Vascularized Tissue Organoids
Abstract
1. Introduction
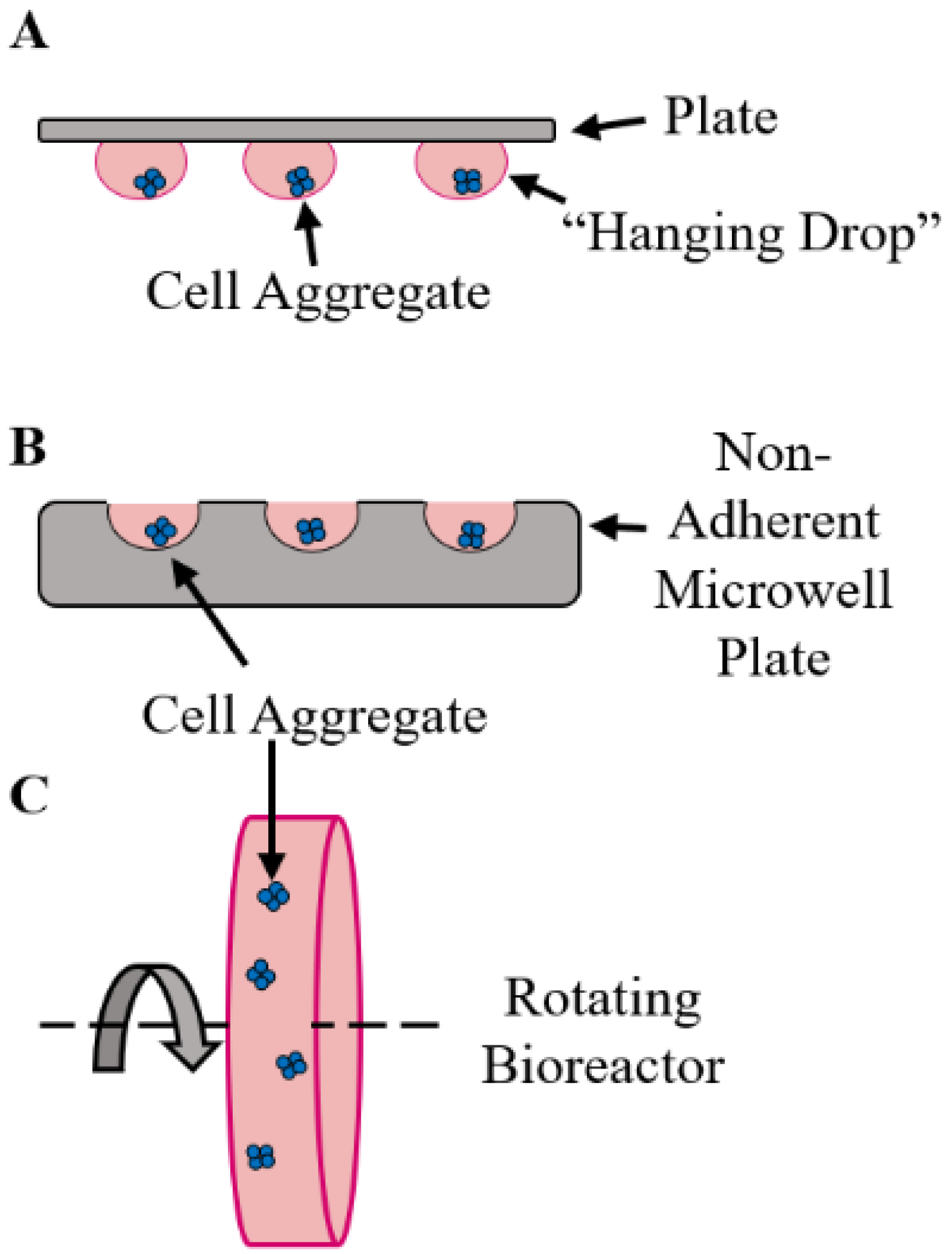
2. Organoid Fabrication Strategies
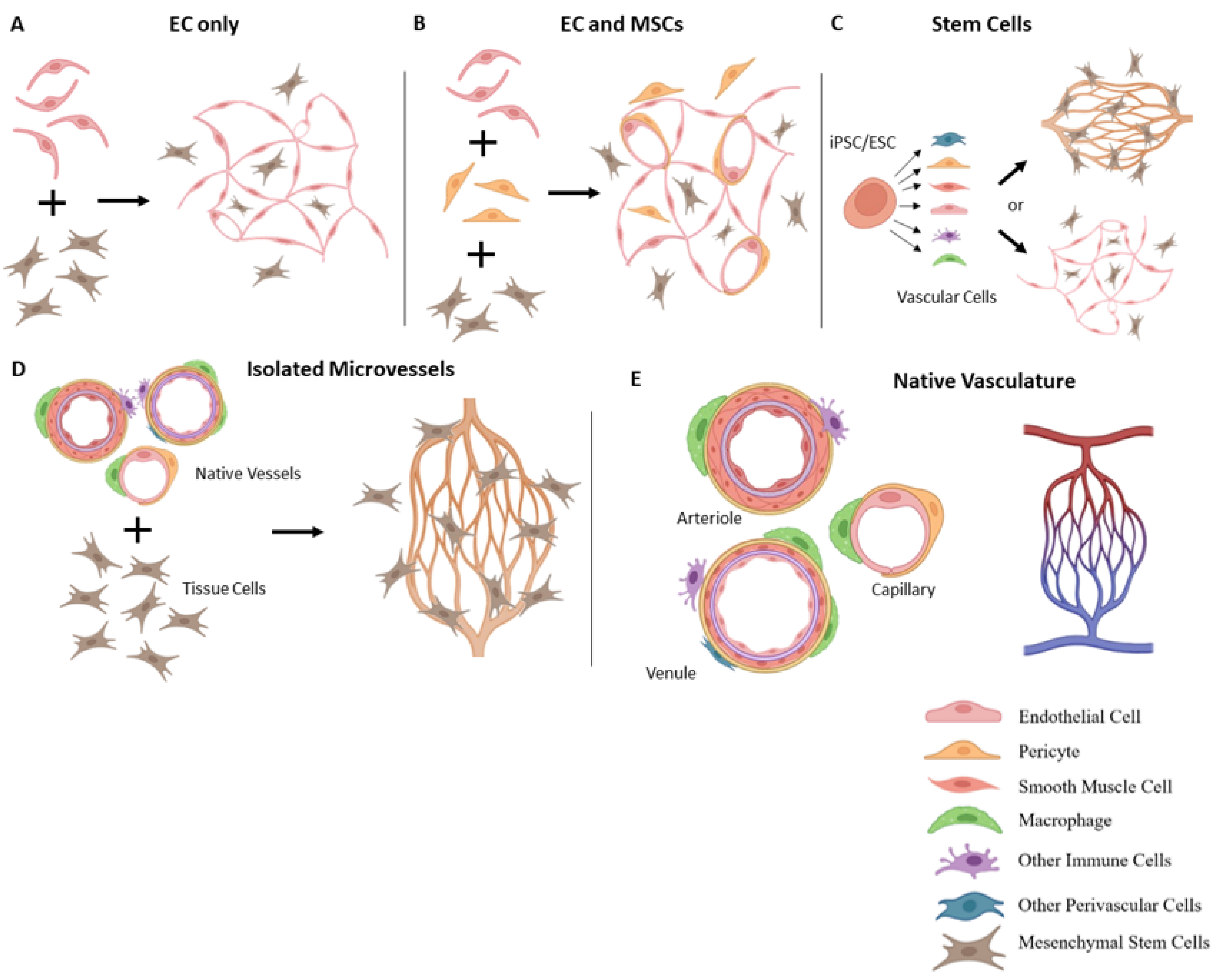
3. Vascularization Strategies
3.1. Vascularization with Endothelial Cells
3.2. Incorporation of Other Vascular Cells
3.3. Stem Cell-Based Vascularization Approaches
3.4. Staging Culture Conditions
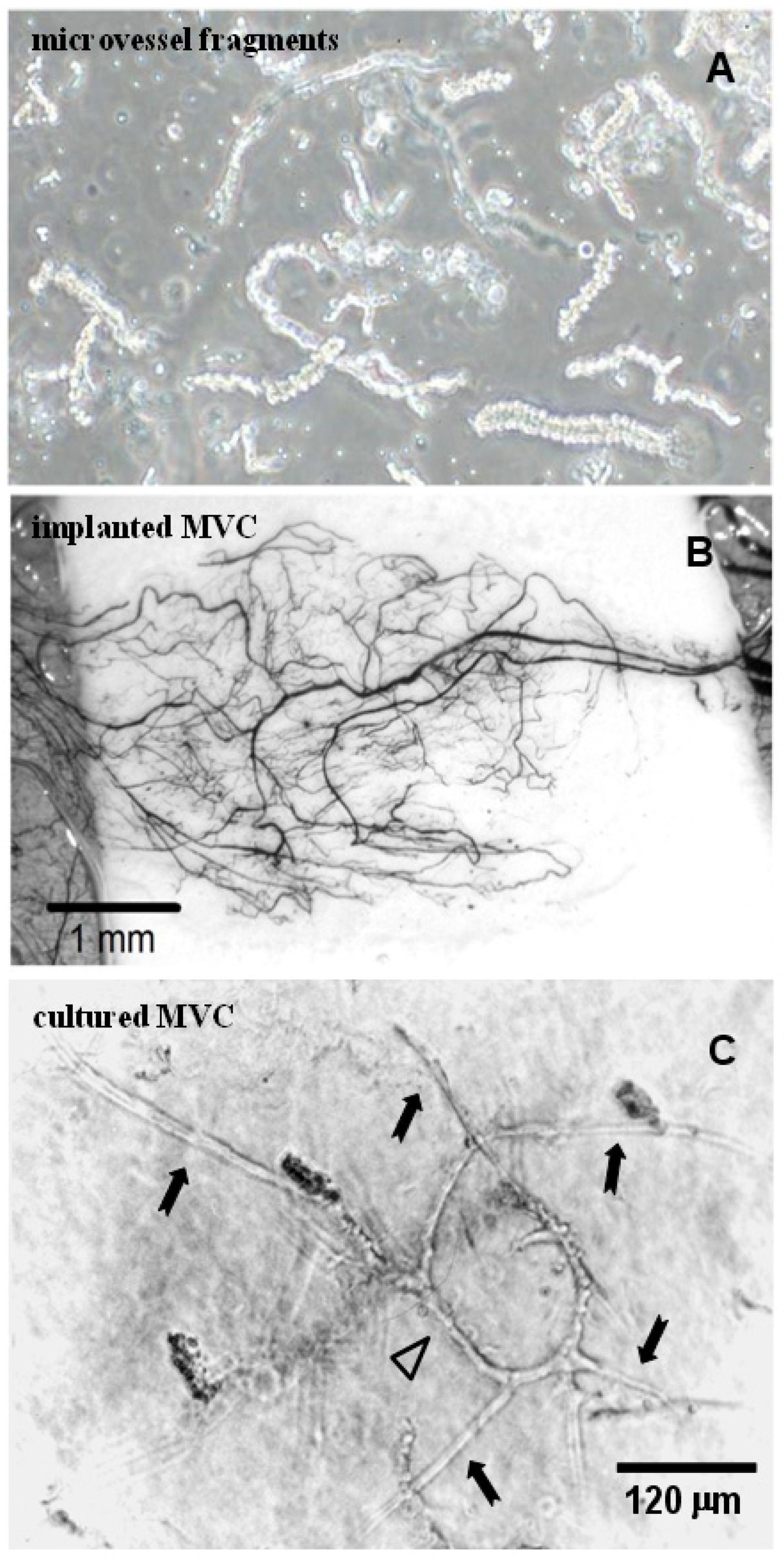
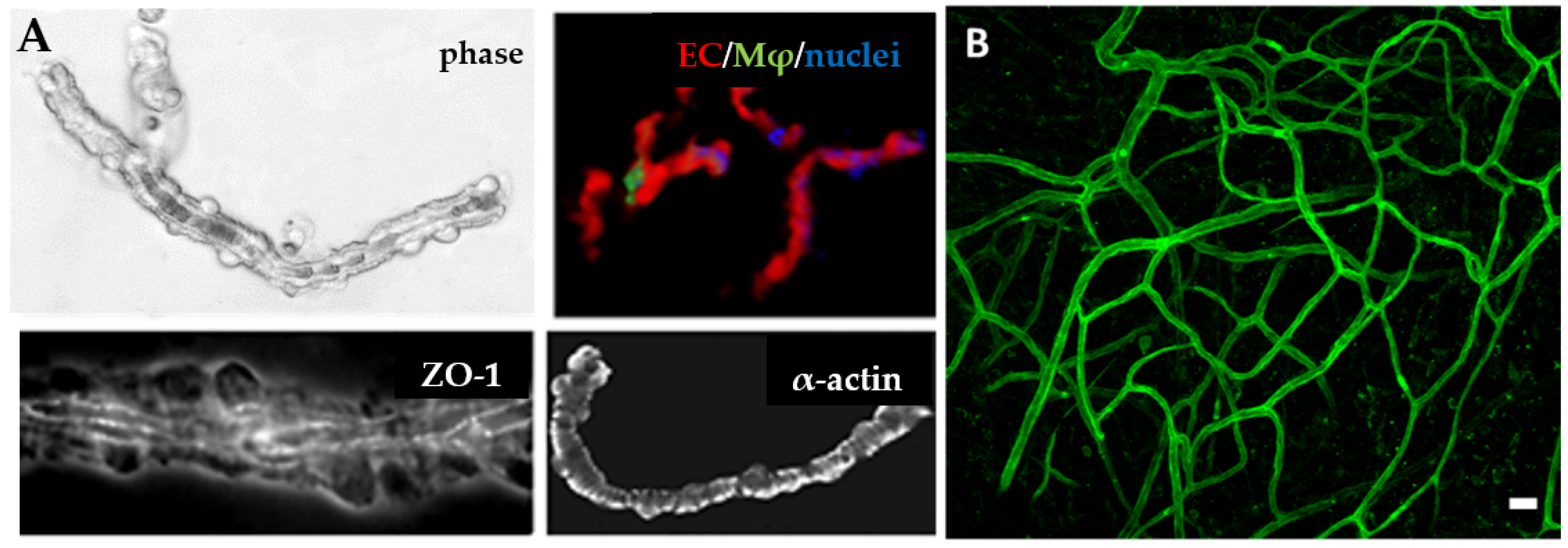
3.5. Isolated Microvessel Fragments

3.6. Matrix Considerations
4. Tissue Specific Considerations
4.1. Liver
4.2. Brain
4.3. Kidney
4.4. Muscle
5. Mechanics of the Microvasculature
5.1. Perfusion and Fluid Mechanics
5.2. Perfusion Strategies
5.3. Other Mechanical Forces
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stegemann, J.P.; Nerem, R.M. Altered Response of Vascular Smooth Muscle Cells to Exogenous Biochemical Stimulation in Two- and Three-Dimensional Culture. Exp. Cell Res. 2003, 283, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Baharvand, H.; Hashemi, S.M.; Kazemi Ashtiani, S.; Farrokhi, A. Differentiation of Human Embryonic Stem Cells into Hepatocytes in 2D and 3D Culture Systems in vitro. Int. J. Dev. Biol. 2006, 50, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.F.; Heng, B.C.; Ge, Z.; Lu, K.; Rufaihah, A.J.; Fan, V.T.; Yeo, J.F.; Cao, T. Comparison of Osteogenesis of Human Embryonic Stem Cells within 2D and 3D Culture Systems. Scand. J. Clin. Lab. Investig. 2008, 68, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.M.; Chen, C.S. Deconstructing the Third Dimension: How 3D Culture Microenvironments Alter Cellular cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef]
- Von Der Mark, K.; Gauss, V.; Von Der Mark, H.; MÜLler, P. Relationship between Cell Shape and Type of Collagen Synthesised as Chondrocytes Lose Their Cartilage Phenotype in Culture. Nature 1977, 267, 531–532. [Google Scholar] [CrossRef]
- Bierwolf, J.; Lutgehetmann, M.; Feng, K.; Erbes, J.; Deichmann, S.; Toronyi, E.; Stieglitz, C.; Nashan, B.; Ma, P.X.; Pollok, J.M. Primary Rat Hepatocyte Culture on 3D Nanofibrous Polymer Scaffolds for Toxicology and Pharmaceutical Research. Biotechnol. Bioeng. 2011, 108, 141–150. [Google Scholar] [CrossRef]
- Beamish, J.A.; He, P.; Molecular, K. Regulation of Contractile Smooth Muscle Cell Phenotype: Implications for Vascular Tissue Engineering. Tissue Eng. Part B 2010, 16, 467–491. [Google Scholar] [CrossRef]
- Asghari, F.; Samiei, M.; Adibkia, K.; Akbarzadeh, A.; Davaran, S. Biodegradable and Biocompatible Polymers for Tissue Engineering Application: A Review. Artif. Cells Nanomed. Biotechnol. 2017, 45, 185–192. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, A.S.; Asif, A.; Yar, M.; Haycock, J.W.; Rehman, I.U. Recent Concepts in Biodegradable Polymers for Tissue Engineering Paradigms: A Critical Review. Int. Mater. Rev. 2018, 64, 91–126. [Google Scholar] [CrossRef]
- Higgins, S.P.; Solan, A.K.; Niklason, L.E. Effects of Polyglycolic Acid on Porcine Smooth Muscle Cell Growth and Differentiation. J. Biomed. Mater. Res. 2003, 67, 295–302. [Google Scholar] [CrossRef]
- Zhu, L.; Fan, Y.; Huang, X.; Chen, T.; Xu, X.; Xu, F.; Gong, Y.; Chen, P. Patent Bibliometric Analysis for Global trend of organoid technologies in the past decade. iScience 2022, 25, 104728. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.; Mayr, U.; Boni, A.; Lukonin, I.; Rempfler, M.; Challet Meylan, L.; Stadler, M.B.; Strnad, P.; Papasaikas, P.; Vischi, D.; et al. Self-Organization and Symmetry Breaking in Intestinal Organoid Development. Nature 2019, 569, 66–72. [Google Scholar] [CrossRef]
- Homan, K.A.; Gupta, N.; Kroll, K.T.; Kolesky, D.B.; Skylar-Scott, M.; Miyoshi, T.; Mau, D.; Valerius, M.T.; Ferrante, T.; Bonventre, J.V.; et al. Flow-Enhanced Vascularization and Maturation of Kidney Organoids in vitro. Nat. Methods 2019, 16, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Takebe, T.; Koike, N.; Sekine, K.; Fujiwara, R.; Amiya, T.; Zheng, Y.W.; Taniguchi, H. Engineering of Human Hepatic Tissue with Functional Vascular Networks. Organogenesis 2014, 10, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Fennema, E.; Rivron, N.; Rouwkema, J.; Van Blitterswijk, C.; De Boer, J. Spheroid Culture as A Tool for Creating 3D Complex Tissues. Trends Biotechnol. 2013, 31, 108–115. [Google Scholar] [CrossRef]
- Liu, D.; Chen, S.; Win Naing, M. A Review of Manufacturing Capabilities of Cell Spheroid Generation Technologies and Future Development. Biotechnol. Bioeng. 2021, 118, 542–554. [Google Scholar] [CrossRef]
- Laschke, M.W.; Menger, M.D. Spheroids as Vascularization Units: From Angiogenesis Research to Tissue Engineering Applications. Biotechnol. Adv. 2017, 35, 782–791. [Google Scholar] [CrossRef]
- Pries, A.R.; Secomb, T.W. Making Microvascular Networks Work: Angiogenesis, Remodeling, and Pruning. Physiology 2014, 29, 446–455. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, L.; Wang, M.; Liu, J.; Zhong, S.; Li, R.; Li, P.; Guo, L.; Fang, A.; Chen, R.; et al. Vascularized Human Cortical Organoids (vOrganoids) Model Cortical Development in vivo. PLoS Biol. 2020, 18, e3000705. [Google Scholar] [CrossRef]
- Inamori, M.; Mizumoto, H.; Kajiwara, T. An Approach for Formation of Vascularized Liver Tissue by Endothelial Cell–Covered Hepatocyte Spheroid Integration. Tissue Eng. Part A 2009, 15, 2029–2037. [Google Scholar] [CrossRef]
