Unforeseen Outcomes Post Treatment for Radiation Induced Trismus: A Case Report
Abstract
:1. Introduction
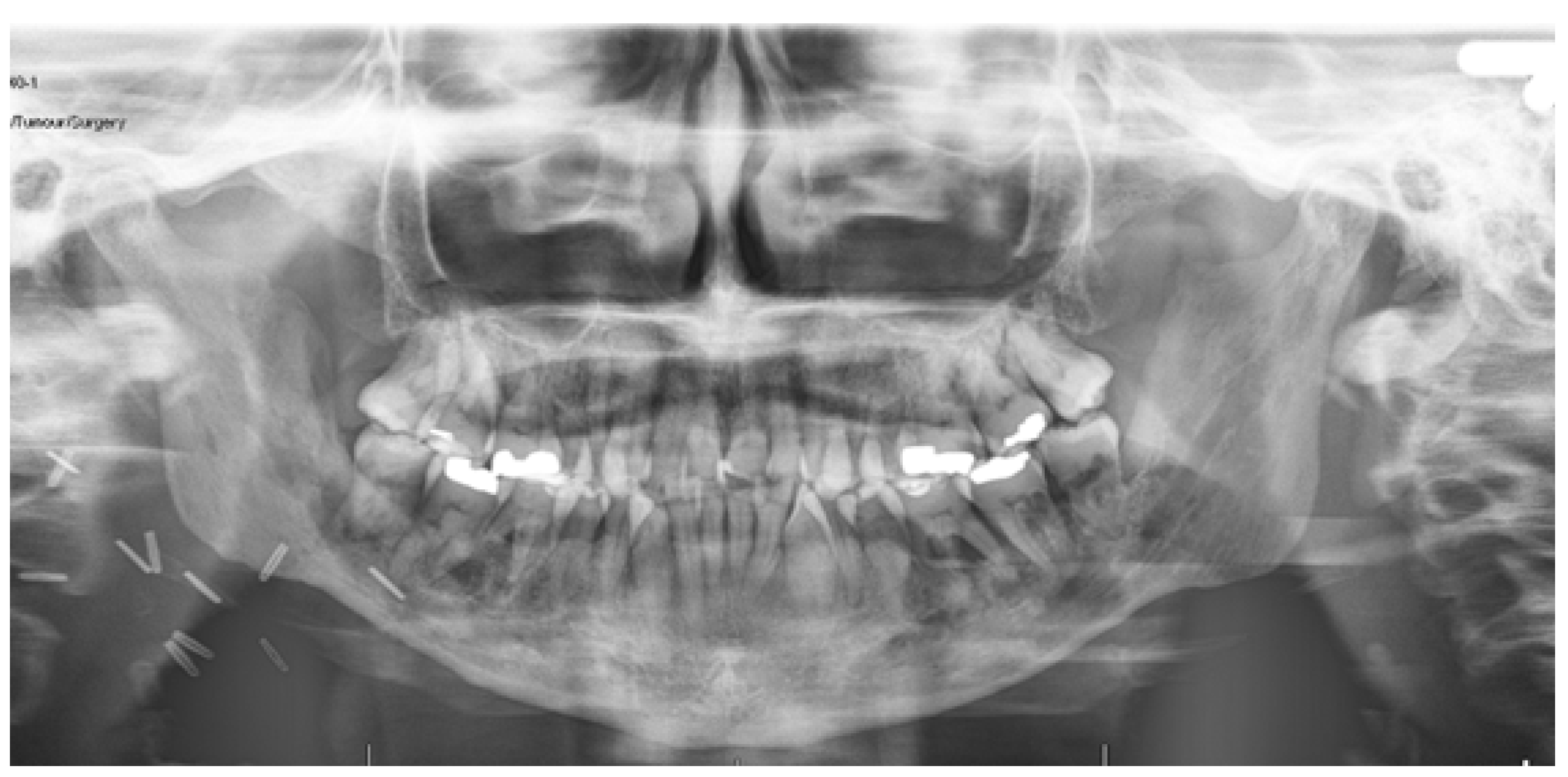
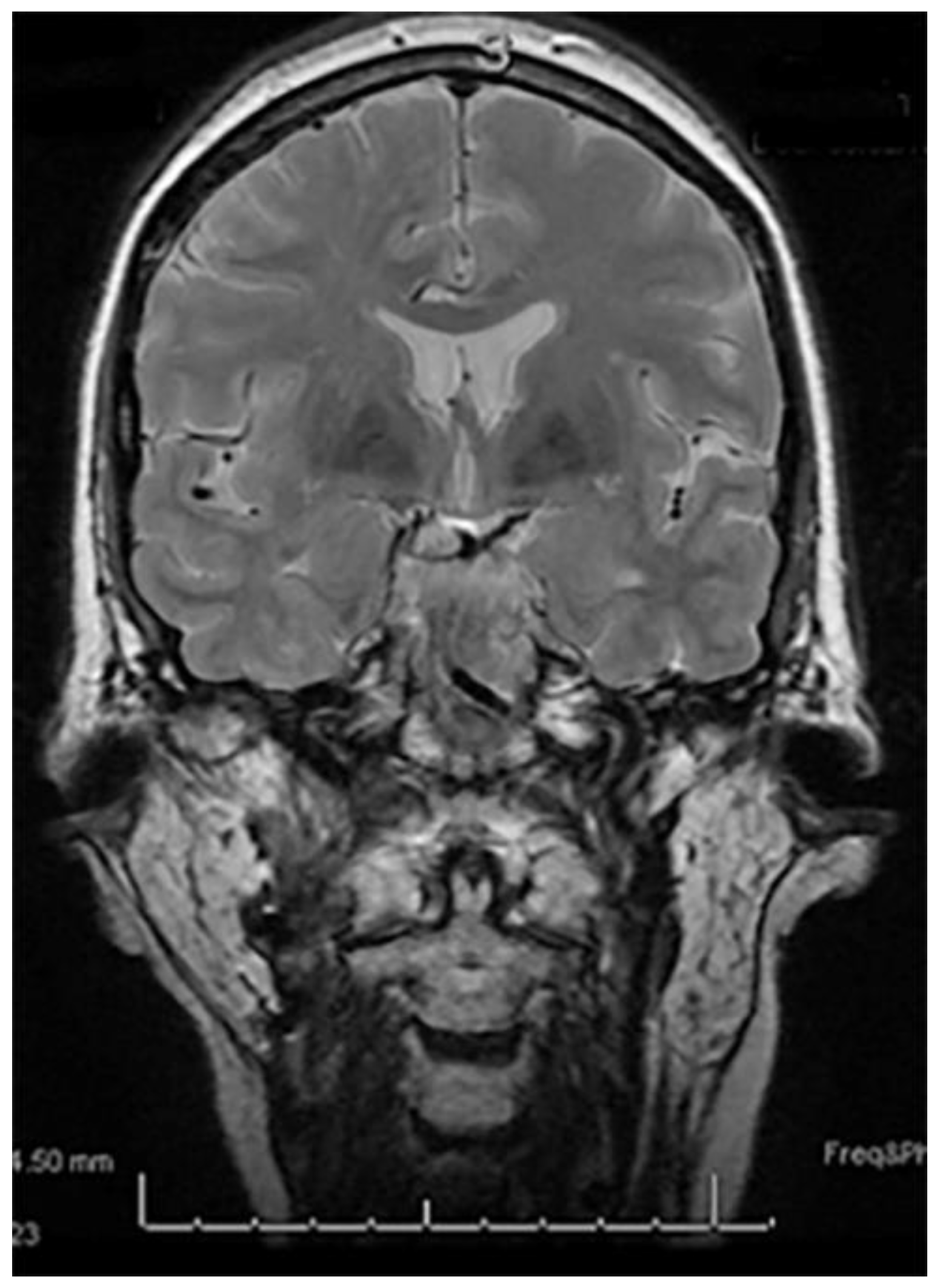
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Alterio, D.; Marvaso, G.; Ferrari, A.; Volpe, S.; Orecchia, R.; Jereczek-Fossa, B.A. Modern radiotherapy for head and neck cancer. Semin. Oncol. 2019, 46, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. Late side effects of radiation treatment for head and neck cancer. Radiat. Oncol. J. 2020, 38, 84–92. [Google Scholar] [CrossRef]
- Sroussi, H.Y.; Epstein, J.B.; Bensadoun, R.-J.; Saunders, D.P.; Lalla, R.V.; Migliorati, C.A.; Heaivilin, N.; Zumsteg, Z.S. Common oral complications of head and neck cancer radiation therapy: Mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. 2017, 6, 2918–2931. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.; Silander, E.; Nyman, J.; Bove, M.; Johansson, L.; Bjork-Eriksson, T.; Hammerlid, E. Impact on quality of life of IMRT versus 3-D conformal radiation therapy in head and neck cancer patients: A case control study. Adv. Radiat. Oncol. 2017, 2, 346–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhandari, S.; Soni, B.W.; Bahl, A.; Ghoshal, S. Radiotherapy-induced oral morbidities in head and neck cancer patients. Spec. Care Dentist. 2020, 40, 238–250. [Google Scholar] [CrossRef]
- Nakajima, T.; Sasakura, H.; Kato, N. Screw-type mouth gag for prevention and treatment of postoperative jaw limitation by fibrous tissue. J. Oral Surg. 1980, 38, 46–50. [Google Scholar]
- Rai, A.; Datarkar, A.; Rai, M. Is buccal fat pad a better option than nasolabial flap for reconstruction of intraoral defects after surgical release of fibrous bands in patients with oral submucous fibrosis? A pilot study: A protocol for the management of oral submucous fibrosis. J. Craniomaxillofac. Surg. 2014, 42, e111–e116. [Google Scholar] [CrossRef]
