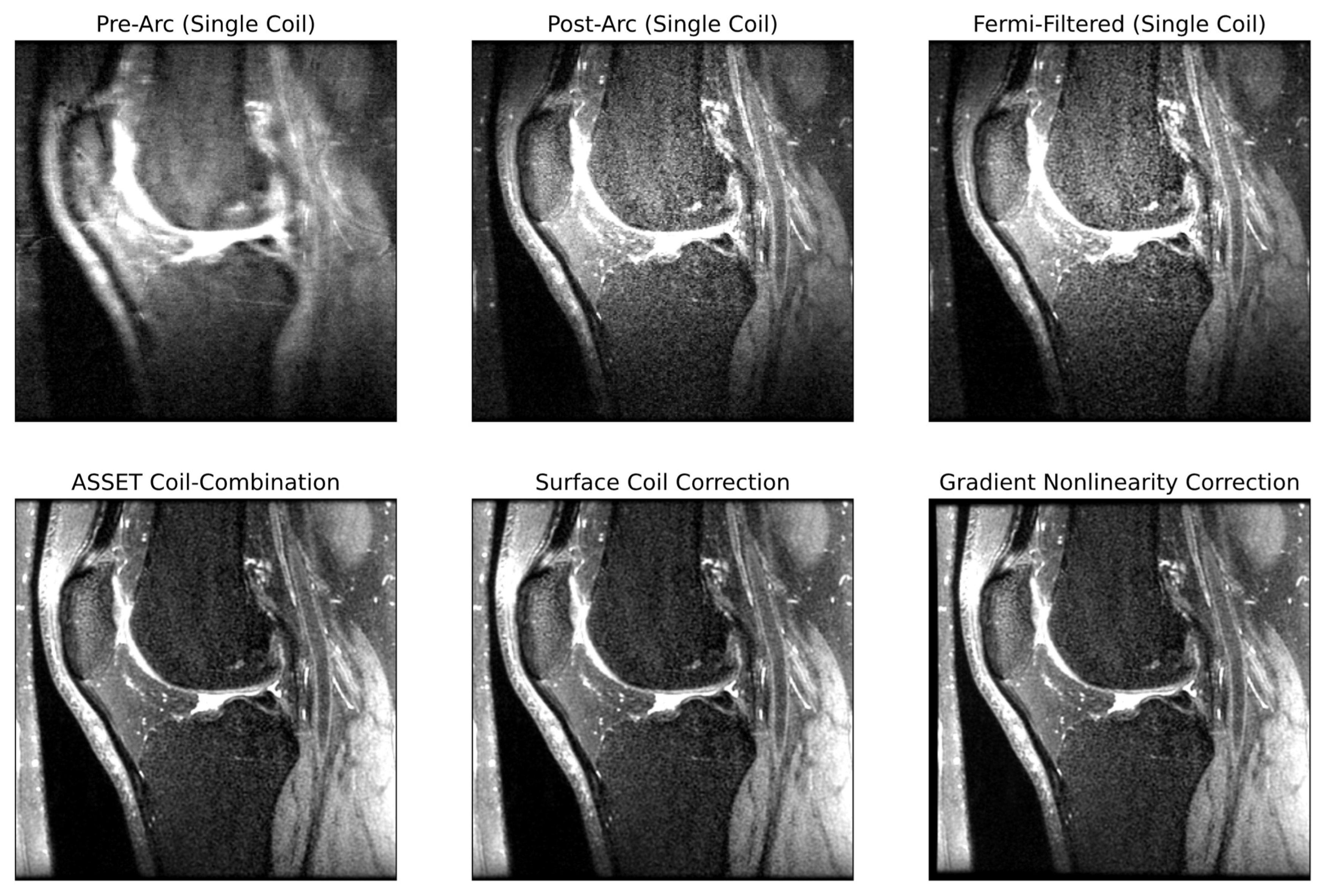
2.2.2. Extraction of ARC-Reconstructed Multicoil Raw k-Space Data
k-Space Post-Processing
Image Space Post-Processing
2.2.3. Ground Truth Segmentation Generation
Cartilage Segmentation Pipeline
Bone Segmentation Pipeline
2.2.4. Selection of Cases for K2S Dataset
2.2.5. Final K2S Dataset Characteristics
2.3. Evaluation Process
2.4. Timeline
- 15 April 2022: Training dataset release
- 30 April 2022: Participant registration close
- 27 June 2022: Release of code used to evaluate submissions
- 6 July 2022: Test dataset release
- 21 July 2022: Submission deadline
- 28 July 2022: Invitation of top 4 teams for in-person presentations
- 18 September 2022: In-person workshop at MICCAI 2022, winners announced
2.5. Overview of Top Submission Methodologies
2.5.1. K-nirsh (University of Tübingen, Tübingen, Germany)
2.5.2. UglyBarnacle (Skolkovo Institute of Science and Technology, Moscow, Russia)
2.5.3. FastMRI-AI (University Medical Center Groningen, Groningen, The Netherlands)
2.5.4. NYU-Knee AI (New York University Grossman School of Medicine, New York, USA)
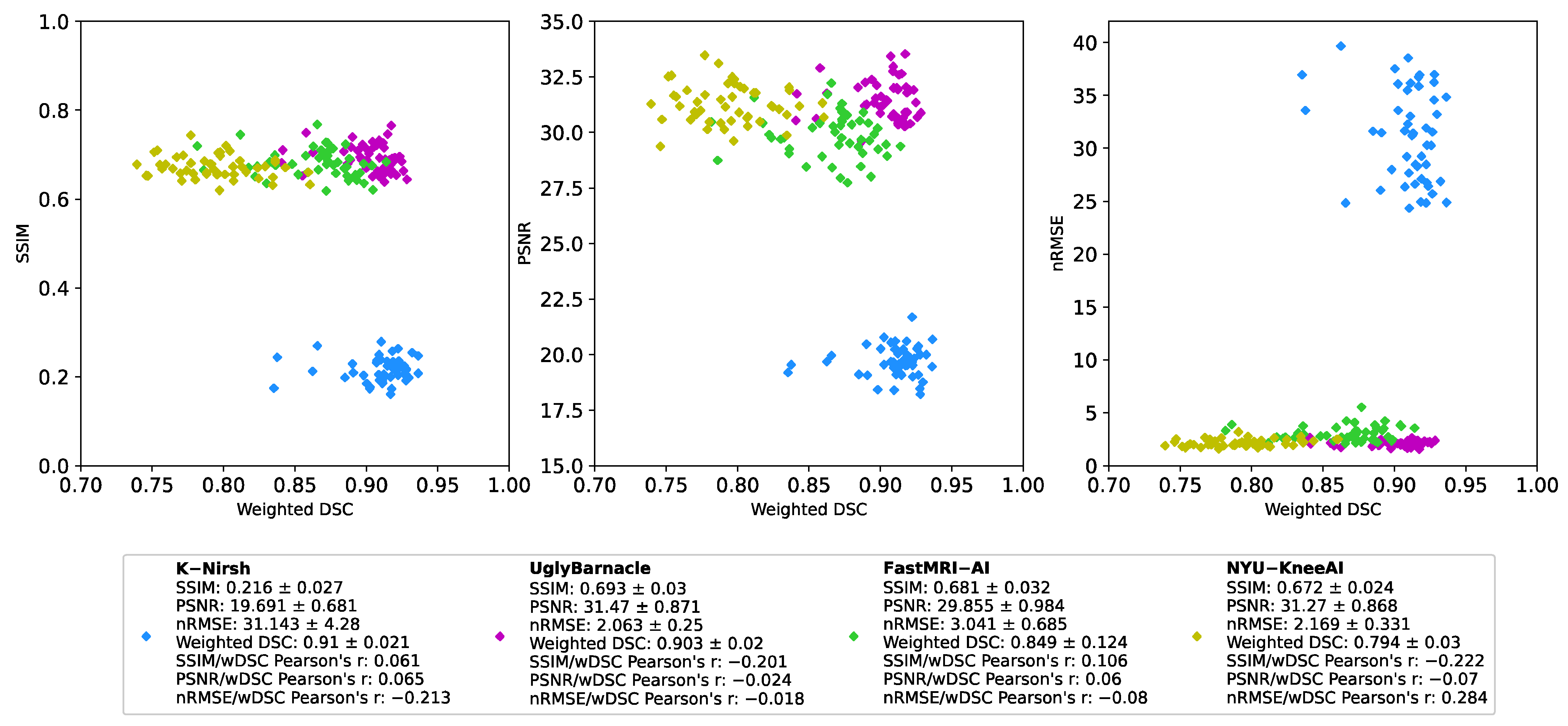
2.6. Further Analysis of Submissions
2.6.1. Intermediate Pipeline Reconstruction Performance
2.6.2. Comparison of Reconstruction and Segmentation Performance
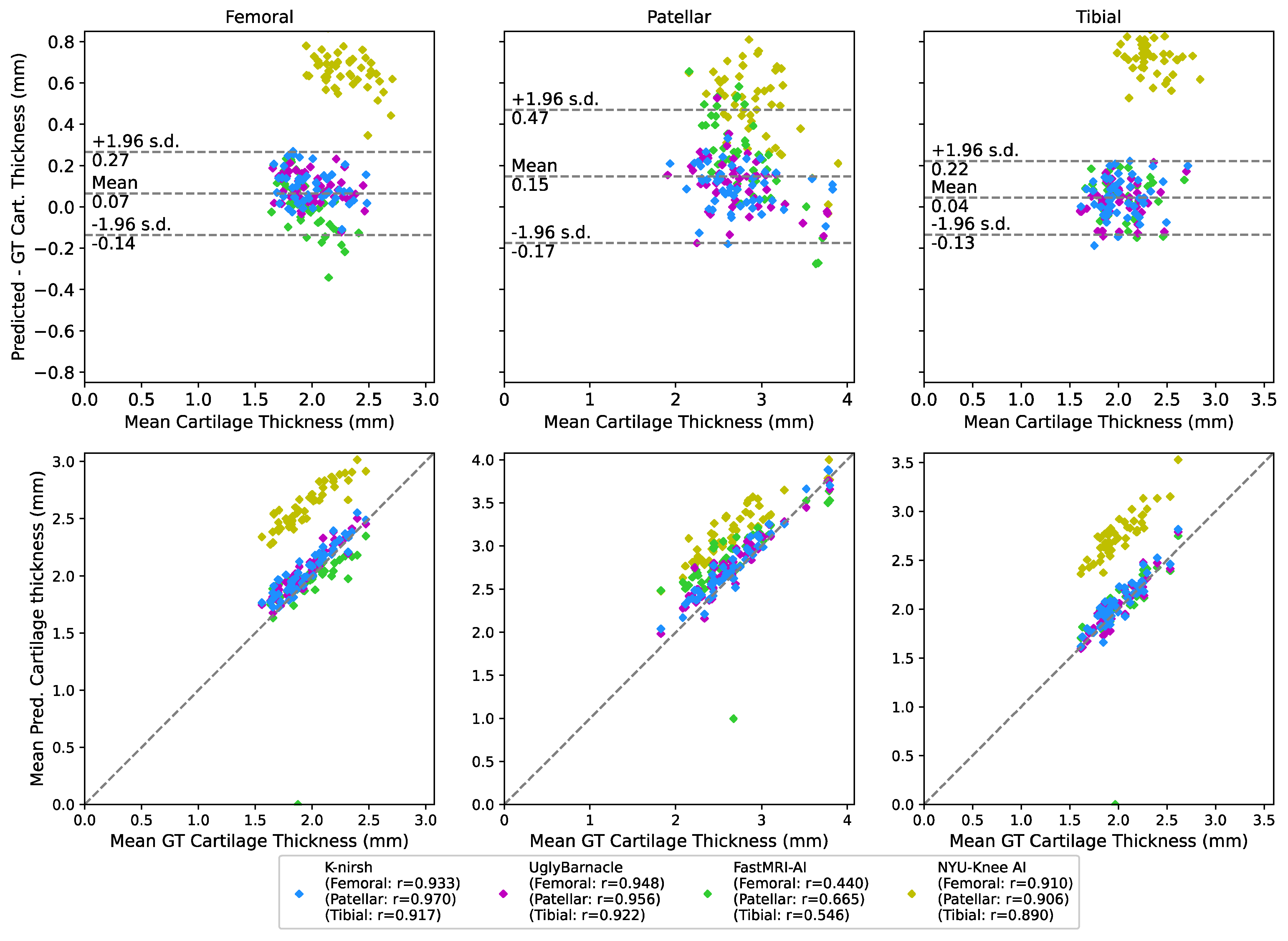
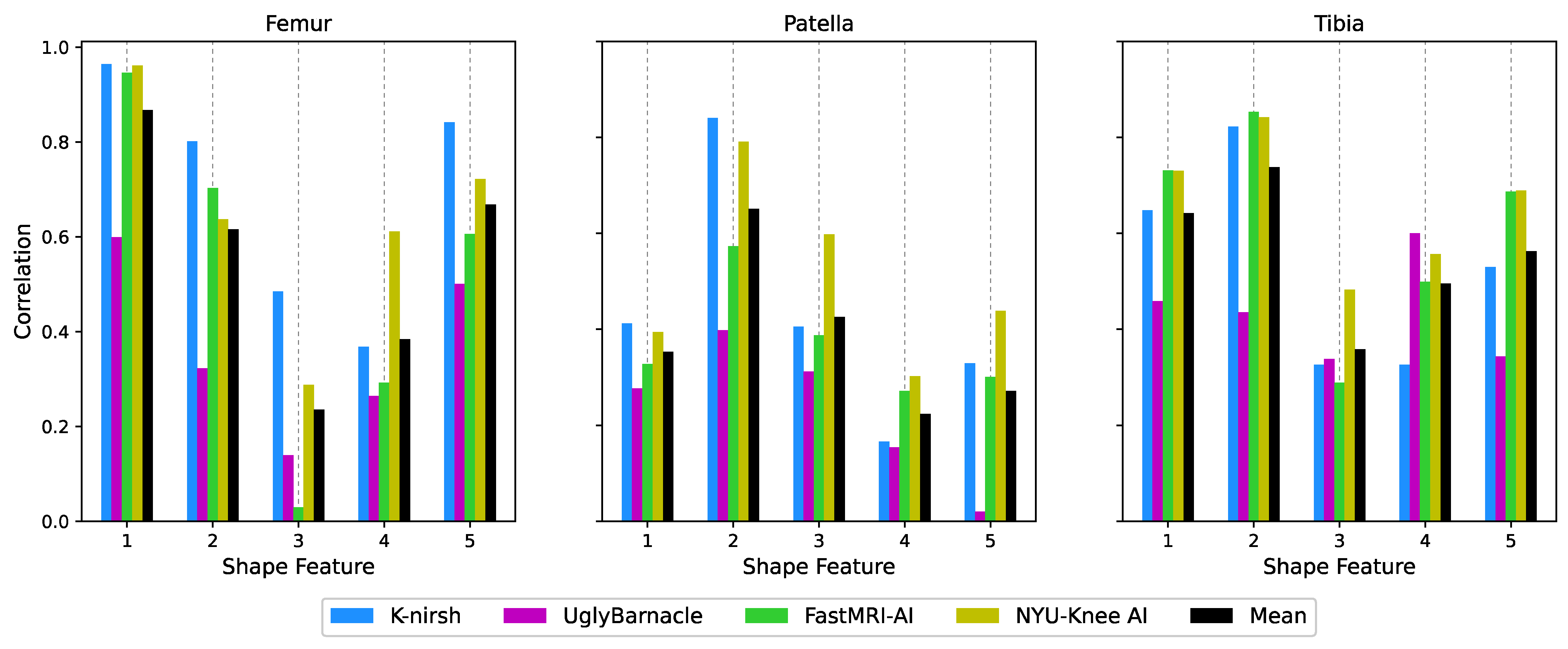
2.6.3. Biomarker Analysis: Cartilage Thickness
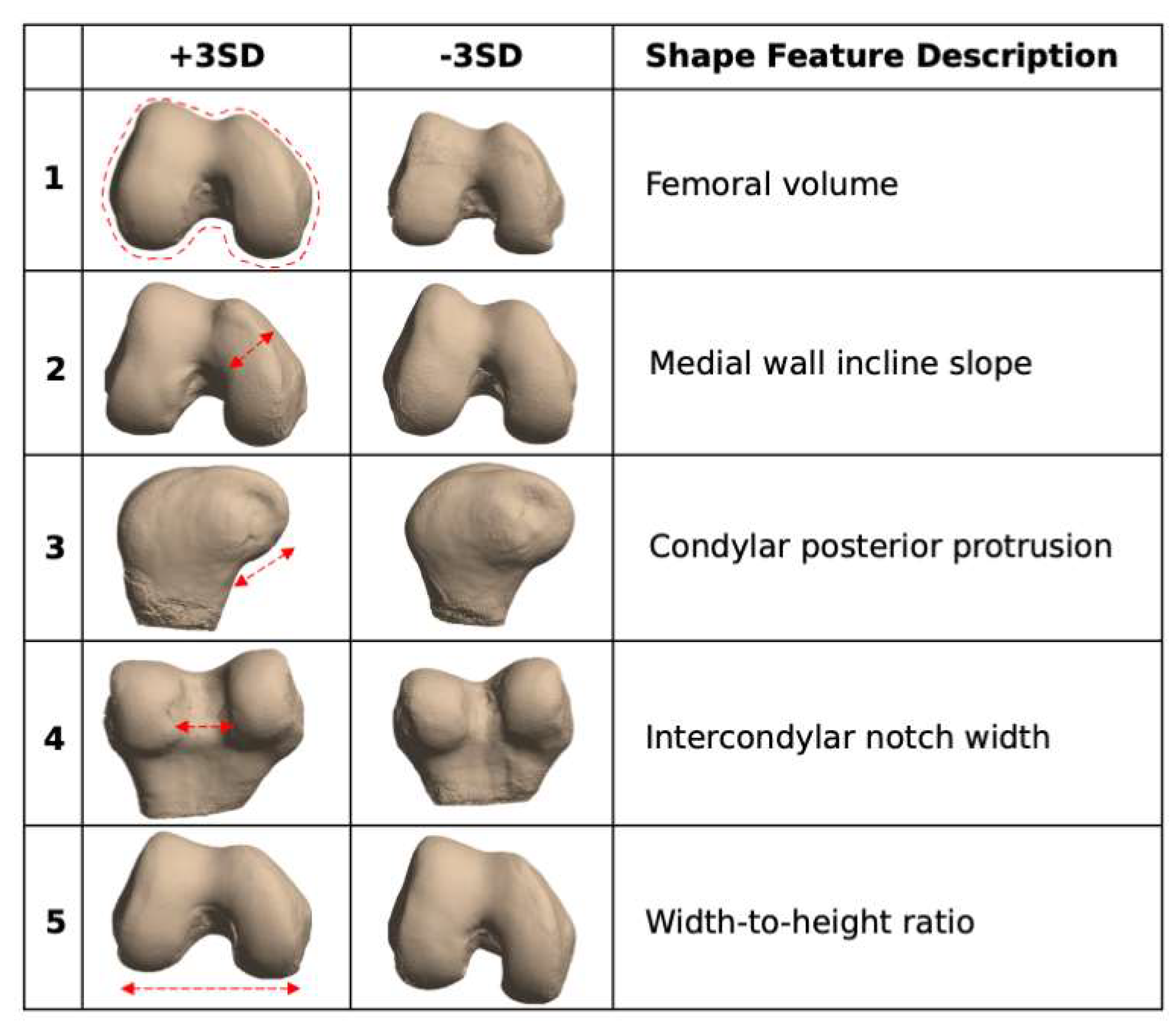
2.6.4. Biomarker Analysis: Bone Shape
3. Results
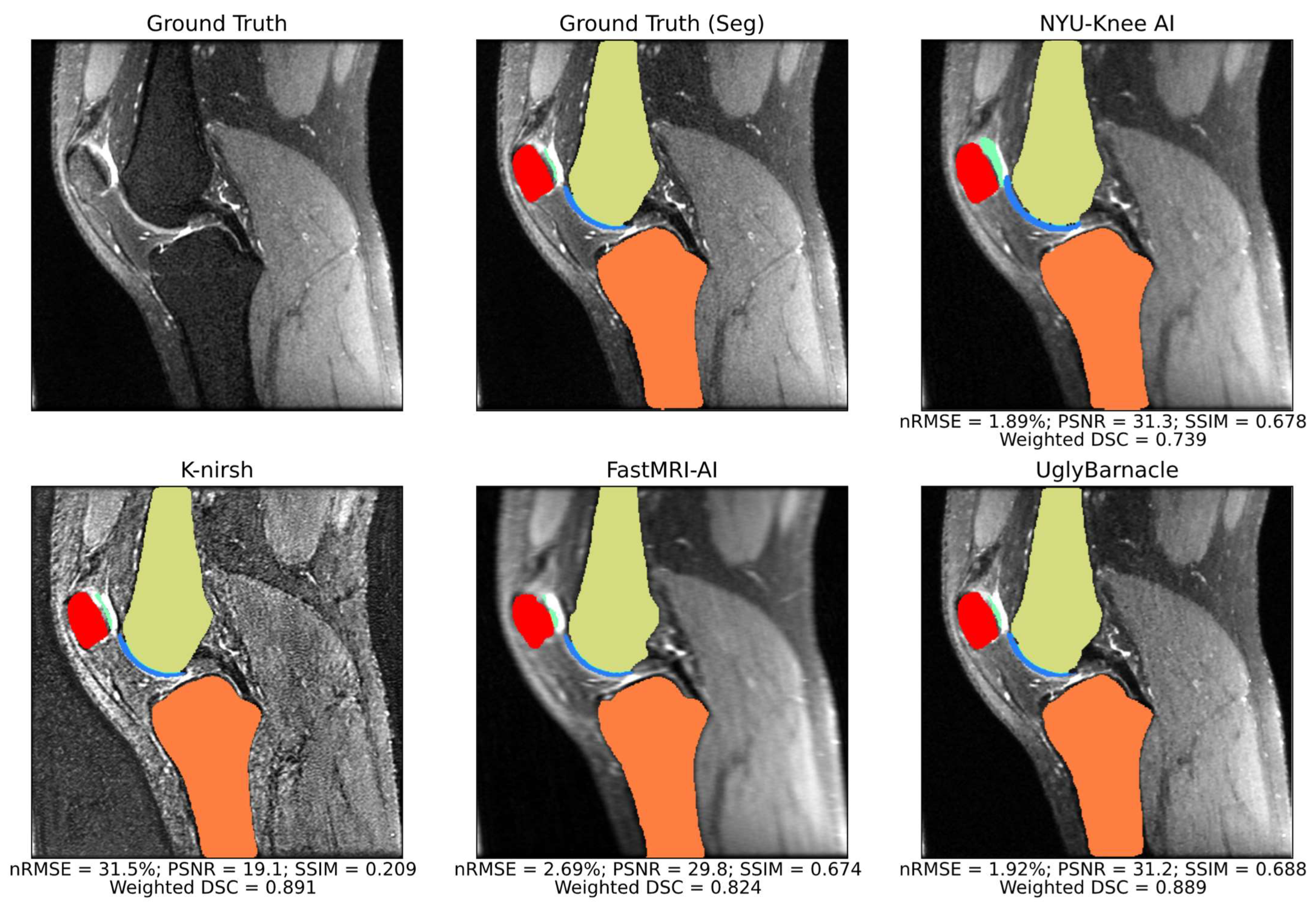
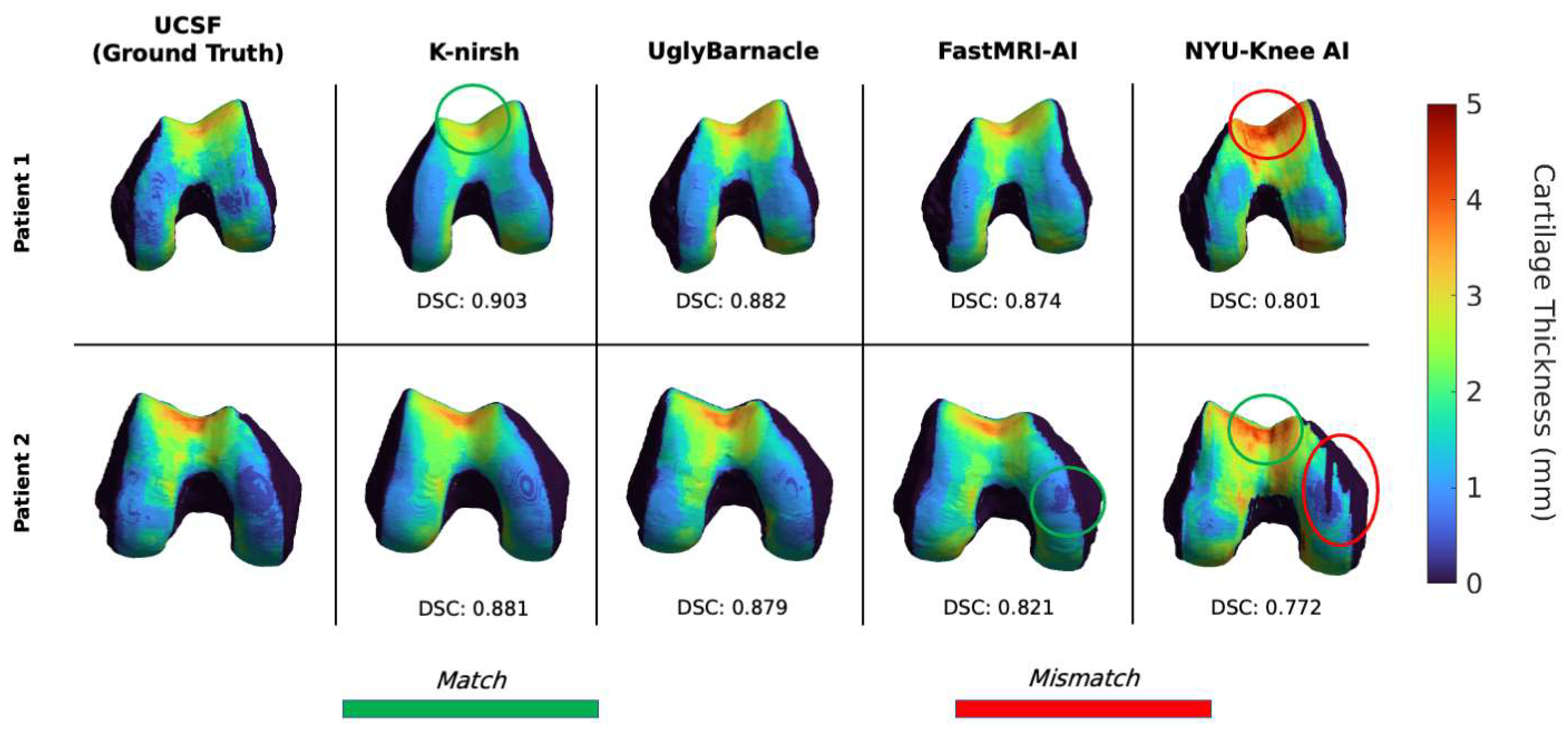
3.1. Segmentation Metrics
3.2. Reconstruction Metrics
3.3. Comparison of Reconstruction and Segmentation Performance
3.4. Biomarker Analysis: Cartilage Thickness
3.5. Biomarker Analysis: Bone Shape
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dean Deyle, G. The Role of MRI in Musculoskeletal Practice: A Clinical Perspective. J. Man. Manip. Ther. 2011, 19, 152–161. [Google Scholar] [CrossRef] [PubMed]
- del Grande, F.; Guggenberger, R.; Fritz, J. Rapid Musculoskeletal MRI in 2021: Value and Optimized Use of Widely Accessible Techniques. AJR 2021, 216, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Delfaut, E.M.; Beltran, J.; Johnson, G.; Rousseau, J.; Marchandise, X.; Cotten, A. Fat Suppression in MR Imaging: Techniques and Pitfalls. RadioGraphics 1999, 19, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Bley, T.A.; Wieben, O.; François, C.J.; Brittain, J.H.; Reeder, S.B. Fat and Water Magnetic Resonance Imaging. J. Magn. Res. Imaging 2010, 31, 4–18. [Google Scholar] [CrossRef]