- Dissanayaka, W.L.; Zhu, L.; Hargreaves, K.M.; Jin, L.; Zhang, C. Scaffold-Free Prevascularized Microtissue Spheroids for Pulp Regeneration. J. Dent. Res. 2014, 93, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Dissanayaka, W.L.; Zhu, L.; Hargreaves, K.M.; Jin, L.; Zhang, C. In vitro Analysis of Scaffold-Free Prevascularized Microtissue Spheroids Containing Human Dental Pulp Cells and Endothelial Cells. J. Endod. 2015, 41, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Wenger, A.; Stahl, A.; Weber, G. Modulation of in vitro Angiogenesis in A Three-Dimensional Spheroidal Coculture model for Bone Tissue Engineering. Tissue Eng. 2004, 10, 1536–1547. [Google Scholar] [CrossRef] [PubMed]
- Figtree, G.A.; Bubb, K.J.; Tang, O.; Kizana, E.; Gentile, C. Vascularized Cardiac Spheroids as Novel 3D in vitro Models to Study Cardiac Fibrosis. Cells Tissues Organs 2017, 204, 191–198. [Google Scholar] [CrossRef]
- Pitaktong, I.; Lui, C.; Lowenthal, J.; Mattson, G.; Jung, W.H.; Bai, Y.; Yeung, E.; Ong, C.S.; Chen, Y.; Gerecht, S.; et al. Early Vascular Cells Improve Microvascularization Within 3D Cardiac Spheroids. Tissue Eng. Part C Methods 2020, 26, 80–90. [Google Scholar] [CrossRef]
- Garzoni, L.R.; Rossi, M.I.; de Barros, A.P.; Guarani, V.; Keramidas, M.; Balottin, L.B.; Adesse, D.; Takiya, C.M.; Manso, P.P.; Otazu, I.B.; et al. Dissecting coronary angiogenesis: 3D co-culture of cardiomyocytes with endothelial or mesenchymal cells. Exp. Cell Res. 2009, 315, 3406–3418. [Google Scholar] [CrossRef]
- Hiramoto, K.; Pai, H.-J.; Ino, K.; Nashimoto, Y.; Shiku, H. Electrochemical measurement of respiratory activity for evaluation of fibroblast spheroids containing endothelial cell networks. Electrochim. Acta 2020, 340, 135979. [Google Scholar] [CrossRef]
- Verseijden, F.; Posthumus-van Sluijs, S.J.; Farrell, E.; van Neck, J.W.; Hovius, S.E.; Hofer, S.O.; van Osch, G.J. Prevascular structures promote vascularization in engineered human adipose tissue constructs upon implantation. Cell Transplant. 2010, 19, 1007–1020. [Google Scholar] [CrossRef]
- Strobel, H.A.; Moss, S.M.; Hoying, J.B. Biofabrication of tissue perfusion systems and microvasculatures, in Rapid Prototyping of Biomaterials. Woodhead Publ. Ser. Biomater. 2020, 205–225. [Google Scholar]
- Hsu, T.W.; Lu, Y.J.; Lin, Y.J.; Huang, Y.T.; Hsieh, L.H.; Wu, B.H.; Lin, Y.C.; Chen, L.C.; Wang, H.W.; Chuang, J.C.; et al. Transplantation of 3D MSC/HUVEC spheroids with neuroprotective and proangiogenic potentials ameliorates ischemic stroke brain injury. Biomaterials 2021, 272, 120765. [Google Scholar] [CrossRef]
- Costa, M.; Cerqueira, M.T.; Santos, T.C.; Sampaio-Marques, B.; Ludovico, P.; Marques, A.P.; Pirraco, R.P.; Reis, R.L. Cell sheet engineering using the stromal vascular fraction of adipose tissue as a vascularization strategy. Acta Biomater. 2017, 55, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, R.A.; Leopoldi, A.; Aichinger, M.; Wick, N.; Hantusch, B.; Novatchkova, M.; Taubenschmid, J.; Hammerle, M.; Esk, C.; Bagley, J.A.; et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature 2019, 565, 505–510. [Google Scholar] [CrossRef]
- Kook, M.G.; Lee, S.E.; Shin, N.; Kong, D.; Kim, D.H.; Kim, M.S.; Kang, H.K.; Choi, S.W.; Kang, K.S. Generation of Cortical Brain Organoid with Vascularization by Assembling with Vascular Spheroid. Int. J. Stem Cells 2022, 15, 85–94. [Google Scholar] [CrossRef]
- Lee, J.H.; Han, Y.S.; Lee, S.H. Long-Duration Three-Dimensional Spheroid Culture Promotes Angiogenic Activities of Adipose-Derived Mesenchymal Stem Cells. Biomol. Ther. 2016, 24, 260–267. [Google Scholar] [CrossRef]
- Bhang, S.H.; Lee, S.; Shin, J.Y.; Lee, T.J.; Kim, B.S. Transplantation of cord blood mesenchymal stem cells as spheroids enhances vascularization. Tissue Eng. Part A 2012, 18, 2138–2147. [Google Scholar] [CrossRef]
- Yamamoto, K.; Tanimura, K.; Watanabe, M.; Sano, H.; Uwamori, H.; Mabuchi, Y.; Matsuzaki, Y.; Chung, S.; Kamm, R.D.; Tanishita, K.; et al. Construction of Continuous Capillary Networks Stabilized by Pericyte-like Perivascular Cells. Tissue Eng. Part A 2019, 25, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.R.; Ueno, Y.; Zheng, Y.W.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef]
- Shah, S.; Kang, K.T. Two-Cell Spheroid Angiogenesis Assay System Using Both Endothelial Colony Forming Cells and Mesenchymal Stem Cells. Biomol. Ther. 2018, 26, 474–480. [Google Scholar] [CrossRef]
- Gurevich, D.B.; Severn, C.E.; Twomey, C.; Greenhough, A.; Cash, J.; Toye, A.M.; Mellor, H.; Martin, P. Live imaging of wound angiogenesis reveals macrophage orchestrated vessel sprouting and regression. EMBO J. 2018, 37, e97786. [Google Scholar] [CrossRef]
- Spiller, K.L.; Anfang, R.R.; Spiller, K.J.; Ng, J.; Nakazawa, K.R.; Daulton, J.W.; Vunjak-Novakovic, G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 2014, 35, 4477–4488. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.M.; Suresh, V.; Ying, G.; West, J.L. M0 and M2 Macrophages Enhance Vascularization of Tissue Engineering Scaffolds. Regen. Eng. Transl. Med. 2018, 4, 51–61. [Google Scholar] [CrossRef]
- Kreimendahl, F.; Marquardt, Y.; Apel, C.; Bartneck, M.; Zwadlo-Klarwasser, G.; Hepp, J.; Jockenhoevel, S.; Baron, J.M. Macrophages significantly enhance wound healing in a vascularized skin model. J. Biomed. Mater. Res. A 2019, 107, 1340–1350. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Freytes, D.O.; Vunjak-Novakovic, D. Vunjak-Novakovic, G. Macrophages Modulate Engineered Human Tissues for Enhanced Vascularization and Healing. Ann. Biomed. Eng. 2015, 43, 616–627. [Google Scholar] [CrossRef]