- Ahmed Djae, K.; Li, Z.; Li, Z.B. Temporalis muscle flap for immediate reconstruction of maxillary defects: Review of 39 cases. Int. J. Oral Maxillofac. Surg. 2011, 40, 715–721. [Google Scholar] [CrossRef]
- Clauser, L.; Curioni, C.; Spanio, S. The use of the temporalis muscle flap in facial and craniofacial reconstructive surgery. A review of 182 cases. J. Craniomaxillofac. Surg. 1995, 23, 203–214. [Google Scholar] [CrossRef]
- Abed, H.; Reilly, D.; Burke, M.; Daly, B. Patients with head and neck cancers’ oral health knowledge, oral health-related quality of life, oral health status, and adherence to advice on discharge to primary dental care: A prospective observational study. Spec. Care Dentist. 2019, 39, 593–602. [Google Scholar] [CrossRef]
- Nabil, S.; Samman, N. Incidence and prevention of osteoradionecrosis after dental extraction in irradiated patients: A systematic review. Int. J. Oral Maxillofac. Surg. 2011, 40, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Duncan, G.G.; Epstein, J.B.; Tu, D.; El Sayed, S.; Bezjak, A.; Ottaway, J.; Pater, J. Quality of life, mucositis, and xerostomia from radiotherapy for head and neck cancers: A report from the NCIC CTG HN2 randomized trial of an antimicrobial lozenge to prevent mucositis. Head Neck 2005, 27, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Lalla, R.V.; Treister, N.; Sollecito, T.; Schmidt, B.; Patton, L.L.; Mohammadi, K.; Hodges, J.S.; Brennan, M.T.; OraRad Study Group. Oral complications at 6 months after radiation therapy for head and neck cancer. Oral Dis. 2017, 23, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Elting, L.S.; Cooksley, C.D.; Chambers, M.S.; Garden, A.S. Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1110–1120. [Google Scholar] [CrossRef]
- Chen, J.A.; Wang, C.C.; Wong, Y.K.; Wang, C.P.; Jiang, R.S.; Lin, J.C.; Chen, C.C.; Liu, S.A. Osteoradionecrosis of mandible bone in patients with oral cancer--associated factors and treatment outcomes. Head Neck 2016, 38, 762–768. [Google Scholar] [CrossRef]