- Aydıngöz, Ü.; Yıldız, A.E.; Ergen, F.B. Zero Echo Time Musculoskeletal MRI: Technique, Optimization, Applications, and Pitfalls. RadioGraphics 2022, 42, 1398–1414. [Google Scholar] [CrossRef]
- Larson, P.E.Z.; Han, M.; Krug, R.; Jakary, A.; Nelson, S.J.; Vigneron, D.B.; Henry, R.G.; McKinnon, G.; Kelley, D.A.C. Ultrashort Echo Time and Zero Echo Time MRI at 7T. Magma 2016, 29, 359–370. [Google Scholar] [CrossRef]
- Afsahi, A.M.; Ma, Y.; Jang, H.; Jerban, S.; Chung, C.B.; Chang, E.Y.; Du, J. Ultrashort Echo Time Magnetic Resonance Imaging Techniques: Met and Unmet Needs in Musculoskeletal Imaging. J. Magn. Reson. Imaging 2022, 55, 1597–1612. [Google Scholar] [CrossRef]
- Chang, E.Y.; Du, J.; Chung, C.B. UTE Imaging in the Musculoskeletal System. J. Magn. Reson. Imaging 2015, 41, 870. [Google Scholar] [CrossRef]
- Yoon, J.H.; Nickel, M.D.; Peeters, J.M.; Lee, J.M. Rapid Imaging: Recent Advances in Abdominal MRI for Reducing Acquisition Time and Its Clinical Applications. KJR 2019, 20, 1597–1615. [Google Scholar] [CrossRef]
- Esteban, O.; Blair, R.W.; Nielson, D.M.; Varada, J.C.; Marrett, S.; Thomas, A.G.; Poldrack, R.A.; Gorgolewski, K.J. Crowdsourced MRI Quality Metrics and Expert Quality Annotations for Training of Humans and Machines. Sci Data 2019, 6, 30. [Google Scholar] [CrossRef]
- Lustig, M.; Donoho, D.; Pauly, J.M. Sparse MRI: The Application of Compressed Sensing for Rapid MR Imaging. Magn. Reson. Med. 2007, 58, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Iuga, A.I.; Abdullayev, N.; Weiss, K.; Haneder, S.; Brüggemann-Bratke, L.; Maintz, D.; Rau, R.; Bratke, G. Accelerated MRI of the Knee. Quality and Efficiency of Compressed Sensing. Eur. J. Radiol. 2020, 132, 109273. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.C. Compressed Sensing MRI: A Review from Signal Processing Perspective. BMC Biomed. Eng. 2019, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Anderson, M.; Tamir, J.I.; Lustig, M.; Li, K. Compressed Sensing MRI Reconstruction on Intel HARPv2. In Proceedings of the 27th IEEE International Symposium on Field-Programmable Custom Computing Machines, San Diego, CA, USA, 28 April –1 May 2019; pp. 254–257. [Google Scholar] [CrossRef]