- Strobel, H.A.; LaBelle, S.A.; Krishnan, L.; Dale, J.; Rauff, A.; Poulson, A.M.; Bader, N.; Beare, J.; Aliaj, K.; Weiss, J.A.; et al. Stromal cells promote neovascular invasion across tissue interfaces. Front. Physiol. 2020, 11, 1026. [Google Scholar] [CrossRef] [PubMed]
- Bingle, L.; Lewis, C.E.; Corke, K.P.; Reed, M.W.; Brown, N.J. Macrophages promote angiogenesis in human breast tumour spheroids in vivo. Br. J. Cancer 2006, 94, 101–107. [Google Scholar] [CrossRef]
- Rama-Esendagli, D.; Yilmaz, G.; Esendagli, G.; Guc, D. Spheroid formation and invasion capacity are differentially influenced by co-cultures of fibroblast and macrophage cells in breast cancer. Mol. Biol. Rep. 2014, 41, 2885–2892. [Google Scholar] [CrossRef]
- Tevis, K.M.; Cecchi, R.J.; Colson, Y.L.; Grinstaff, M.W. Mimicking the tumor microenvironment to regulate macrophage phenotype and assessing chemotherapeutic efficacy in embedded cancer cell/macrophage spheroid models. Acta Biomater. 2017, 50, 271–279. [Google Scholar] [CrossRef]
- Donzelli, E.; Salvade, A.; Mimo, P.; Vigano, M.; Morrone, M.; Papagna, R.; Carini, F.; Zaopo, A.; Miloso, M.; Baldoni, M.; et al. Mesenchymal stem cells cultured on a collagen scaffold: In vitro osteogenic differentiation. Arch. Oral Biol. 2007, 52, 64–73. [Google Scholar] [CrossRef]
- Qian, S.-W.; Li, X.; Zhang, Y.-Y.; Huang, H.-Y.; Liu, Y.; Sun, X.; Tang, Q.-Q. Characterization of adipocyte differentiation from human mesenchymal stem cells in bone marrow. BMC Dev. Biol. 2010, 10, 47. [Google Scholar] [CrossRef]
- Strobel, H.A.; Gerton, T.; Hoying, J.B. Vascularized adipocyte organoid model using isolated human microvessel fragments. Biofabrication 2021. [Google Scholar] [CrossRef]
- Solorio, L.D.; Fu, A.S.; Hernandez-Irizarry, R.; Alsberg, E. Chondrogenic differentiation of human mesenchymal stem cell aggregates via controlled release of TGF-beta1 from incorporated polymer microspheres. J. Biomed. Mater. Res. A 2010, 92, 1139–1144. [Google Scholar] [PubMed]
- Ansboro, S.; Hayes, J.S.; Barron, V.; Browne, S.; Howard, L.; Greiser, U.; Lalor, P.; Shannon, F.; Barry, F.P.; Pandit, A.; et al. A chondromimetic microsphere for in situ spatially controlled chondrogenic differentiation of human mesenchymal stem cells. J. Control Release 2014, 179, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Niklason, L.E. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs). FASEB J. 2008, 22, 1635–1648. [Google Scholar] [CrossRef]
- Todeschi, M.R.; El Backly, R.; Capelli, C.; Daga, A.; Patrone, E.; Introna, M.; Mastrogiacomo, M. Transplanted Umbilical Cord Mesenchymal Stem Cells Modify the In Vivo Microenvironment Enhancing Angiogenesis and Leading to Bone Regeneration. Stem Cells Dev. 2015, 24, 157–1581. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, L.; Scott, P.G.; Tredget, E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007, 25, 2648–2659. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef]
- Gao, M.L.; Lei, X.L.; Han, F.; He, K.W.; Jin, S.Q.; Zhang, Y.Y.; Jin, Z.B. Patient-Specific Retinal Organoids Recapitulate Disease Features of Late-Onset Retinitis Pigmentosa. Front. Cell Dev. Biol. 2020, 8, 128. [Google Scholar] [CrossRef]
- Yamanaka, S. Patient-specific pluripotent stem cells become even more accessible. Cell Stem Cell 2010, 7, 1–2. [Google Scholar] [CrossRef]
- Takasato, M.; Er, P.X.; Chiu, H.S.; Maier, B.; Baillie, G.J.; Ferguson, C.; Parton, R.G.; Wolvetang, E.J.; Roost, M.S.; Chuva de Sousa Lopes, S.M.; et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 2015, 526, 564–568. [Google Scholar] [CrossRef]
- Liu, Y.; Berendsen, A.D.; Jia, S.; Lotinun, S.; Baron, R.; Ferrara, N.; Olsen, B.R. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J. Clin. Investig. 2012, 122, 3101–3113. [Google Scholar] [CrossRef]
- Ham, O.; Jin, Y.B.; Kim, J.; Lee, M.O. Blood vessel formation in cerebral organoids formed from human embryonic stem cells. Biochem. Biophys. Res. Commun. 2020, 521, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Ogunshola, O.O.; Stewart, W.B.; Mihalcik, V.; Solli, T.; Madri, J.A.; Ment, L.R. Neuronal VEGF expression correlates with angiogenesis in postnatal developing rat brain. Dev. Brain Res. 2000, 119, 139–153. [Google Scholar] [CrossRef]
- Chang, S.H.; Kanasaki, K.; Gocheva, V.; Blum, G.; Harper, J.; Moses, M.A.; Shih, S.C.; Nagy, J.A.; Joyce, J.; Bogyo, M.; et al. VEGF-A induces angiogenesis by perturbing the cathepsin-cysteine protease inhibitor balance in venules, causing basement membrane degradation and mother vessel formation. Cancer Res. 2009, 69, 4537–4544. [Google Scholar] [CrossRef]
- Pham, M.T.; Pollock, K.M.; Rose, M.D.; Cary, W.A.; Stewart, H.R.; Zhou, P.; Nolta, J.A.; Waldau, B. Generation of human vascularized brain organoids. Neuroreport 2018, 29, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Y.; Ju, X.C.; Li, Y.; Zeng, P.M.; Wu, J.; Zhou, Y.Y.; Shen, L.B.; Dong, J.; Chen, Y.J.; Luo, Z.G. Generation of vascularized brain organoids to study neurovascular interactions. Elife 2022, 11, e76707. [Google Scholar] [CrossRef]