- Iqbal, Z.; Kyzas, P. Analysis of the critical dose of radiation therapy in the incidence of Osteoradionecrosis in head and neck cancer patients: A case series. BDJ Open 2020, 6, 18. [Google Scholar] [CrossRef]
- Louise Kent, M.; Brennan, M.T.; Noll, J.L.; Fox, P.C.; Burri, S.H.; Hunter, J.C.; Lockhart, P.B. Radiation-induced trismus in head and neck cancer patients. Support. Care Cancer 2008, 16, 305–309. [Google Scholar] [CrossRef]
- Lang, K.; Held, T.; Meixner, E.; Tonndorf-Martini, E.; Ristow, O.; Moratin, J.; Bougatf, N.; Freudlsperger, C.; Debus, J.; Adeberg, S. Frequency of osteoradionecrosis of the lower jaw after radiotherapy of oral cancer patients correlated with dosimetric parameters and other risk factors. Head Face Med. 2022, 18, 7. [Google Scholar] [CrossRef]
- Curi, M.M.; Dib, L.L. Osteoradionecrosis of the jaws: A retrospective study of the background factors and treatment in 104 cases. J. Oral Maxillofac. Surg. 1997, 55, 540–544, discussion 545–546. [Google Scholar] [CrossRef]
- Seo, M.H.; Eo, M.Y.; Myoung, H.; Kim, S.M.; Lee, J.H. The effects of pentoxifylline and tocopherol in jaw osteomyelitis. J. Korean Assoc. Oral Maxillofac. Surg. 2020, 46, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Dijkstra, P.U.; Huisman, P.M.; Roodenburg, J.L. Criteria for trismus in head and neck oncology. Int. J. Oral Maxillofac. Surg. 2006, 35, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Watters, A.L.; Cope, S.; Keller, M.N.; Padilla, M.; Enciso, R. Prevalence of trismus in patients with head and neck cancer: A systematic review with meta-analysis. Head Neck 2019, 41, 3408–3421. [Google Scholar] [CrossRef] [PubMed]