- Glockner, J.F.; Hu, H.H.; Stanley, D.W.; Angelos, L.; King, K. Parallel MR Imaging: A User’s Guide. RadioGraphics 2005, 25, 1279–1297. [Google Scholar] [CrossRef] [PubMed]
- Pruessmann, K.P.; Weiger, M.; Scheidegger, M.B.; Boesiger, P. SENSE: Sensitivity Encoding for Fast MRI. Magn. Reson. Med. 1999, 42, 952–962. [Google Scholar] [CrossRef]
- Griswold, M.A.; Jakob, P.M.; Heidemann, R.M.; Nittka, M.; Jellus, V.; Wang, J.; Kiefer, B.; Haase, A. Generalized Autocalibrating Partially Parallel Acquisitions (GRAPPA). Magn. Res. Med. 2002, 47, 1202–1210. [Google Scholar] [CrossRef]
- Liu, F.; Feng, L.; Kijowski, R. MANTIS: Model-Augmented Neural NeTwork with Incoherent k-Space Sampling for Efficient MR Parameter Mapping. Magn. Reson. Med. 2019, 82, 174–188. [Google Scholar] [CrossRef]
- Shimron, E.; de Goyeneche, A.; Wang, K.; Halgren, A.; Syed, A.B.; Vasanawala, S.; Lustig, M. BladeNet: Rapid PROPELLER Acquisition and Reconstruction for High Spatio-Temporal Resolution Abdominal MRI. In Proceedings of the 31st Annual International Society for Magnetic Resonance in Medicine, London, UK, 7–12 May 2022; p. 684. [Google Scholar]
- Tippareddy, C.; Zhao, W.; Sunshine, J.L.; Griswold, M.; Ma, D.; Badve, C. Magnetic Resonance Fingerprinting: An Overview. EJNMMI 2021, 48, 4189–4200. [Google Scholar] [CrossRef]
- Boyacioglu, R.; Wang, C.; Ma, D.; McGivney, D.F.; Yu, X.; Griswold, M.A. 3D Magnetic Resonance Fingerprinting with Quadratic RF Phase. Magn. Reson. Med. 2021, 85, 2084–2094. [Google Scholar] [CrossRef]
- Zibetti, M.V.W.; Sharafi, A.; Otazo, R.; Regatte, R.R. Accelerating 3D-T1ρ Mapping of Cartilage Using Compressed Sensing with Different Sparse and Low Rank Models. Magn. Reson. Med. 2018, 80, 1475–1491. [Google Scholar] [CrossRef]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. LNCS 2015, 9351, 234–241. [Google Scholar] [CrossRef]
- Baccouche, A.; Garcia-Zapirain, B.; Castillo Olea, C.; Elmaghraby, A.S. Connected-UNets: A Deep Learning Architecture for Breast Mass Segmentation. NPJ Breast Cancer 2021, 7, 151. [Google Scholar] [CrossRef] [PubMed]