- Lee, H.N.; Choi, Y.Y.; Kim, J.W.; Lee, Y.S.; Choi, J.W.; Kang, T.; Kim, Y.K.; Chung, B.G. Effect of biochemical and biomechanical factors on vascularization of kidney organoid-on-a-chip. Nano Converg. 2021, 8, 35. [Google Scholar] [CrossRef]
- Hoying, J.B.; Boswell, C.A.; Williams, S.K. Angiogenic Potential of Microvessel Fragments Established in Three-Dimensional Collagen Gels. In Vitro Cell. Dev. Biol.-Anim. 1996, 32, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.S.; Greer, K.A.; Stiening, C.M.; Chen, H.Y.; Kidd, K.R.; Schwartz, M.A.; Sullivan, C.J.; Rekapally, H.; Hoying, J.B. Implanted microvessels progress through distinct neovascularization phenotypes. Microvasc. Res. 2010, 79, 10–20. [Google Scholar] [CrossRef]
- Nunes, S.S.; Rekapally, H.; Chang, C.C.; Hoying, J.B. Vessel arterial-venous plasticity in adult neovascularization. PLoS ONE 2011, 6, e27332. [Google Scholar] [CrossRef]
- Utzinger, U.; Baggett, B.; Weiss, J.A.; Hoying, J.B.; Edgar, L.T. Large-scale time series microscopy of neovessel growth during angiogenesis. Angiogenesis 2015, 18, 219–232. [Google Scholar] [CrossRef]
- Shepherd, B.R.; Chen, H.Y.; Smith, C.M.; Gruionu, G.; Williams, S.K.; Hoying, J.B. Rapid perfusion and network remodeling in a microvascular construct after implantation. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.S.; Krishnan, L.; Gerard, C.S.; Dale, J.R.; Maddie, M.A.; Benton, R.L.; Hoying, J.B. Angiogenic potential of microvessel fragments is independent of the tissue of origin and can be influenced by the cellular composition of the implants. Microcirculation 2010, 17, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Hiscox, A.M.; Stone, A.L.; Limesand, S.; Hoying, J.B.; Williams, S.K. An islet-stabilizing implant constructed using a preformed vasculature. Tissue Eng. Part A 2008, 14, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, L.; Underwood, C.J.; Maas, S.; Ellis, B.J.; Kode, T.C.; Hoying, J.B.; Weiss, J.A. Effect of mechanical boundary conditions on orientation of angiogenic microvessels. Cardiovasc. Res. 2008, 78, 324–332. [Google Scholar] [CrossRef]
- Krishnan, L.; Weiss, J.A.; Wessman, M.D.; Hoying, J.D. Design and application of a test system for viscoelastic characterization of collagen gels. Tissue Eng. 2004, 10, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, N.D.; Andreou, S.; Hoying, J.B.; Utzinger, U. Live imaging of collagen remodeling during angiogenesis. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H3198–H3206. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Krishnan, L.; Nunes, S.S.; Church, K.H.; Edgar, L.T.; Boland, E.D.; Weiss, J.A.; Williams, S.K.; Hoying, J.B. Determinants of Microvascular Network Topologies in Implanted Neovasculatures. Arterioscler. Thromb. Vasc. Biol. 2011, 32, 5–14. [Google Scholar] [CrossRef]
- Moss, S.M.; Schilp, J.; Yaakov, M.; Cook, M.; Schuschke, E.; Hanke, B.; Strobel, H.A.; Hoying, J.B. Point-of-use, automated fabrication of a 3D human liver model supplemented with human adipose microvessels. SLAS Discov. 2022, 27, 358–368. [Google Scholar] [CrossRef]
- Moss, S.M.; Ortiz-Hernandez, M.; Levin, D.; Richburg, C.A.; Gerton, T.; Cook, M.; Houlton, J.J.; Rizvi, Z.H.; Goodwin, P.C.; Golway, M.; et al. A Biofabrication Strategy for a Custom-Shaped, Non-Synthetic Bone Graft Precursor with a Prevascularized Tissue Shell. Front. Bioeng. Biotechnol. 2022, 10, 838415. [Google Scholar] [CrossRef]
- Vartanian, K.B.; Chen, H.Y.; Kennedy, J.; Beck, S.K.; Ryaby, J.T.; Wang, H.; Hoying, J.B. The non-proteolytically active thrombin peptide TP508 stimulates angiogenic sprouting. J. Cell Physiol. 2006, 206, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Nunes, S.S.; Sibole, S.C.; Krishnan, L.; Williams, S.K.; Weiss, J.A.; Hoying, J.B. Angiogenesis in a microvascular construct for transplantation depends on the method of chamber circulation. Tissue Eng. Part A 2010, 16, 795–805. [Google Scholar] [CrossRef]
- Carter, W.B.; Uy, K.; Ward, M.D. and Hoying, J.B. Parathyroid-induced angiogenesis is VEGF-dependent. Surgery 2000, 128, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Carter, W.B. HER2 signaling—Induced microvessel dismantling. Surgery 2001, 130, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.; Ader, I.; Creff, J.; Lemenager, H.; Achard, P.; Casteilla, L.; Sensebe, L.; Carriere, A.; Deschaseaux, F. Human adipose stromal-vascular fraction self-organizes to form vascularized adipose tissue in 3D cultures. Sci. Rep. 2019, 9, 7250. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, A.J.; Krishnan, L.; Sullivan, C.J.; Williams, S.K.; Hoying, J.B. Microvascular repair: Post-angiogenesis vascular dynamics. Microcirculation 2012, 19, 676–695. [Google Scholar]
- Nalbach, L.; Muller, D.; Wrublewsky, S.; Metzger, W.; Menger, M.D.; Laschke, M.W.; Ampofo, E. Microvascular fragment spheroids: Three-dimensional vascularization units for tissue engineering and regeneration. J. Tissue Eng. 2021, 12, 20417314211035593. [Google Scholar] [CrossRef]
- Takahashi, Y.; Sekine, K.; Kin, T.; Takebe, T.; Taniguchi, H. Self-Condensation Culture Enables Vascularization of Tissue Fragments for Efficient Therapeutic Transplantation. Cell Rep. 2018, 23, 1620–1629. [Google Scholar] [CrossRef]
- Stegemann, J.P.; Hong, H.; Nerem, R.M. Mechanical, biochemical, and extracellular matrix effects on vascular smooth muscle cell phenotype. J. Appl. Physiol. 2005, 98, 2321–2327. [Google Scholar] [CrossRef]