- Bhrany, A.D.; Izzard, M.; Wood, A.J.; Futran, N.D. Coronoidectomy for the treatment of trismus in head and neck cancer patients. Laryngoscope 2007, 117, 1952–1956. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, K.; Tanaka, T. Trismus in patients with malignant tumours in the head and neck. J. Laryngol. Otol. 1993, 107, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Browne, J.D.; Butler, S.; Rees, C. Functional outcomes and suitability of the temporalis myofascial flap for palatal and maxillary reconstruction after oncologic resection. Laryngoscope 2011, 121, 1149–1159. [Google Scholar] [CrossRef]
- Moreau, A.; Benassarou, M.A.; Benslama, L.; Goudot, P.; Schoumann, T. Anterior pedicle temporalis muscle flap interposition in the treatment of TMJ disorders. J. Stomatol. Oral Maxillofacial. Surg. 2018, 119, 325–327. [Google Scholar] [CrossRef]
- Yazdani, J.; Ali Ghavimi, M.; Pourshahidi, S.; Ebrahimi, H. Comparison of Clinical Efficacy of Temporalis Myofascial Flap and Dermal Graft as Interpositional Material in Treatment of Temporomandibular Joint Ankylosis. J. Craniofac. Surg. 2010, 21, 1218–1220. [Google Scholar] [CrossRef]
- Matsuo, K.; Palmer, J.B. Anatomy and Physiology of Feeding and Swallowing: Normal and Abnormal. Phys. Med. Rehabil. Clin. N. Am. 2008, 19, 691–707. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivam, A.; Garg, A.; Sillifant, P. Unforeseen Outcomes Post Treatment for Radiation Induced Trismus: A Case Report. Medicines 2022, 9, 31. https://doi.org/10.3390/medicines9050031
Sivam A, Garg A, Sillifant P. Unforeseen Outcomes Post Treatment for Radiation Induced Trismus: A Case Report. Medicines. 2022; 9(5):31. https://doi.org/10.3390/medicines9050031
Chicago/Turabian StyleSivam, Akash, Ankit Garg, and Paul Sillifant. 2022. "Unforeseen Outcomes Post Treatment for Radiation Induced Trismus: A Case Report" Medicines 9, no. 5: 31. https://doi.org/10.3390/medicines9050031
APA StyleSivam, A., Garg, A., & Sillifant, P. (2022). Unforeseen Outcomes Post Treatment for Radiation Induced Trismus: A Case Report. Medicines, 9(5), 31. https://doi.org/10.3390/medicines9050031