- Hess, M.; Allaire, B.; Gao, K.T.; Tibrewala, R.; Inamdar, G.; Bharadwaj, U.; Chin, C.; Pedoia, V.; Bouxsein, M.; Anderson, D.; et al. Deep Learning for Multi-Tissue Segmentation and Fully Automatic Personalized Biomechanical Models from BACPAC Clinical Lumbar Spine MRI. Pain Med. 2022; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Z.; Shi, H.; Deng, Y.; Zhou, G.; Tang, S. A Deep Learning-Based Method for Knee Articular Cartilage Segmentation in MRI Images. In Proceedings of the 10th International Conference on Control, Automation and Information Sciences ICCAIS, Xi′an, China, 14–17 October 2021; pp. 690–694. [Google Scholar] [CrossRef]
- Iriondo, C.; Liu, F.; Calivà, F.; Kamat, S.; Majumdar, S.; Pedoia, V. Towards Understanding Mechanistic Subgroups of Osteoarthritis: 8-Year Cartilage Thickness Trajectory Analysis. J. Orthop. Res. 2021, 39, 1305–1317. [Google Scholar] [CrossRef]
- Namiri, N.K.; Flament, I.; Astuto, B.; Shah, R.; Tibrewala, R.; Caliva, F.; Link, T.M.; Pedoia, V.; Majumdar, S. Deep Learning for Hierarchical Severity Staging of Anterior Cruciate Ligament Injuries from Mri. Radiol. Artif. Intell. 2020, 2, e190207. [Google Scholar] [CrossRef]
- Jamaludin, A.; Kadir, T.; Zisserman, A. SpineNet: Automatically Pinpointing Classification Evidence in Spinal MRIs. Med. Image Anal. 2017, 41, 63–73. [Google Scholar] [CrossRef]
- Gao, K.T.; Pedoia, V.; Young, K.A.; Kogan, F.; Koff, M.F.; Gold, G.E.; Potter, H.G.; Majumdar, S. Multiparametric MRI Characterization of Knee Articular Cartilage and Subchondral Bone Shape in Collegiate Basketball Players. J. Orthop. Res. 2021, 39, 1512–1522. [Google Scholar] [CrossRef]
- Calivá, F.; Leynes, A.P.; Shah, R.; Bharadwaj, U.U.; Majumdar, S.; Larson, P.E.Z.; Pedoia, V. Breaking Speed Limits with Simultaneous Ultra-Fast MRI Reconstruction and Tissue Segmentation. Proc. Mach. Learn. Res. 2020, 121, 94–110. [Google Scholar]
- Fienup, J.R. Invariant Error Metrics for Image Reconstruction. Appl. Opt. 1997, 36, 8352–8357. [Google Scholar] [CrossRef]
- Horé, A.; Ziou, D. Is There a Relationship between Peak-Signal-to-Noise Ratio and Structural Similarity Index Measure? IET Image Process. 2013, 7, 12–24. [Google Scholar] [CrossRef]
- Dosselmann, R.; Yang, X.D. A Comprehensive Assessment of the Structural Similarity Index. Signal Image Video Process. 2009, 5, 81–91. [Google Scholar] [CrossRef]
- Peterfy, C.G.; Schneider, E.; Nevitt, M. The Osteoarthritis Initiative: Report on the Design Rationale for the Magnetic Resonance Imaging Protocol for the Knee. Osteoarthr. Cartil. 2008, 16, 1433–1441. [Google Scholar] [CrossRef]