- Hakkinen, K.M.; Harunaga, J.S.; Doyle, A.D.; Yamada, K.M. Direct comparisons of the morphology, migration, cell adhesions, and actin cytoskeleton of fibroblasts in four different three-dimensional extracellular matrices. Tissue Eng. Part A 2011, 17, 713–724. [Google Scholar] [CrossRef]
- Sellaro, T.L.; Ravindra, A.K.; Stolz, D.B.; Badylak, S.F. Maintenance of hepatic sinusoidal endothelial cell phenotype in vitro using organ-specific extracellular matrix scaffolds. Tissue Eng. 2007, 13, 2301–2310. [Google Scholar] [CrossRef]
- Robinet, A.; Fahem, A.; Cauchard, J.-H.; Huet, E.; Vincent, L.; Lorimier, S.; Antonicelli, F.; Soria, C.; Crepin, M.; Hornebeck, W.; et al. Elastin-derived peptides enhance angiogenesis by promoting endothelial cell migration and tubulogenesis through upregulation of MT1-MMP. J. Cell Sci. 2004, 118, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Neve, A.; Cantatore, F.P.; Maruotti, N.; Corrado, A.; Ribatti, D. Extracellular matrix modulates angiogenesis in physiological and pathological conditions. Biomed. Res. Int. 2014, 2014, 756078. [Google Scholar] [CrossRef] [PubMed]
- Mundel, T.M.; Kalluri, R. Type IV collagen-derived angiogenesis inhibitors. Microvasc. Res. 2007, 74, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Simon-Assmann, P.; Orend, G.; Mammadova-Bach, E.; Spenle, C.; Lefebvre, O. Role of laminins in physiological and pathological angiogenesis. Int. J. Dev. Biol. 2011, 55, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Yarali, Z.B.; Onak, G.; Karaman, O. Effect of Integrin Binding Peptide on Vascularization of Scaffold-Free Microtissue Spheroids. Tissue Eng. Regen. Med. 2020, 17, 595–605. [Google Scholar] [CrossRef]
- Kim, J.W.; Nam, S.A.; Yi, J.; Kim, J.Y.; Lee, J.Y.; Park, S.Y.; Sen, T.; Choi, Y.M.; Lee, J.Y.; Kim, H.L.; et al. Kidney Decellularized Extracellular Matrix Enhanced the Vascularization and Maturation of Human Kidney Organoids. Adv. Sci. 2022, 9, e2103526. [Google Scholar] [CrossRef]
- Nguyen, J.; Lin, Y.Y.; Gerecht, S. The next generation of endothelial differentiation: Tissue-specific ECs. Cell Stem Cell 2021, 28, 1188–1204. [Google Scholar] [CrossRef]
- DeLeve, L.D.; Maretti-Mira, A.C. Liver Sinusoidal Endothelial Cell: An Update. Semin. Liver Dis. 2017, 37, 377–387. [Google Scholar]
- Ganesan, L.P.; Mohanty, S.; Kim, J.; Clark, K.R.; Robinson, J.M.; Anderson, C.L. Rapid and efficient clearance of blood-borne virus by liver sinusoidal endothelium. PLoS Pathog. 2011, 7, e1002281. [Google Scholar] [CrossRef]
- Breiner, K.M.; Schaller, H.; Knolle, P.A. Endothelial cell-mediated uptake of a hepatitis B virus: A new concept of liver targeting of hepatotropic microorganisms. Hepatology 2001, 34 Pt 1, 803–808. [Google Scholar] [CrossRef]
- Li, R.; Oteiza, A.; Sorensen, K.K.; McCourt, P.; Olsen, R.; Smedsrod, B.; Svistounov, D. Role of liver sinusoidal endothelial cells and stabilins in elimination of oxidized low-density lipoproteins. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G71–G81. [Google Scholar] [CrossRef]
- Yao, Z.; Mates, J.M.; Cheplowitz, A.M.; Hammer, L.P.; Maiseyeu, A.; Phillips, G.S.; Wewers, M.D.; Rajaram, M.V.; Robinson, J.M.; Anderson, C.L.; et al. Blood-Borne Lipopolysaccharide Is Rapidly Eliminated by Liver Sinusoidal Endothelial Cells via High-Density Lipoprotein. J. Immunol. 2016, 197, 2390–2399. [Google Scholar] [CrossRef]
- Wu, J.; Meng, Z.; Jiang, M.; Zhang, E.; Trippler, M.; Broering, R.; Bucchi, A.; Krux, F.; Dittmer, U.; Yang, D.; et al. Toll-like receptor-induced innate immune responses in non-parenchymal liver cells are cell type-specific. Immunology 2010, 129, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.S.; Nolan, D.J.; Butler, J.M.; James, D.; Babazadeh, A.O.; Rosenwaks, Z.; Mittal, V.; Kobayashi, H.; Shido, K.; Lyden, D.; et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature 2010, 468, 310–315. [Google Scholar] [CrossRef]
- Herrnberger, L.; Hennig, R.; Kremer, W.; Hellerbrand, C.; Goepferich, A.; Kalbitzer, H.R.; Tamm, E.R. Formation of fenestrae in murine liver sinusoids depends on plasmalemma vesicle-associated protein and is required for lipoprotein passage. PLoS ONE 2014, 9, e115005. [Google Scholar] [CrossRef]
- Xie, G.; Wang, X.; Wang, L.; Wang, L.; Atkinson, R.D.; Kanel, G.C.; Gaarde, W.A.; Deleve, L.D. Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats. Gastroenterology 2012, 142, 918–927.e6. [Google Scholar] [CrossRef] [PubMed]
- Rockey, D.C.; Chung, J.J. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: Endothelial dysfunction in portal hypertension. Gastroenterology 1998, 114, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Horn, T.; Christoffersen, P.; Henrikse, J.H. Alcoholic Liver Injury: Defenestration in Noncirrhotic Livers- A Scanning Electron Microscopic Study. Hepatology 1987, 7, 77–82. [Google Scholar] [CrossRef]
- DeLeve, L.D.; Wang, X.; Hu, L.; McCuskey, M.K.; McCuskey, R.S. Rat liver sinusoidal endothelial cell phenotype is maintained by paracrine and autocrine regulation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G757–G763. [Google Scholar] [CrossRef]
- Kim, Y.; Rajagopalan, P. 3D hepatic cultures simultaneously maintain primary hepatocyte and liver sinusoidal endothelial cell phenotypes. PLoS ONE 2010, 5, e15456. [Google Scholar] [CrossRef]
- March, S.; Hui, E.E.; Underhill, G.H.; Khetani, S.; Bhatia, S.N. Microenvironmental regulation of the sinusoidal endothelial cell phenotype in vitro. Hepatology 2009, 50, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.D.; Schirmer, K.; Munst, B.; Heinz, S.; Ghafoory, S.; Wolfl, S.; Simon-Keller, K.; Marx, A.; Oie, C.I.; Ebert, M.P.; et al. In Vitro Generation of Functional Liver Organoid-Like Structures Using Adult Human Cells. PLoS ONE 2015, 10, e0139345. [Google Scholar] [CrossRef] [PubMed]
- Yap, K.K.; Gerrand, Y.W.; Dingle, A.M.; Yeoh, G.C.; Morrison, W.A.; Mitchell, G.M. Liver sinusoidal endothelial cells promote the differentiation and survival of mouse vascularised hepatobiliary organoids. Biomaterials 2020, 251, 120091. [Google Scholar] [CrossRef]
- Tsang, H.Y.; Yi Lo, P.H.; Ho Lee, K.K. Generation of liver organoids from human induced pluripotent stem cells as liver fibrosis and steatosis models. bioRxiv 2021, in press. [Google Scholar] [CrossRef]
- Li, J.; Xing, F.; Chen, F.; He, L.; So, K.F.; Liu, Y.; Xiao, J. Functional 3D Human Liver Bud Assembled from MSC-Derived Multiple Liver Cell Lineages. Cell Transplant. 2019, 28, 510–521. [Google Scholar] [CrossRef]
- Koui, Y.; Kido, T.; Ito, T.; Oyama, H.; Chen, S.W.; Katou, Y.; Shirahige, K.; Miyajima, A. An In Vitro Human Liver Model by iPSC-Derived Parenchymal and Non-parenchymal Cells. Stem Cell Rep. 2017, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Cakir, B.; Xiang, Y.; Tanaka, Y.; Kural, M.H.; Parent, M.; Kang, Y.J.; Chapeton, K.; Patterson, B.; Yuan, Y.; He, C.S.; et al. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 2019, 16, 1169–1175. [Google Scholar] [CrossRef]
- Song, L.; Yuan, X.; Jones, Z.; Griffin, K.; Zhou, Y.; Ma, T.; Li, Y. Assembly of Human Stem Cell-Derived Cortical Spheroids and Vascular Spheroids to Model 3-D Brain-like Tissues. Sci. Rep. 2019, 9, 5977. [Google Scholar] [CrossRef]
- Mansour, A.A.; Goncalves, J.T.; Bloyd, C.W.; Li, H.; Fernandes, S.; Quang, D.; Johnston, S.; Parylak, S.L.; Jin, X.; Gage, F.H. An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol. 2018, 36, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Guo, Y.; Zhu, Y.; Qin, J. Engineering stem cell-derived 3D brain organoids in a perfusable organ-on-a-chip system. RSC Adv. 2018, 8, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J. Inflammatory Mediators and Modulation of Blood–Brain Barrier Permeability. Cell. Mol. Neurobiol. 2000, 20, 131–147. [Google Scholar] [CrossRef]
- Jen, K.-Y.; Haragsim, L.; Laszik, Z.G. Kidney Microvasculature in Health and Disease. Exp. Model. Ren. Dis. 2011, 169, 51–72. [Google Scholar]
- Satchell, S.C.; Braet, F. Glomerular endothelial cell fenestrations: An integral component of the glomerular filtration barrier. Am. J. Physiol. Ren. Physiol. 2009, 296, F947–F956. [Google Scholar] [CrossRef] [PubMed]
- Ballermann, B.J. Glomerular endothelial cell differentiation. Kidney Int. 2005, 67, 1668–1671. [Google Scholar] [CrossRef]
- Morizane, R.; Lam, A.Q.; Freedman, B.S.; Kishi, S.; Valerius, M.T.; Bonventre, J.V. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol. 2015, 33, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.Q.; Freedman, B.S.; Morizane, R.; Lerou, P.H.; Valerius, M.T.; Bonventre, J.V. Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers. J. Am. Soc. Nephrol. 2014, 25, 1211–1225. [Google Scholar] [CrossRef] [PubMed]
- Krogh, A. The number and distribution of capillaries in muscles with calculations of the oxygen pressure head necessary for supplying the tissue. J. Physiol. 1919, 52, 409–415. [Google Scholar] [CrossRef]
- Kusters, Y.H.A.M.; Barrett, E.J. Muscle microvasculature’s structural and functional specializations facilitate muscle metabolism. Am. J. Physiology. Endocrinol. Metab. 2016, 310, E379–E387. [Google Scholar] [CrossRef] [PubMed]
- Andersen, P.; Saltin, B. Maximal perfusion of skeletal muscle in man. J. Pysiol. 1985, 366, 233–249. [Google Scholar] [CrossRef]
- Kubínová, L.; Janácek, J.; Ribaric, S.; Cebasek, V.; Erzen, I. Three-dimensional study of the capillary supply of skeletal muscle fibres using confocal microscopy. J. Muscle Cell Motil. 2001, 22, 217–227. [Google Scholar] [CrossRef]
- Egginton, S.; Zhou, A.-L.; Brown, M.D.; Hudlická, O. Unorthodox angiogenesis in skeletal muscle. Cardiovasc. Res. 2001, 49, 634–646. [Google Scholar] [CrossRef]
- Gholobova, D.; Terrie, L.; Gerard, M.; Declercq, H.; Thorrez, L. Vascularization of tissue-engineered skeletal muscle constructs. Biomaterials 2020, 235, 119708. [Google Scholar] [CrossRef] [PubMed]
- Okano, T.; Matsuda, T. Tissue Engineered Skeletal Muscle: Preparation of Highly Dense, Highly Oriented Hybrid Muscular Tissues. Cell Transplant. 1998, 7, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.A.; Smiley, B.L.; Mills, J.; Vandenburgh, H.H. Mechanical stimulation improves tissue-engineered human skeletal muscle. Am. J. Physiol. Cell Physiol. 2002, 283, C1557–C1565. [Google Scholar] [CrossRef]
- Brown, M.D. Exercise and coronary vascular remodelling in the healthy heart. Exp. Physiol. 2003, 88, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Efthimiadou, A.; Asimakopoulos, B.; Nikolettos, N.; Giatromanolaki, A.; Sivridis, E.; Lialiaris, T.S.; Papachristou, D.N.; Kontoleon, E. The Angiogenetic Effect of Intramuscular Administration of VEGF on Muscle. The Influence of Exercise on Angiogenesis. In Vivo 2004, 18, 825–830. [Google Scholar] [PubMed]