- Segal, N.A.; Nevitt, M.C.; Gross, K.D.; Hietpas, J.; Glass, N.A.; Lewis, C.E.; Torner, J.C. The Multicenter Osteoarthritis Study: Opportunities for Rehabilitation Research. PM&R 2013, 5, 647–654. [Google Scholar] [CrossRef]
- Schiratti, J.B.; Dubois, R.; Herent, P.; Cahané, D.; Dachary, J.; Clozel, T.; Wainrib, G.; Keime-Guibert, F.; Lalande, A.; Pueyo, M.; et al. A Deep Learning Method for Predicting Knee Osteoarthritis Radiographic Progression from MRI. Arthritis Res. Ther. 2021, 23, 262. [Google Scholar] [CrossRef] [PubMed]
- Tolpadi, A.A.; Lee, J.J.; Pedoia, V.; Majumdar, S. Deep Learning Predicts Total Knee Replacement from Magnetic Resonance Images. Sci. Rep. 2020, 10, 6371. [Google Scholar] [CrossRef]
- Morales, A.G.; Lee, J.J.; Caliva, F.; Iriondo, C.; Liu, F.; Majumdar, S.; Pedoia, V. Uncovering Associations between Data-Driven Learned QMRI Biomarkers and Chronic Pain. Sci. Rep. 2021, 11, 21989. [Google Scholar] [CrossRef]
- Muckley, M.J.; Riemenschneider, B.; Radmanesh, A.; Kim Member, S.; Jeong, G.; Ko, J.; Jun, Y.; Shin, H.; Hwang, D.; Mostapha, M.; et al. Results of the 2020 FastMRI Challenge for Machine Learning MR Image Reconstruction HHS Public Access. IEEE Trans. Med. Imaging 2021, 40, 2306–2317. [Google Scholar] [CrossRef]
- Ramzi, Z.; Starck, J.L.; Ciuciu, P. Density Compensated Unrolled Networks for Non-Cartesian MRI Reconstruction. In Proceedings of the International Symposium on Biomedical Imaging, Nice, France, 13–16 April 2021; pp. 1443–1447. [Google Scholar] [CrossRef]
- Fabian, Z.; Soltanolkotabi, M. HUMUS-Net: Hybrid Unrolled Multi-Scale Network Architecture for Accelerated MRI Reconstruction. ArXiv 2022, arXiv:2203.08213. [Google Scholar] [CrossRef]
- Shimron, E.; Tamir, J.I.; Wang, K.; Lustig, M. Implicit Data Crimes: Machine Learning Bias Arising from Misuse of Public Data. Proc. Natl. Acad. Sci. USA 2022, 119, e2117203119. [Google Scholar] [CrossRef]
- Eelbode, T.; Bertels, J.; Berman, M.; Vandermeulen, D.; Maes, F.; Bisschops, R.; Blaschko, M.B. Optimization for Medical Image Segmentation: Theory and Practice When Evaluating With Dice Score or Jaccard Index. IEEE Trans. Med. Imaging 2020, 39, 3679–3690. [Google Scholar] [CrossRef]