- Tsagalou, E.P.; Anastasiou-Nana, M.; Agapitos, E.; Gika, A.; Drakos, S.G.; Terrovitis, J.V.; Ntalianis, A.; Nanas, J.N. Depressed coronary flow reserve is associated with decreased myocardial capillary density in patients with heart failure due to idiopathic dilated cardiomyopathy. J. Am. Coll. Cardiol. 2008, 52, 1391–1398. [Google Scholar] [CrossRef]
- Kitsuka, T.; Itoh, M.; Amamoto, S.; Arai, K.I.; Oyama, J.; Node, K.; Toda, S.; Morita, S.; Nishida, T.; Nakayama, K. 2-Cl-C.OXT-A stimulates contraction through the suppression of phosphodiesterase activity in human induced pluripotent stem cell-derived cardiac organoids. PLoS ONE 2019, 14, e0213114. [Google Scholar] [CrossRef]
- Filippo Buono, M.; von Boehmer, L.; Strang, J.; Hoerstrup, S.P.; Emmert, M.Y.; Nugraha, B. Human Cardiac Organoids for Modeling Genetic Cardiomyopathy. Cells 2020, 9, 1733. [Google Scholar] [CrossRef]
- Archer, C.R.; Sargeant, R.; Basak, J.; Pilling, J.; Barnes, J.R.; Pointon, A. Characterization and Validation of a Human 3D Cardiac Microtissue for the Assessment of Changes in Cardiac Pathology. Sci. Rep. 2018, 8, 10160. [Google Scholar] [CrossRef]
- Hossler, F.E.; Monson, F.C. Microvasculature of the Rabbit Urinary Bladder. Anat. Rec. 1995, 263, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Hu, W.; Matulay, J.T.; Silva, M.V.; Owczarek, T.B.; Kim, K.; Chua, C.W.; Barlow, L.J.; Kandoth, C.; Williams, A.B.; et al. Tumor Evolution and Drug Response in Patient-Derived Organoid Models of Bladder Cancer. Cell 2018, 173, 515–528.e17. [Google Scholar] [CrossRef] [PubMed]
- Mullenders, J.; de Jongh, E.; Brousali, A.; Roosen, A.; Blom, J.P.A.; Begthel, H.; Korving, J.; Jonges, T.; Kranenburg, O.; Meijer, R.; et al. Mouse and human urothelial cancer organoids: A tool for bladder cancer research. Proc. Natl. Acad. Sci. USA 2019, 116, 4567–4574. [Google Scholar] [CrossRef]
- Chary, S.R.; Jain, R.K. Direct Measurement of Interstitial Convection and Diffusion of Albumin in Normal and Neoplastic Tissues by Fluorescence Photobleaching. Proc. Natl. Acad. Sci. USA 1989, 86, 5385–5389. [Google Scholar] [CrossRef]
- Dafni, H.; Israely, T.; Bhujwalla, Z.M.; Benjamin, L.E.; Neeman, M. Overexpression of Vascular Endothelial Growth Factor 165 Drives Peritumor Interstitial Convection and Induces Lymphatic Drain: Magnetic Resonance Imaging, Confocal Microscopy, and Histological Tracking of Triple-labeled Albumin. Cancer Res. 2002, 62, 6731–6739. [Google Scholar] [PubMed]
- Galiea, P.A.; Nguyen, D.-H.T.; Choi, C.K.; Cohen, D.M.; Janmey, P.A.; Chen, C.S. Fluid shear stress threshold regulates angiogenic sprouting. Proc. Natl. Acad. Sci. USA 2014, 111, 7968–7973. [Google Scholar] [CrossRef] [PubMed]
- Wragg, J.W.; Durant, S.; McGettrick, H.M.; Sample, K.M.; Egginton, S.; Bicknell, R. Shear stress regulated gene expression and angiogenesis in vascular endothelium. Microcirculation 2014, 21, 290–300. [Google Scholar] [CrossRef]
- Abe, Y.; Watanabe, M.; Chung, S.; Kamm, R.D.; Tanishita, K.; Sudo, R. Balance of interstitial flow magnitude and vascular endothelial growth factor concentration modulates three-dimensional microvascular network formation. APL Bioeng. 2019, 3, 036102. [Google Scholar] [CrossRef]
- Hernandez Vera, R.; Genove, E.; Alvarez, L.; Borros, S.; Kamm, R.; Lauffenburger, D.; Semino, C.E. Interstitial fluid flow intensity modulates endothelial sprouting in restricted Src-activated cell clusters during capillary morphogenesis. Tissue Eng. Part A 2009, 15, 175–185. [Google Scholar] [CrossRef]
- Chiu, J.J.; Chen, L.J.; Lee, P.L.; Lee, C.I.; Lo, L.W.; Usami, S.; Chien, S. Shear stress inhibits adhesion molecule expression in vascular endothelial cells induced by coculture with smooth muscle cells. Blood 2003, 101, 2667–2674. [Google Scholar] [CrossRef]
- Szmitko, P.E.; Wang, C.H.; Weisel, R.D.; de Almeida, J.R.; Anderson, T.J.; Verma, S. New markers of inflammation and endothelial cell activation: Part I. Circulation 2003, 108, 1917–1923. [Google Scholar] [CrossRef] [PubMed]
- Ando, J.; Tsubi, H.; Korenaga, R.; Takada, Y.; Toyama-Sorimachi, N.; Miyasaka, M.; Kamiya, A. Shear stress inhibits adhesion of cultured mouse endothelial cells to lymhocytes by downregulating VCAM-1 expression. Am. J. Physiol. 1994, 267, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.D.; Grazul-Bilska, A.T.; Reynolds, L.P.; Johnson, M.L.; Redmer, D.A. Isolation and Characterization of Ovine Luteal Pericytes and Effects of Nitric Oxide on Pericyte Expression of Angiogenic Factors. Endocrine 2006, 29, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Tamilarasan, K.P.; Kolluru, G.K.; Muley, A.; Nair, C.M.; Omanakuttan, A.; Murty, K.V.G.K.; Chatterjee, S. Activated pericyte attenuates endothelial functions: Nitric oxide-cGMP rescues activated pericyte-associated endothelial dysfunctions. Biochem. Cell Biol. 2007, 85, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Bassaneze, V.; Barauna, V.G.; Lavini-Ramos, C.; Kalil, J.; Schettert, I.T.; Miyakawa, A.A.; Krieger, J.E. Shear Stress Induces Nitric Oxide–Mediated Vascular Endothelial Growth Factor Production in Human Adipose Tissue Mesenchymal Stem Cells. Stem Cells Dev. 2010, 19, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Kubes, P.; Granger, N. Nitric oxide modulates microvascular permeability. Am. Physiol. Soc. 1992, 262, H611–H615. [Google Scholar] [CrossRef]
- Duran, W.N.; Beuve, A.V.; Sanchez, F.A. Nitric oxide, S-nitrosation, and endothelial permeability. IUBMB Life 2013, 65, 819–826. [Google Scholar] [CrossRef]