- Brau, A.C.S.; Beatty, P.J.; Skare, S.; Bammer, R. Comparison of Reconstruction Accuracy and Efficiency among Autocalibrating Data-Driven Parallel Imaging Methods. Magn. Res. Med. 2008, 59, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Hsu, E.W. Noise Removal in Magnetic Resonance Diffusion Tensor Imaging. Magn. Res. Med. 2005, 54, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Noël, P.; Bammer, R.; Reinhold, C.; Haider, M.A. Parallel Imaging Artifacts in Body Magnetic Resonance Imaging. Can. Assoc. Radiol. J. 2009, 60, 91. [Google Scholar] [CrossRef] [PubMed]
- Liney, G.P.; Owen, S.C.; Beaumont, A.K.E.; Lazar, V.R.; Manton, D.J.; Beavis, A.W. Commissioning of a New Wide-Bore MRI Scanner for Radiotherapy Planning of Head and Neck Cancer. Brit. J. Radiol. 2013, 86, 20130150. [Google Scholar] [CrossRef]
- Thomas, M.S.; Greenwood, R.; Nolan, C.; Malcolm, P.N.; Toms, A.P. Optimizing MRI of Small Joints and Extremities. Clin. Radiol. 2014, 69, e414–e421. [Google Scholar] [CrossRef]
- Sekihara, K.; Kuroda, M.; Kohno, H. Image Restoration from Non-Uniform Magnetic Field Influence for Direct Fourier NMR Imaging. Phys. Med. Biol 1984, 29, 15–24. [Google Scholar] [CrossRef]
- Astuto, B.; Flament, I.; Namiri, N.K.; Shah, R.; Bharadwaj, U.; Link, T.M.; Bucknor, M.D.; Pedoia, V.; Majumdar, S. Automatic Deep Learning–Assisted Detection and Grading of Abnormalities in Knee MRI Studies. Radiol. Artif. Intell. 2021, 3, e200165. [Google Scholar] [CrossRef]
- Milletari, F.; Navab, N.; Ahmadi, S.A. V-Net: Fully Convolutional Neural Networks for Volumetric Medical Image Segmentation. In Proceedings of the 2016 4th International Conference on 3D Vision, Stanford, CA, USA, 25–28 October 2016; pp. 565–571. [Google Scholar] [CrossRef]
- Isensee, F.; Jaeger, P.F.; Kohl, S.A.A.; Petersen, J.; Maier-Hein, K.H. NnU-Net: A Self-Configuring Method for Deep Learning-Based Biomedical Image Segmentation. Nat. Methods 2020, 18, 203–211. [Google Scholar] [CrossRef]
- Saha, A.; Hosseinzadeh, M.; Huisman, H. End-to-End Prostate Cancer Detection in BpMRI via 3D CNNs: Effects of Attention Mechanisms, Clinical Priori and Decoupled False Positive Reduction. Med. Image Anal. 2021, 73, 102155. [Google Scholar] [CrossRef]
- Zibetti, M.V.W.; Johnson, P.M.; Sharafi, A.; Hammernik, K.; Knoll, F.; Regatte, R.R. Rapid Mono and Biexponential 3D-T1p Mapping of Knee Cartilage Using Variational Networks. Sci. Rep. 2020, 10, 19144. [Google Scholar] [CrossRef]
- Hammernik, K.; Klatzer, T.; Kobler, E.; Recht, M.P.; Sodickson, D.K.; Pock, T.; Knoll, F. Learning a Variational Network for Reconstruction of Accelerated MRI Data. Magn. Res. Med. 2017, 79, 3055–3071. [Google Scholar] [CrossRef] [PubMed]
- Zibetti, M.V.W.; Knoll, F.; Regatte, R.R. Alternating Learning Approach for Variational Networks and Undersampling Pattern in Parallel MRI Applications. IEEE Trans. Comput. Imaging 2022, 8, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Norman, B.; Pedoia, V.; Majumdar, S. Use of 2D U-Net Convolutional Neural Networks for Automated Cartilage and Meniscus Segmentation of Knee MR Imaging Data to Determine Relaxometry and Morphometry. Radiology 2018, 288, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimkhani, S.; Jaward, M.H.; Cicuttini, F.M.; Dharmaratne, A.; Wang, Y.; de Herrera, A.G.S. A Review on Segmentation of Knee Articular Cartilage: From Conventional Methods towards Deep Learning. Artif. Intell. Med. 2020, 106, 101851. [Google Scholar] [CrossRef] [PubMed]
- Uecker, M.; Lai, P.; Murphy, M.J.; Virtue, P.; Elad, M.; Pauly, J.M.; Vasanawala, S.S.; Lustig, M. ESPIRiT—An Eigenvalue Approach to Autocalibrating Parallel MRI: Where SENSE Meets GRAPPA. Magn. Res. Med. 2014, 71, 990. [Google Scholar] [CrossRef]
- Schober, P.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Bahadir, C.D.; Wang, A.Q.; Dalca, A.V.; Sabuncu, M.R. Deep-Learning-Based Optimization of the Under-Sampling Pattern in MRI. IEEE Trans Comput Imaging 2020, 6, 1139–1152. [Google Scholar] [CrossRef]
- Razumov, A.; Rogov, O.Y.; Dylov, D.V. Optimal MRI Undersampling Patterns for Pathology Localization. Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). In Proceedings of the Medical Image Computing and Computer Assisted Intervention–MICCAI, Singapore, 18–22 September 2022; 13436 LNCS. pp. 768–779. [Google Scholar] [CrossRef]



| MR Acquisition Information | ||
|---|---|---|
| Scanner: GE Discovery MR750 3T Scanner (GE Healthcare, Milwaukee, WI) Gradient System Max Strength: 50 mT/m Max Slew Rate: 200 mT/m/ms | ||
| Coil: 18-channel knee transmit/receive coil (Quality Electrodynamics (QED), Mayfield Village, OH) | ||
| TR/TE: 1002/29 ms | FOV: 150 mm | Slice Thickness: 0.6 mm (0.6 mm spacing between slices) |
| Flip Angle: 90 | SAR: 0.0939 | Echo Train Length: 36 |
| Frequency: 128 | Bandwidth: 244 | ARC [45]: 4 (R = 2 in ky, kz) |
| Acquisition Matrix: 256 × 256 × 200 | Image Dimensions: 512 × 512 × 200 Resolution: 0.586 mm × 0.586 mm × 0.6 mm Voxel Size: 0.293 mm × 0.293 mm × 0.6 mm | |