- Ridnour, L.A.; Isenberg, J.S.; Espey, M.G.; Thomas, D.D.; Roberts, D.D.; Wink, D.A. Nitric oxide regulates angiogenesis through a functional switch involving thrombospondin-1. Proc. Natl. Acad. Sci. USA 2005, 102, 13147–13152. [Google Scholar] [CrossRef]
- Ziche, M.; Morbidelli, L.; Masini, E.; Amerini, S.; Granger, H.J.; Maggi, C.A.; Geppetti, P.; Ledda, F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J. Clin. Investig. 1994, 94, 2036–2044. [Google Scholar] [CrossRef]
- Archer, S.L.; Huang, J.M.; Hampl, V.; Nelson, D.P.; Shultz, P.J.; Weir, E.K. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 1994, 91, 7583–7587. [Google Scholar] [CrossRef]
- Beyer, A.M.; Durand, M.J.; Hockenberry, J.; Gamblin, T.C.; Phillips, S.C.; Gutterman, D.D. An acute rise in intraluminal pressure shifts the mediator of flow-mediated dilation from nitric oxide to hydrogen peroxide in human arterioles. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1587–H1593. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.J.; Dora, K.A. EDH: Endothelium-dependent hyperpolarization and microvascular signalling. Acta Physiol. 2017, 219, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Munn, L.L. Fluid forces control endothelial sprouting. Proc. Natl. Acad. Sci. USA 2011, 108, 15342–15347. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.B.; Whisler, J.A.; Jeon, J.S.; Kamm, R.D. Mechanisms of tumor cell extravasation in an in vitro microvascular network platform. Integr. Biol. 2013, 5, 1262–1271. [Google Scholar] [CrossRef]
- Moya, M.L.; Hsu, Y.H.; Lee, A.P.; Hughes, C.C.; George, S.C. In vitro perfused human capillary networks. Tissue Eng. Part C Methods 2013, 19, 730–737. [Google Scholar] [CrossRef]
- Wang, X.; Phan, D.T.; Sobrino, A.; George, S.C.; Hughes, C.C.; Lee, A.P. Engineering anastomosis between living capillary networks and endothelial cell-lined microfluidic channels. Lab Chip 2016, 16, 282–290. [Google Scholar] [CrossRef]
- Zhang, B.; Korolj, A.; Lai, B.F.L.; Radisic, M. Advances in organ-on-a-chip engineering. Nat. Rev. Mater. 2018, 3, 257–278. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Elkhammas, E.; Hasan, H. Translating advances in organ-on-a-chip technology for supporting organs. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 107, 2006–2018. [Google Scholar] [CrossRef]
- Azizipour, N.; Avazpour, R.; Rosenzweig, D.H.; Sawan, M.; Ajji, A. Evolution of Biochip Technology: A Review from Lab-on-a-Chip to Organ-on-a-Chip. Micromachines 2020, 11, 599. [Google Scholar] [CrossRef]
- Ma, C.; Peng, Y.; Li, H.; Chen, W. Organ-on-a-Chip: A New Paradigm for Drug Development. Trends Pharmacol. Sci. 2021, 42, 119–133. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, L.; Wang, X.; Feng, L.; Wu, J.; Zhu, X.; Wen, W.; Gong, X. Organ-on-a-chip: Recent breakthroughs and future prospects. Biomed. Eng. Online 2020, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Nashimoto, Y.; Okada, R.; Hanada, S.; Arima, Y.; Nishiyama, K.; Miura, T.; Yokokawa, R. Vascularized cancer on a chip: The effect of perfusion on growth and drug delivery of tumor spheroid. Biomaterials 2020, 229, 119547. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, D.; Li, J.; Yang, S.; Xu, J.; Yokota, H.; Zhang, P. Wnt3a involved in the mechanical loading on improvement of bone remodeling and angiogenesis in a postmenopausal osteoporosis mouse model. FASEB J. 2019, 33, 8913–8924. [Google Scholar] [CrossRef] [PubMed]
- Kasper, G.; Dankert, N.; Tuischer, J.; Hoeft, M.; Gaber, T.; Glaeser, J.D.; Zander, D.; schirschmann, M.T.; Thompson, T.; Matziolis, G.; et al. Mesenchymal stem cells regulate angiogenesis according to their mechanical environment. Stem Cells 2007, 25, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, B.; Engelbrecht, L.K.; Fernandez, P.; Hutterer, F.P.; Raich, M.K.; Scheel, C.H.; Bausch, A.R. Mechanical plasticity of collagen directs branch elongation in human mammary gland organoids. Nat. Commun. 2021, 12, 2759. [Google Scholar] [CrossRef]
- Edgar, L.T.; Underwood, C.J.; Guilkey, J.E.; Hoying, J.B.; Weiss, J.A. Extracellular matrix density regulates the rate of neovessel growth and branching in sprouting angiogenesis. PLoS ONE 2014, 9, e85178. [Google Scholar] [CrossRef]
- Santos, L.; Fuhrmann, G.; Juenet, M.; Amdursky, N.; Horejs, C.M.; Campagnolo, P.; Stevens, M.M. Extracellular Stiffness Modulates the Expression of Functional Proteins and Growth Factors in Endothelial Cells. Adv. Healthc. Mater. 2015, 4, 2056–2063. [Google Scholar] [CrossRef]
- Zhang, S.; Kan, E.L.; Kamm, R.D. Integrating functional vasculature into organoid culture: A biomechanical perspective. APL Bioeng. 2022, 6, 030401. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strobel, H.A.; Moss, S.M.; Hoying, J.B. Vascularized Tissue Organoids. Bioengineering 2023, 10, 124. https://doi.org/10.3390/bioengineering10020124
Strobel HA, Moss SM, Hoying JB. Vascularized Tissue Organoids. Bioengineering. 2023; 10(2):124. https://doi.org/10.3390/bioengineering10020124
Chicago/Turabian StyleStrobel, Hannah A., Sarah M. Moss, and James B. Hoying. 2023. "Vascularized Tissue Organoids" Bioengineering 10, no. 2: 124. https://doi.org/10.3390/bioengineering10020124
APA StyleStrobel, H. A., Moss, S. M., & Hoying, J. B. (2023). Vascularized Tissue Organoids. Bioengineering, 10(2), 124. https://doi.org/10.3390/bioengineering10020124