| Cartilage | Bone | Full | |||||
|---|---|---|---|---|---|---|---|
| Team | Femoral | Tibial | Patellar | Femur | Tibia | Patella | Weighted DSC |
| K-nirsh | 0.904 ± 0.014 | 0.899 ± 0.015 | 0.910 ± 0.034 | 0.989 ± 0.002 | 0.985 ± 0.004 | 0.966 ± 0.012 | 0.910 ± 0.021 |
| UglyBarnacle | 0.895 ± 0.016 | 0.890 ± 0.017 | 0.903 ± 0.032 | 0.984 ± 0.004 | 0.980 ± 0.004 | 0.961 ± 0.015 | 0.903 ± 0.021 |
| FastMRI-AI | 0.845 ± 0.124 | 0.862 ± 0.126 | 0.843 ± 0.124 | 0.964 ± 0.078 | 0.952 ± 0.138 | 0.834 ± 0.306 | 0.849 ± 0.123 |
| NYU-Knee AI | 0.798 ± 0.029 | 0.756 ± 0.04 | 0.796 ± 0.043 | 0.980 ± 0.004 | 0.975 ± 0.005 | 0.939 ± 0.014 | 0.795 ± 0.030 |
| Team | nRMSE | PSNR | SSIM |
|---|---|---|---|
| K-nirsh | 31.2 ± 4.26 | 19.7 ± 0.68 | 0.217 ± 0.059 |
| UglyBarnacle | 2.07 ± 0.25 | 31.5 ± 0.87 | 0.693 ± 0.043 |
| FastMRI-AI | 3.05 ± 0.68 | 29.8 ± 0.99 | 0.681 ± 0.061 |
| NYU-Knee AI | 2.18 ± 0.33 | 31.3 ± 0.87 | 0.672 ± 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolpadi, A.A.; Bharadwaj, U.; Gao, K.T.; Bhattacharjee, R.; Gassert, F.G.; Luitjens, J.; Giesler, P.; Morshuis, J.N.; Fischer, P.; Hein, M.; et al. K2S Challenge: From Undersampled K-Space to Automatic Segmentation. Bioengineering 2023, 10, 267. https://doi.org/10.3390/bioengineering10020267
Tolpadi AA, Bharadwaj U, Gao KT, Bhattacharjee R, Gassert FG, Luitjens J, Giesler P, Morshuis JN, Fischer P, Hein M, et al. K2S Challenge: From Undersampled K-Space to Automatic Segmentation. Bioengineering. 2023; 10(2):267. https://doi.org/10.3390/bioengineering10020267
Chicago/Turabian StyleTolpadi, Aniket A., Upasana Bharadwaj, Kenneth T. Gao, Rupsa Bhattacharjee, Felix G. Gassert, Johanna Luitjens, Paula Giesler, Jan Nikolas Morshuis, Paul Fischer, Matthias Hein, and et al. 2023. "K2S Challenge: From Undersampled K-Space to Automatic Segmentation" Bioengineering 10, no. 2: 267. https://doi.org/10.3390/bioengineering10020267
APA StyleTolpadi, A. A., Bharadwaj, U., Gao, K. T., Bhattacharjee, R., Gassert, F. G., Luitjens, J., Giesler, P., Morshuis, J. N., Fischer, P., Hein, M., Baumgartner, C. F., Razumov, A., Dylov, D., Lohuizen, Q. v., Fransen, S. J., Zhang, X., Tibrewala, R., de Moura, H. L., Liu, K., ... Pedoia, V. (2023). K2S Challenge: From Undersampled K-Space to Automatic Segmentation. Bioengineering, 10(2), 267. https://doi.org/10.3390/bioengineering10020267










